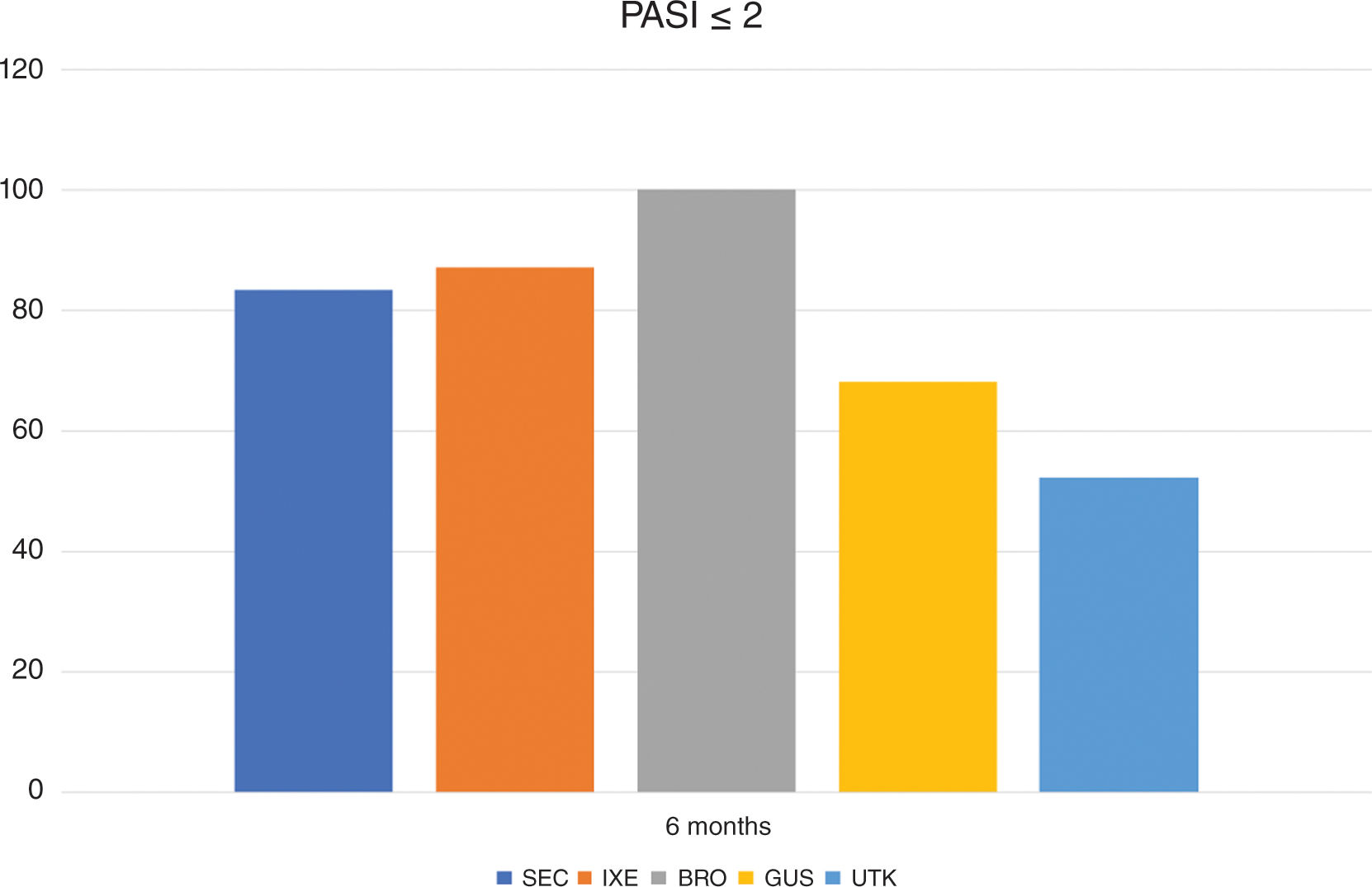Combinations of topical (TT) and biological therapies (BT) are a common thing in the routine clinical practice. However, the scientific medical literature on how TT is, actually, used after the initiation of BT is scarce, particularly in combination with anti-IL17, or anti-IL23.
ObjectivesTo describe the frequency of the concomitant use of TT + BT at baseline and after a 6-month course of several drugs (anti-IL17, ustekinumab, and anti-IL23). Our secondary endpoints are to describe the type of topical therapy used, compare the frequency of use of TT among the different groups of BT, describe the survival of topical therapy in these patients, and identify the factors that can impact the use or discontinuation of topical therapy in these patients (clinical response, quality of life, type of drug, etc.).
