Clear cell acanthoma (CCA) is an uncommon lesion histologically characterized by the presence of epidermal acanthosis with keratinocytes containing clear cytoplasm. Although many single cases of CCA have been described, few case series have been published. The aim of this study was to describe the clinical characteristics of CCA in our practice setting.
Material and methodsRetrospective study of patients diagnosed with CCA at Hospital Universitario de Bellvitge in Barcelona, Spain, between 1995 and 2021. We conducted a chart review to record age, sex, number and location of lesions, diameter, time since onset, clinical characteristics, suspected clinical diagnosis, and treatment.
ResultsSeventy patients (30 women and 40 men) with a mean (SD) age of 62 (13) years were diagnosed with CCA during the study period. Median (interquartile range) time since onset was 2 (4) years and median lesion diameter was 6 (5)mm. One woman had multiple lesions. Lesions were located on the lower extremities in 57 patients (81%), the posterior aspect of the trunk in 8 (11%), the anterior aspect of the trunk in 4 (5%), and the upper extremities in 1 (1%). CCA was clinically suspected in 40% of patients seen by dermatologists.
ConclusionsCCA presents as an erythematous, dome-shaped lesion with pinpoint vessels and an epidermal collarette. The accuracy of clinical diagnosis has improved relative to earlier series, possibly due to a better clinical understanding of this lesion and a greater use of dermoscopy.
El acantoma de células claras (ACC) es una lesión poco frecuente caracterizada histológicamente por la presencia de acantosis epidérmica a expensas de queratinocitos de citoplasma claro. Aunque se han descrito muchos casos clínicos de forma individual, se han publicado pocas series de pacientes con ACC. Nuestro objetivo fue analizar las características clínicas del ACC en nuestra población.
Material y métodosEstudio retrospectivo de los pacientes con ACC diagnosticados en el Hospital Universitario de Bellvitge en Barcelona, España, entre 1995-2021. Se revisaron las historias clínicas para obtener los siguientes datos: edad, sexo, localización, número de lesiones, diámetro, tiempo de evolución, características clínicas de los tumores, diagnóstico clínico de sospecha y tratamiento realizado.
ResultadosSetenta pacientes con ACC fueron incluidos en el estudio (30 mujeres y 40 varones, edad media: 62 años, DE: 13). La mediana del tiempo de evolución fue de 2 años, rango intercuartílico (RIQ): 4 y la del diámetro 6mm, RIQ: 5. Una paciente presentó lesiones múltiples. Las lesiones se localizaron en las extremidades inferiores en 57 pacientes (81%), en la cara posterior del tronco en 8 pacientes (11%), la cara anterior del tronco en 4 pacientes (5%) y en las extremidades superiores en un paciente (1%). Se planteó el diagnóstico clínico de ACC en el 40% de los pacientes atendidos por dermatólogos.
ConclusionesEl aspecto clínico característico del ACC es el de una lesión cupuliforme eritematosa con punteado vascular y collarete epidérmico. La proporción de ACC diagnosticados clínicamente ha mejorado respecto a series antiguas, quizás por un mayor conocimiento clínico de la entidad y un mayor uso de la dermatoscopia.
Clear cell acanthoma (CCA) was first described by Degos et al.1 in 1962. It is an uncommon benign lesion of unknown origin that typically manifests as a dome-shaped red-brown papule characterized histologically by the presence of epidermal acanthosis with pale glycogen-containing keratinocytes.2
While many individual cases have been reported, few extensive series of cases of CCA have been published.3–5
Our objective was to review the clinical characteristics of patients diagnosed with CCA in our population.
Material and MethodsWe performed a retrospective observational study of cases classed as CCA in the database of the histopathology department of our hospital between 1995 and 2021. Ours is an 800-bed university hospital that provides health care to a catchment population of approximately 1000000 people. We reviewed the clinical histories and collected the following data: age at diagnosis, sex, location of the lesions (lower limbs, upper limbs, anterior trunk, posterior trunk, head, and neck), number of lesions, diameter of the tumor (mm), time between onset and diagnosis (years), the suspected clinical diagnosis (CCA, seborrheic keratosis, Bowen disease, angioma, dermatofibroma, basal cell carcinoma, squamous cell carcinoma, melanocytic nevus, other), and treatment (surgical excision, electrocautery, cryotherapy). As for the clinical characteristics of the tumor, we also recorded the presence or absence of the following: hemispherical shape, erythematous appearance, pain, pinpoint vessels, epidermal collarette and whether this was painful.