Materials and methodsThis was a retrospective, observational, and single-center study of patients with moderate-to-severe psoriasis treated with anti-IL17 (secukinumab, ixekizumab), anti-IL17R (brodalumab), ustekinumab, and guselkumab from January 2015 through December 2020.
ResultsWe included a total of 138 patients. When treatment started, 82.7% were on TT (55% daily), and after 6 months, 86.6% had discontinued TT. Regarding the analysis by type of drug, at 6 months, we found that 100% of the patients with BRO had discontinued topical treatment. We did not find any significant differences in the frequency of use of TT based on the BT used during the 6-month course of treatment. The estimated mean course of TT was 4.3 months (SD, 6.7). Also, the estimated mean course of TT was significantly shorter in the group of patients who achieved PASI100 (2.8 months vs. 8.1 months).
ConclusionsIn our cohort, we saw a significant decrease in the frequency of use of TT at 6 months after starting BT in the routine clinical practice. This reduction occurred earlier in patients who improved their objective clinical response and quality of life.
Las combinaciones de tratamiento tópico (TT) y terapia biológica (TB) son frecuentes en la práctica clínica. Sin embargo, la literatura en este ámbito acerca de cómo se emplea el TT tras el inicio de la TB es escasa, en particular respecto al TT combinado con anti-interleucina (IL)-17 o anti-IL-23.
ObjetivosDescribir la frecuencia de uso del TT concomitante a la TB al inicio y tras seis meses de tratamiento con distintos fármacos (anti-IL-17, ustekinumab [UTK] y anti-IL-23). Nuestros objetivos secundarios son: definir el tipo de terapia tópica empleada, comparar la recurrencia de uso de tópicos entre los distintos grupos de TB, describir la supervivencia del TT en estos pacientes y buscar factores que condicionen el uso o suspensión del mismo en estos sujetos (respuesta clínica, calidad de vida, tipo de fármaco, etc.).
Material y métodosEstudio retrospectivo observacional y unicéntrico en pacientes con psoriasis moderada-grave, tratados con anti-IL-17 (secukinumab [SEC], ixekizumab [IXE]), anti-IL-17R (brodalumab [BRO]), UTK y guselkumab [GUS] entre enero de 2015 y diciembre de 2020.
ResultadosIncluimos a 138 pacientes; al inicio del tratamiento, 82,7% empleaban TT (55% diariamente) y, tras seis meses, 86,6% había suspendido el TT. Respecto al análisis según el fármaco, a los seis meses destaca que 100% de los sujetos con BRO interrumpieron el TT. No encontramos diferencias significativas respecto a la frecuencia de uso de TT según la TB empleada a los seis meses de tratamiento. El tiempo medio estimado de utilización de TT fue de 4,3 meses (desviación estándar [DE] 6,7) y fue significativamente menor en el grupo de pacientes que alcanzaron el Psoriasis Area Severity Index (PASI)100 (2,8 vs. 8,1 meses).
ConclusionesEn nuestra cohorte, observamos una disminución significativa en la frecuencia de uso de TT a los seis meses después de iniciar una TB en la práctica clínica. Esta reducción fue más temprana en aquellos pacientes que experimentaron tanto una mejoría en la respuesta clínica objetiva como en la calidad de vida.
Psoriasis is a chronic and immune-mediated disease that affects between 1% and 4% of the adult population worldwide.1 Approximately 80% of patients with this condition have a mild form that can be controlled exclusively with topical treatment (TT). The remaining patients have moderate-to-severe psoriasis, which sometimes requires systemic therapies, either conventional or biological, for disease control.2 The progressively increasing effectiveness of biological therapies (BT) allows us to set demanding clinical and quality of life goals for individuals with moderate-to-severe psoriasis.3 However, sometimes, as monotherapy, BT does not achieve total clearance of the disease or does so immediately. The persistence of residual skin lesions can worsen the patients’ quality of life despite the clinical response being considered acceptable. Therefore, generally, in the routine clinical practice, after starting BT, prior TT is not discontinued, allowing individuals to use it on areas with persistent disease activity.
The combination of BT and TT could speed up the time to optimal response, extend the maintenance of the initial response to BT, provide proper treatment for refractory lesions in partial responders, and favor a reduction in the dose and side effects of BT.4
Currently, the interleukin (IL)-23/T helper (Th)17 axis is considered the main pathogenic pathway in the management of psoriasis, achieving excellent clinical responses with agents targeting this pathway. The greater efficacy of anti-IL-17 and anti-IL-23/p19 drug—compared to anti-TNF and anti-IL-12/IL-23 p40—would suggest that patients on these drugs would require fewer TT. Similarly, the speed of response of anti-IL-17 drugs5 would predict that subjects would discontinue TT earlier than with an anti-IL-23/p19 or anti-IL-23/p40.
Although clinical trials quantify the use of TT by patients, the frequency of TT use after the initiation of BT in the routine clinical practice has been poorly described. Furthermore, the factors impacting the use of TT in subjects treated with BT are not clearly established. Some studies demonstrate the benefit of combining TT with classical systemic treatments6 and with an anti-TNF.4,7–11. However, we have not found literature in the routine clinical practice on the combined regimen of topical drugs with anti-IL-17 or anti-IL-23.
Our main objective was to describe the frequency of concomitant use of TT and BT at baseline and after 6 months of treatment with various drugs (anti-IL-17, ustekinumab [UTK], and anti-IL-23). Our secondary objectives were to define the type of topical therapy used, compare the recurrence of topical use among different BT groups, describe TT survival, and look for factors impacting the use or discontinuation of TT in these patients.
Materials and methodsStudy designWe conducted a retrospective, observational study with patients older 18 years old with moderate-to-severe psoriasis, treated at the psoriasis monographic office of Hospital de la Princesa, Madrid, Spain with anti-IL-17 (secukinumab [SEC], ixekizumab [IXE]) and anti-IL-17R (brodalumab [BRO]), UTK, and guselkumab [GUS], from January 2015 through December 2020. We included subjects who initiated BT during this period and with, at least, a 6-month 6-follow-up. The study was approved by Hospital de la Princesa Research and Ethics Committee (CEIM 8/21).
Study variablesAt the first visit, sociodemographic data and clinical parameters were recorded (Appendix A of the supplementary data). As indices of severity and quality of life impact, the Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI) were collected at each visit. PASI75 and 90 were calculated for each patient to identify whether they had achieved these responses at each visit. We also coded whether the patients achieved absolute PASI ≤ 2 or not. Regarding the variable of interest (the use of TT), information was drawn from the data collected form the patients’ health records at each session. The frequency of use was translated as an ordinal variable, as follows: no use, use for 1 or 2 days/week, 3 to 4 days/week, 5 to 6 days/week, and daily use.
A combined variable was created that included parameters of clinical response (PASI) and quality of life (DLQI) to evaluate differences in the use of topicals in patients with good objective control who also showed improvement in their quality of life. This variable is based on the so-called “happyPASI” created by Van den Reek et al. to include quality of life parameters in the monitoring of drug use.12 These variables are referred to in this study as happyPASI2 (PASI <2 and DLQI <5) and happyPASI100 (PASI100 and DLQI 0 or 1).
Statistical analysisStatistical analysis was performed using SPSS/PC software (version 18.0 for Windows, SPSS Inc., Chicago, IL, United States). A Shapiro-Wilk test was conducted to check for normality. Descriptive studies of the different variables included were conducted as well.
Additionally, a Kaplan-Meyer study was conducted to determine the speed at which patients discontinued TT, considering censored data those from patients who had not discontinued TT until the date of the last follow-up, followed by Cox regression to analyze the variables associated with such discontinuation. P values <.05 were considered statistically significant.
ResultsA total of 138 patients treated with the following drugs were included: SEC (n=39), IXE (n=26), BRO (n=10), GUS (n=37), and UTK (n=26).
Regarding the demographic data of the overall cohort, our participants had the following parameters: mean age, 52.5 years (standard deviation [SD,], 15.4); median body mass index [BMI], 27.6 (interquartile range [IQR] 5.69); median weight, 80kg (IQR 23); and mean height, 169.8cm (SD, 9.4). The mean number of previous treatments, both systemic and biological drugs, was 3.6 (SD, 2.5). Regarding disease severity, the study participants had a mean baseline PASI of 12.6 (SD, 7.8) and a mean baseline DLQI of 12.1 (SD, 7.8).
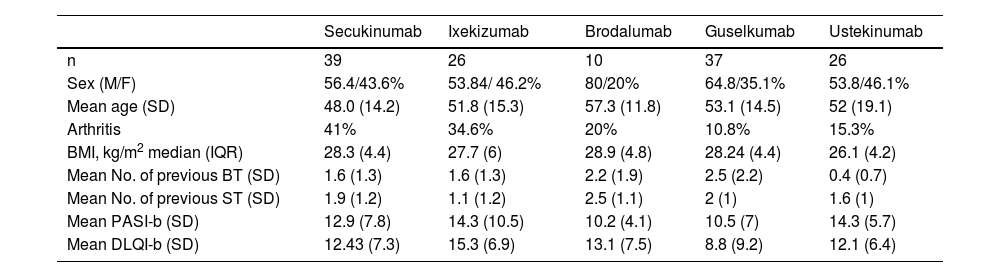
Table 1 shows the demographic data of the cohort based on the BT used.