The data obtained were analyzed using SPSS Statistics for Windows, Version 17.0 (SPSS Inc.). Categorical variables were compared using the Fisher exact test. Continuous variables were compared using the t test when normally distributed (confirmed by the Kolmogorov–Smirnov test). If the distribution was not normal, the Mann-Whitney test was applied. Statistical significance was set at P<.05. The parameters were compared according to sex.
ResultsWe identified 70 patients with a histologic diagnosis of CCA (30 women, 40 men; mean [SD] age, 62 [13] years [range, 31–96 years]). The median (interquartile range [IQR]) time to diagnosis was 2 (4) years. The diameter of the lesions ranged between 4mm and 35mm, with a median (IQR) of 6 (5)mm.
One patient had developed multiple lesions (8 in total, all on the lower limbs). The lesions were located on the lower limbs in 57 cases (81%), posterior trunk in 8, anterior trunk in 4, and upper limbs in 1. No lesions were detected on the head, neck, or acral skin.
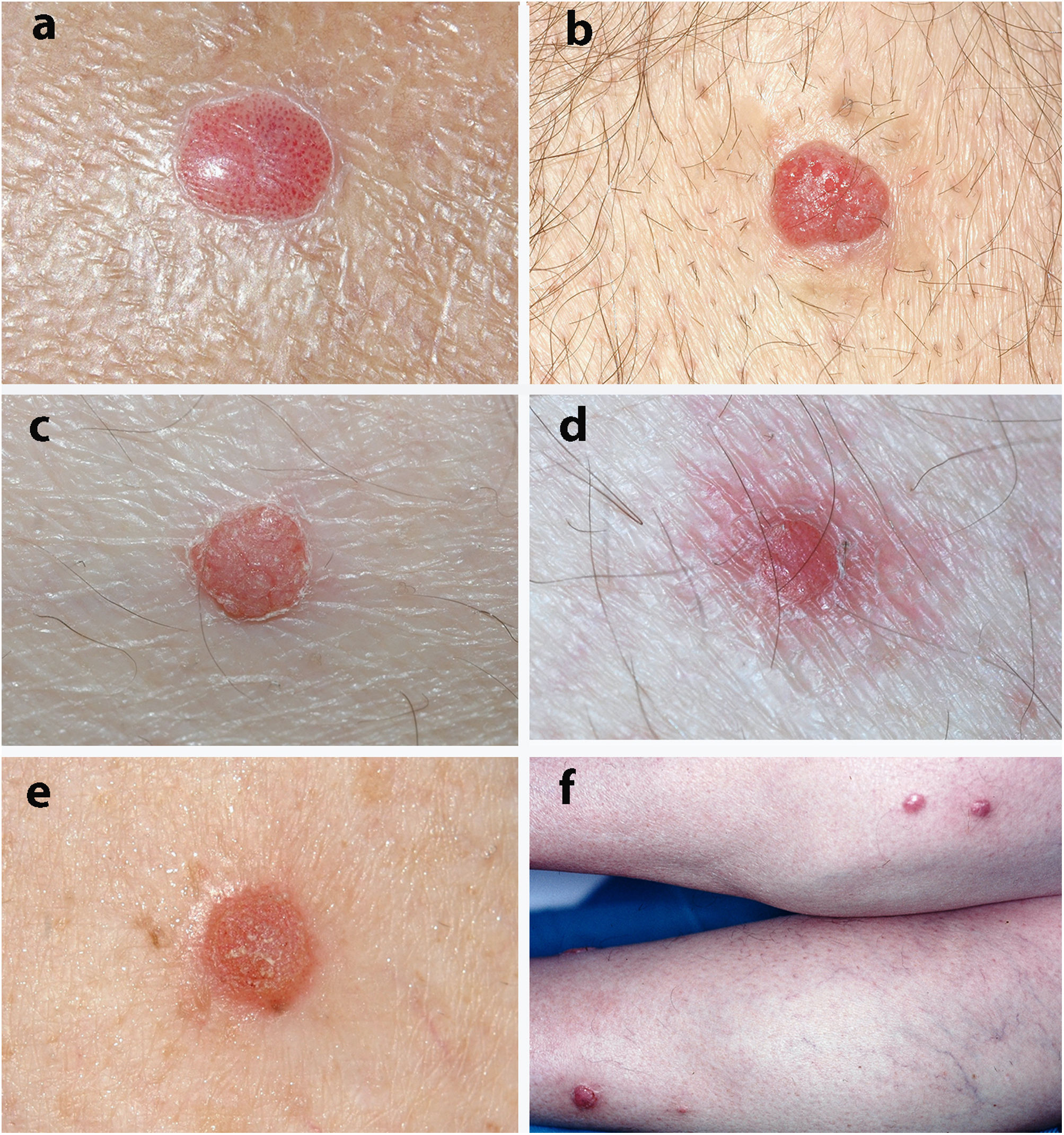
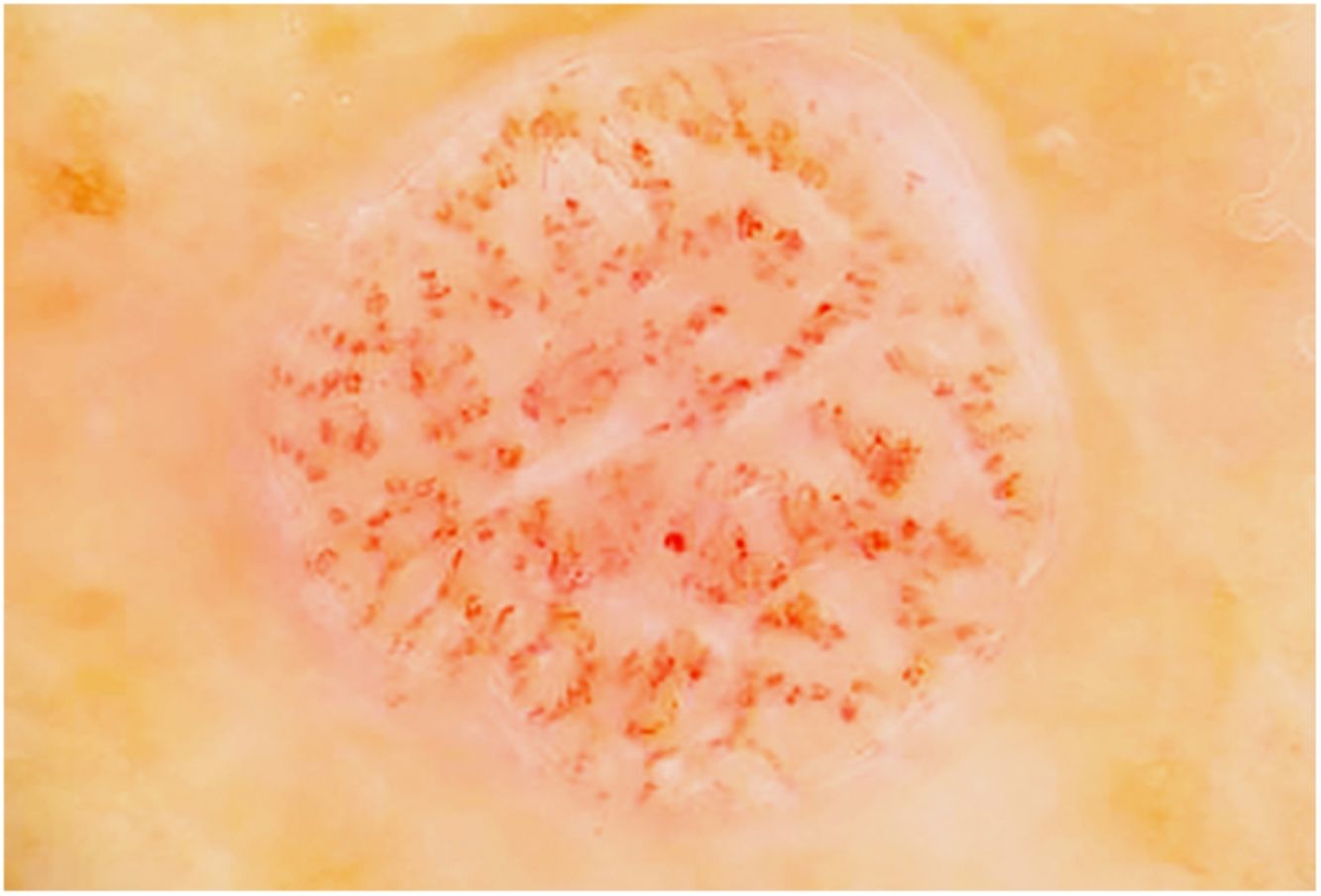
The most frequent clinical manifestation was that of a dome-shaped nodular lesion with pinpoint vessels and a peripheral epidermal collarette (12 cases). A further 12 cases involved erythematous nodules or papules, 3 cases involved pigmented nodules, 3 scaly nodules, 3 that were vascular in appearance, 3 that were of normal color, 2 violaceous cases, and a psoriasiform case. In 4 cases, the clinical history was remarkable in that the lesions tended to bleed. Only 1 lesion was described as painful.