Sociodemographic and baseline clinical characteristics based on the type of BT used.
| Secukinumab | Ixekizumab | Brodalumab | Guselkumab | Ustekinumab | |
|---|---|---|---|---|---|
| n | 39 | 26 | 10 | 37 | 26 |
| Sex (M/F) | 56.4/43.6% | 53.84/ 46.2% | 80/20% | 64.8/35.1% | 53.8/46.1% |
| Mean age (SD) | 48.0 (14.2) | 51.8 (15.3) | 57.3 (11.8) | 53.1 (14.5) | 52 (19.1) |
| Arthritis | 41% | 34.6% | 20% | 10.8% | 15.3% |
| BMI, kg/m2 median (IQR) | 28.3 (4.4) | 27.7 (6) | 28.9 (4.8) | 28.24 (4.4) | 26.1 (4.2) |
| Mean No. of previous BT (SD) | 1.6 (1.3) | 1.6 (1.3) | 2.2 (1.9) | 2.5 (2.2) | 0.4 (0.7) |
| Mean No. of previous ST (SD) | 1.9 (1.2) | 1.1 (1.2) | 2.5 (1.1) | 2 (1) | 1.6 (1) |
| Mean PASI-b (SD) | 12.9 (7.8) | 14.3 (10.5) | 10.2 (4.1) | 10.5 (7) | 14.3 (5.7) |
| Mean DLQI-b (SD) | 12.43 (7.3) | 15.3 (6.9) | 13.1 (7.5) | 8.8 (9.2) | 12.1 (6.4) |
BMI, body mass index; BT, biological therapy; DLQI, Dermatology Life Quality Index; IQR, interquartile range; PASI, Psoriasis Area Severity Index; SD, standard deviation; ST, systemic therapy.
In the overall cohort, after 6 months of treatment, 51.1% of patients achieved PASI100; 56.6% achieved PASI90, and 74.6% achieved PASI ≤ 2.
The progression of the PASI <2 response based on the treatment group is shown in Figure 1.
At 6 months, 50.4% of patients achieved complete clearance, along with minimal or absent impact on quality of life (DLQI 0 or 1), the variable called happyPASI100. In addition, 60.4% achieved happyPASI <2.
Frequency of topical useRegarding the type of TT used for body and extremities, 100% used fixed combinations of calcipotriol/betamethasone. Among them, 75% also used corticosteroids, mainly clobetasol propionate 0.05%. A total of 40% opted for mometasone lotion on the scalp. Other products were used very rarely. Given the homogeneity in the use of topical products and that it remained consistent during different visits, from now on, all 3 will be grouped under the term TT and analyzed together.
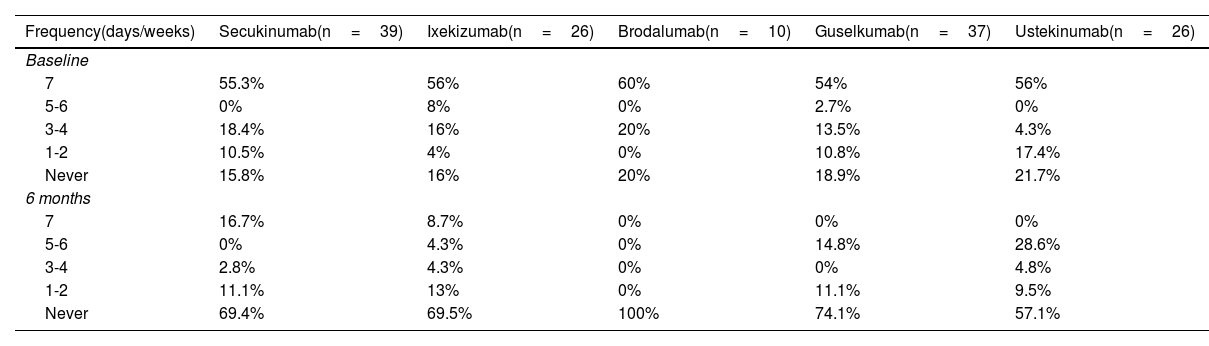
The overall frequency rate of baseline TT use was: 55%, daily; 2.7%, 5 to 6 days per week; 16.4%, 3 to 4 days a week; 8.2%, 1 to 2 days per week, and 17.3% did not use TT. On the other hand, 6 months after the start of BT, only 5.7% of patients were using TT daily, while 86.8% reported not using it.
Regarding the analysis by drug, at 6 months we saw that 100% of patients on BRO suspended TT. Table 2 specifies the frequency of TT use based on the BT used.
Frequency of topical use at baseline and at 6 months based on treatment group.
| Frequency(days/weeks) | Secukinumab(n=39) | Ixekizumab(n=26) | Brodalumab(n=10) | Guselkumab(n=37) | Ustekinumab(n=26) |
|---|---|---|---|---|---|
| Baseline | |||||
| 7 | 55.3% | 56% | 60% | 54% | 56% |
| 5-6 | 0% | 8% | 0% | 2.7% | 0% |
| 3-4 | 18.4% | 16% | 20% | 13.5% | 4.3% |
| 1-2 | 10.5% | 4% | 0% | 10.8% | 17.4% |
| Never | 15.8% | 16% | 20% | 18.9% | 21.7% |
| 6 months | |||||
| 7 | 16.7% | 8.7% | 0% | 0% | 0% |
| 5-6 | 0% | 4.3% | 0% | 14.8% | 28.6% |
| 3-4 | 2.8% | 4.3% | 0% | 0% | 4.8% |
| 1-2 | 11.1% | 13% | 0% | 11.1% | 9.5% |
| Never | 69.4% | 69.5% | 100% | 74.1% | 57.1% |
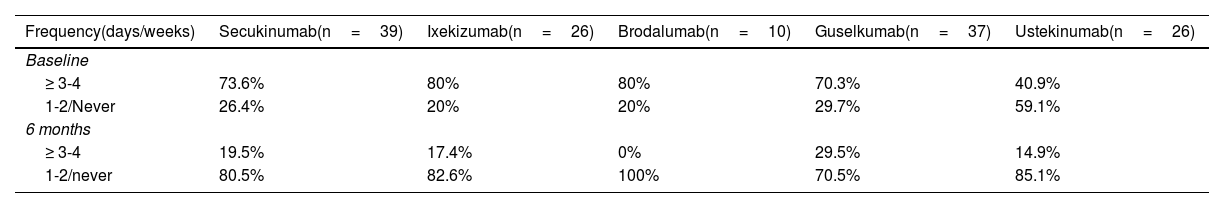
When categorizing the different frequencies of TT use into 2 categories, we saw that at the beginning of therapy, patients treated with UTK used less TT (chi-square test, p=0.03). We did not obtain significant differences (chi-square test, p=0.479) after 6 months on therapy (table 3).
Frequency of topical use at baseline and at 6 months.
| Frequency(days/weeks) | Secukinumab(n=39) | Ixekizumab(n=26) | Brodalumab(n=10) | Guselkumab(n=37) | Ustekinumab(n=26) |
|---|---|---|---|---|---|
| Baseline | |||||
| ≥ 3-4 | 73.6% | 80% | 80% | 70.3% | 40.9% |
| 1-2/Never | 26.4% | 20% | 20% | 29.7% | 59.1% |
| 6 months | |||||
| ≥ 3-4 | 19.5% | 17.4% | 0% | 29.5% | 14.9% |
| 1-2/never | 80.5% | 82.6% | 100% | 70.5% | 85.1% |
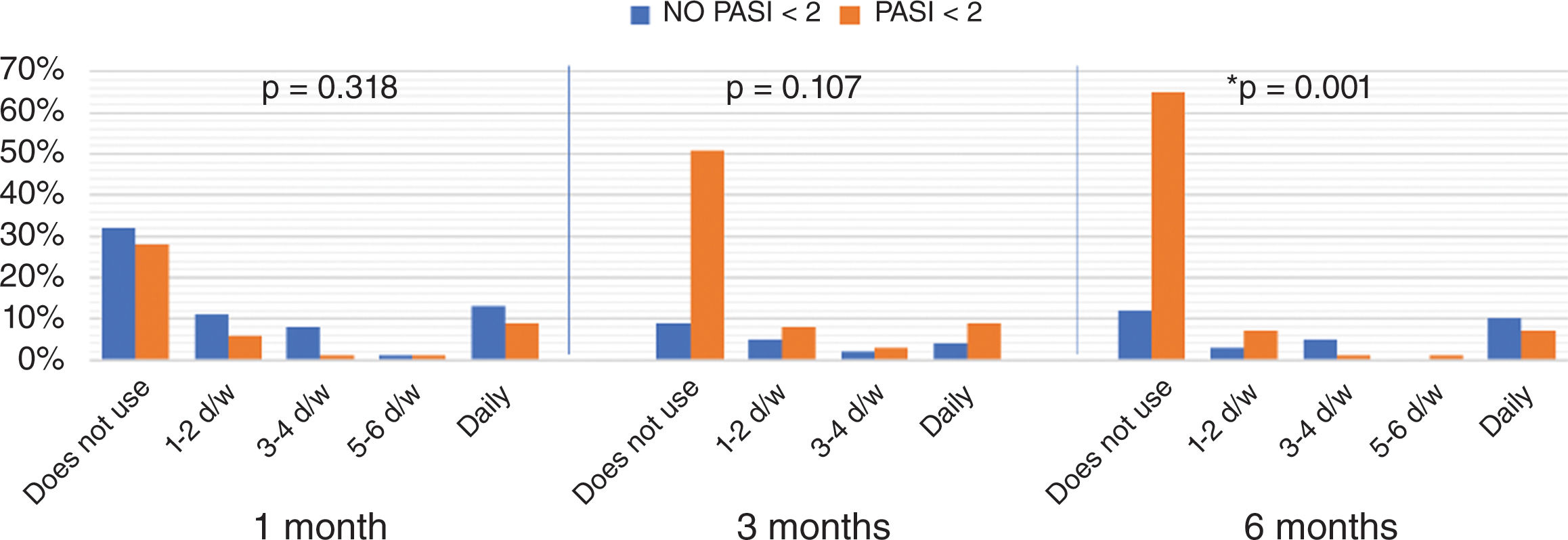
When comparing the distribution of frequencies of topical use between patients who achieved an absolute PASI <2 vs those who did not, a reduction in recurrence was seen, with statistically significant differences from 6 months of BT onwards (fig. 2).
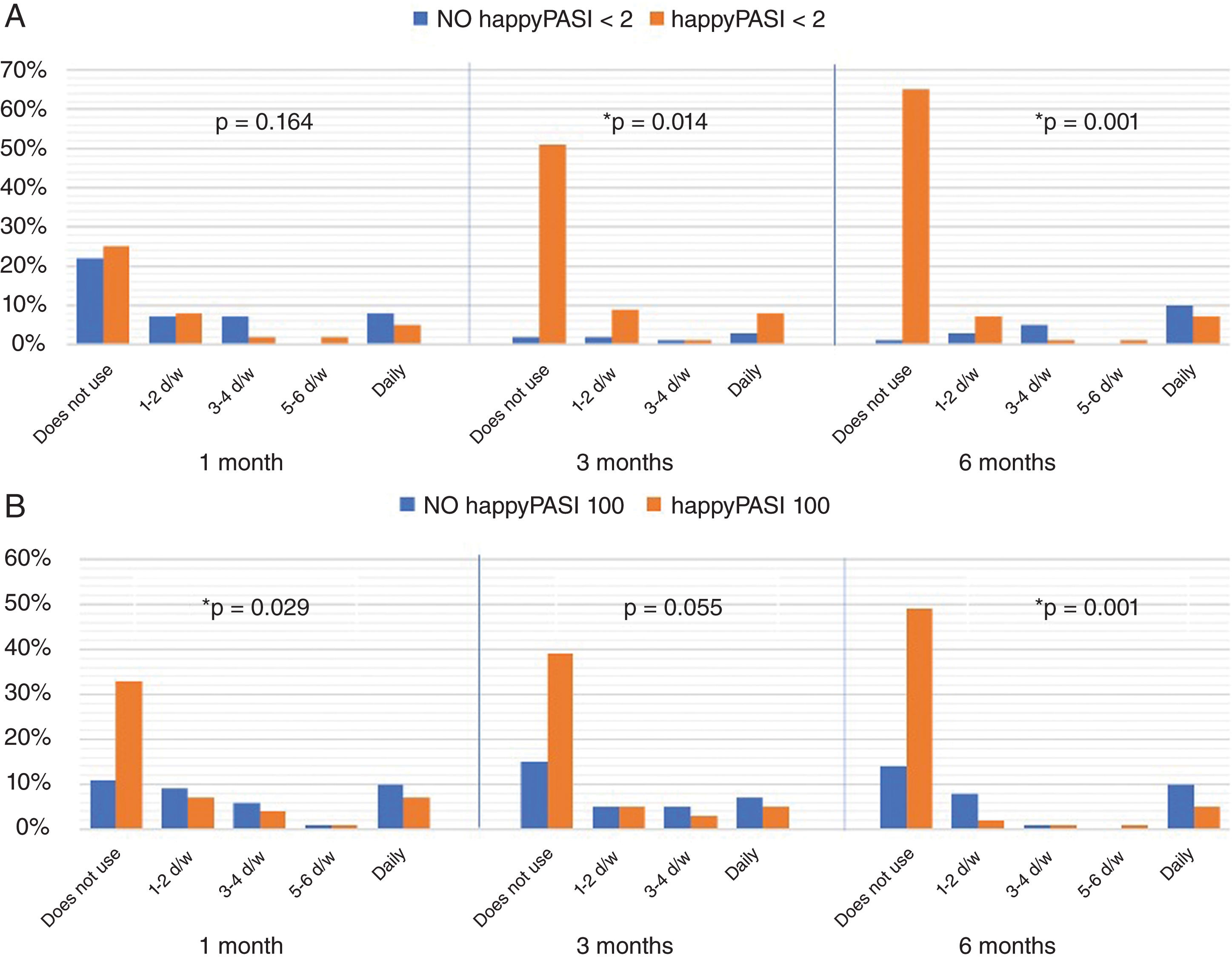
When we added quality of life parameters, we observed that the differences between patients who achieved a happyPASI2 and those who did not were statistically significant from 3 months onwards (fig. 3). Finally, when we compared study participants who achieved a happyPASI100 to those who did not, we noticed significant differences between the 2 groups from the first month of treatment. In the group that achieved happyPASI100, at 1 month, 33% did not use TT (P=.029), at 3 months, 39% (P=.055), and at 6 months, 49% (P<.001) (fig. 3).
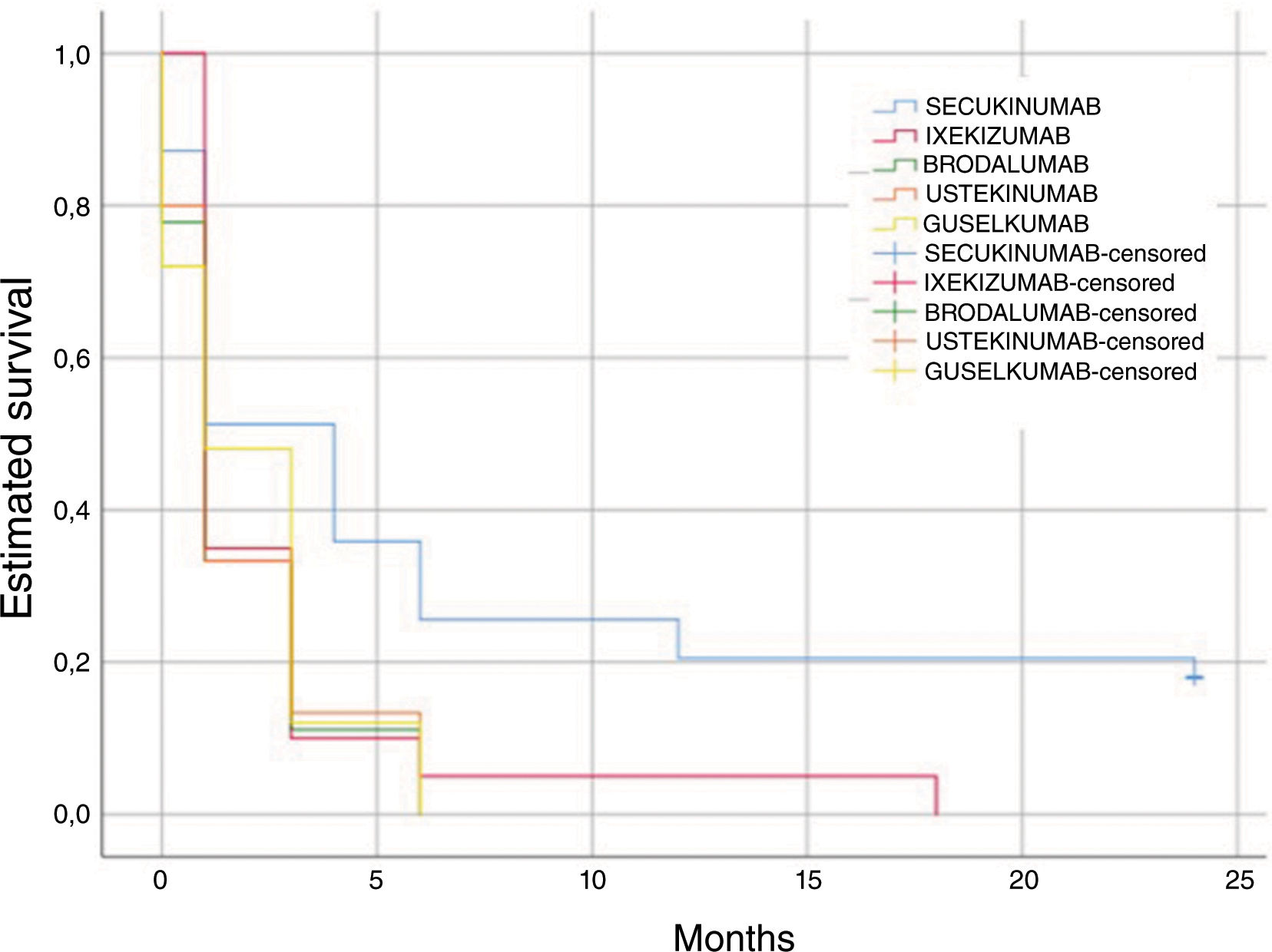
Survival of topical useIn our cohort, the estimated mean time for TT use was 4.3 months (SD, 6.7). Individually, in the subgroup on BRO, a shorter mean time of TT use was seen (1.7 months), followed by UTK (1.8 months), GUS (2 months), IXE (2.6 months), and lastly SEC (7.1 months) (Fig. 4). When comparing different drugs, the differences were statistically significant (log rank, p=0.027).
Additionally, we found that the estimated mean time of TT use was significantly shorter in the group of patients who achieved PASI100 (2.8 vs 8.1 months).
In the 6 month of treatment, gender, age, presence of psoriatic arthritis, location of psoriasis, BMI, baseline PASI, and PASI90 were not associated with TT suspension.
DiscussionWe described a series of 138 patients with moderate-to-severe psoriasis treated with BT along with TT in the routine clinical practice.
In our cohort—6 months into BT with anti-IL-17 and anti-IL-23—the number of patients who did not require TT went up to 86.8%. This represents a 76.8% reduction in topical use in the overall cohort. In all BT groups, a decrease in TT use was observed, with BRO and GUS being the drugs with the highest percentage of participants reporting no need for TT. Specifically, BRO showed 100% TT suspension at 6 months, and it was also associated with a shorter estimated time of TT use. One possible explanation for this could be the association of the IL-17 receptor blocking mechanism with a faster response.5 This could also be associated with the high levels of PASI100 response achieved and maintained in our series in patients on BRO. Despite being the drug with the lowest efficacy in clinical trials, in our series, UTK was the second drug in which subjects showed a faster discontinuation of topical treatments, although the small sample size could impact this result. In the SEC-treated group, we found individuals with PASI100 who reported daily topical use. In these cases, TT could be contributing to controlling areas with minimal disease activity.
Specific literature on the combination of TT and drugs targeting IL-17 and IL-23 is scarce. Only Bernardini et al. included this combination in their study of 60 patients treated with biologics—13.3% of whom were treated with anti-IL-17 without mentioning the specific drug. In this study, a higher percentage of individuals who achieved PASI90 and DLQI ≤ 1 was observed in the group using TT plus anti-IL-17.8 Gooderham et al. reported that, in patients treated with etanercept, failing to achieve a PASI90 response on week 12 predicted TT use.11 In our cohort, we did not find significant differences regarding the PASI90 response but with PASI100. Therefore, we could consider the absence of complete response at 6 months as a predictor of topical use. Probably, some of these patients, despite presenting good control (PASI90), do not achieve complete clearance, and TT helps control the disease in the most resistant plaques. On the other hand, those who achieved complete clearance and a DLQI 0-1 suspended TT earlier than those who did not.
We did not find an association between TT use and age, although greater satisfaction with TT use in older patients has been reported.13 We did not find any differences either in TT use based on sociodemographic variables or those associated with the initial severity of the disease, or the site affected by psoriasis.
LimitationsThe main limitations of the study are its observational and retrospective design. Consequently, some of the data on the frequency of topical use collected in the health records may be incomplete. Another issue is the heterogeneous number of patients included in each treatment group.
However, being a study conducted in a center where specialists involved in patient follow-up systematically collect data on TT frequency of use, we believe the information can be applicable to other psoriatic cohorts on BT. Additionally, the fact of measuring patient satisfaction, apart from their clinical response, may allow us to deduce preferences and improve the feeling of empowerment.
ConclusionsIn our cohort, we observed a significant decrease in the frequency of TT use at 6 months after initiating BT in clinical practice. This reduction happened earlier in individuals who improved both their objective clinical response and quality of life. Complete clearance is associated with less use of TT, meaning that confirmation of this can be an additional piece of information on efficacy, since achieving PASI100 in biologic monotherapy or at the expense of continuing to apply topical treatments daily makes a big difference to the patient. Compared to other series, sociodemographic variables did not seem to have an impact on the frequency of TT use. Therefore, our results suggest that recording this variable can be a useful tool in the routine clinical practice for clinicians (as an indirect measure of BT effectiveness) and patients alike (as it allows for a more objective quantification of their progress since the start of BT).
FundingNone declared
Conflicts of interestS. Berenguer-Ruiz, M. Romero-Dávila, M. Aparicio-Domínguez, and M. Olivares-Guerrero declared no conflicts of interest whatsoever.
E. Daudén has potential conflicts of interest (serving on advisory boards, consulting, research support, participating in clinical trials, and receiving speaker's fees) with the following pharmaceutical companies: AbbVie, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD, Lilly, and Celgene.
M. Llamas-Velasco has potential conflicts of interest too (serving on advisory boards, consulting, providing research support, participating in clinical trials, and receiving speaker's fees) with the following pharmaceutical companies: AbbVie, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Lilly, Celgene, UCB, and Boehringer Ingelheim.