The most frequent clinical diagnoses were CCA (24 cases), seborrheic keratosis (12 cases), basal cell carcinoma (10 cases), dermatofibroma (9 cases), squamous cell carcinoma (8 cases), angioma (8 cases), and Bowen disease (7 cases).
Sixty cases were treated by dermatologists, and 10 by other specialists. Only the dermatologists proposed a clinical diagnosis of CCA (24 of 60 cases, 40%).
Treatment involved complete surgical excision in 39 patients. The remaining lesions were treated with electrocautery or cryotherapy after histological confirmation of the diagnosis. As CCA is a benign lesion, patients were not clinically followed up, and we only have a report of 1 local recurrence after cryotherapy.
Fig. 1 shows the clinical appearance of some of the lesions.
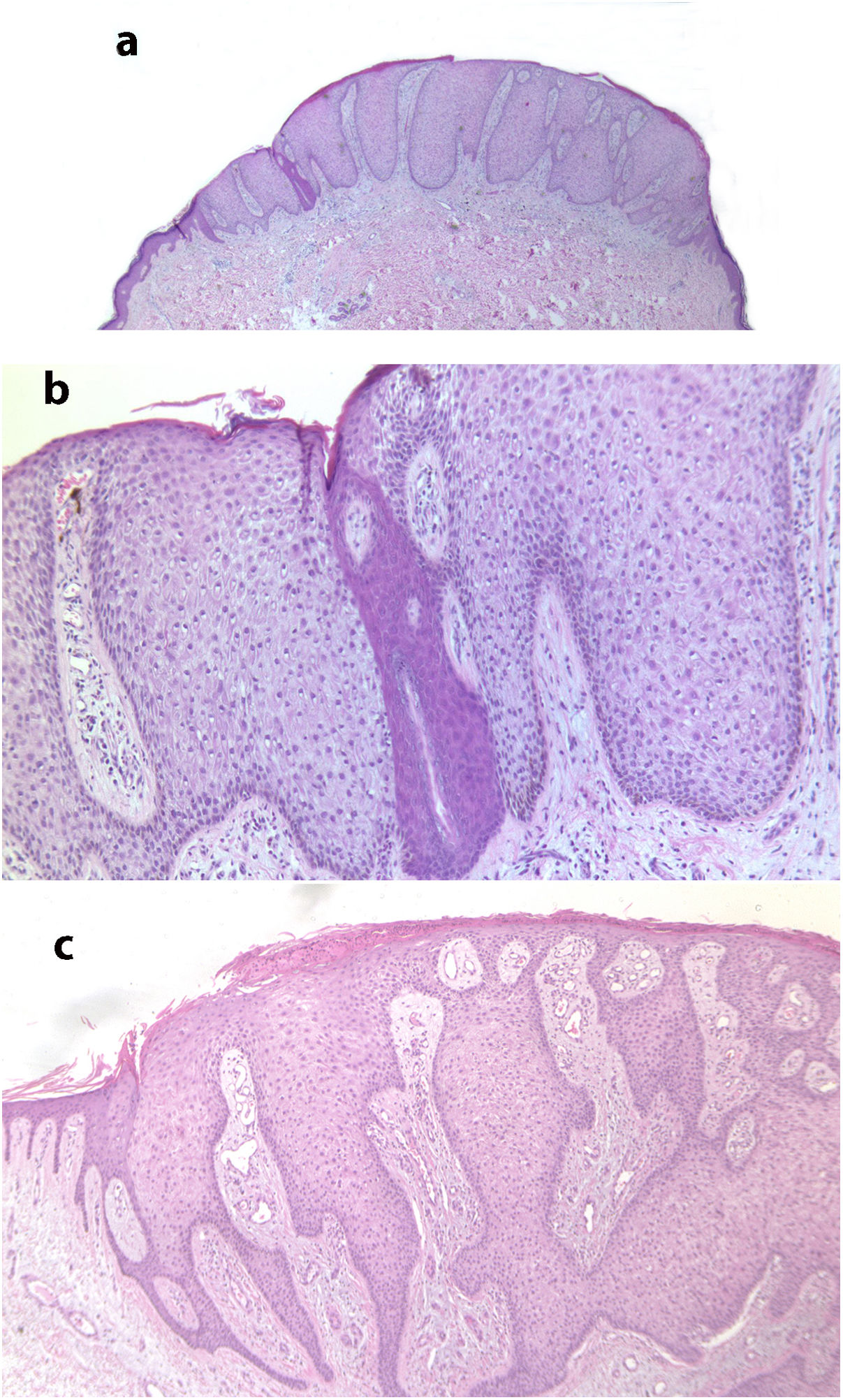
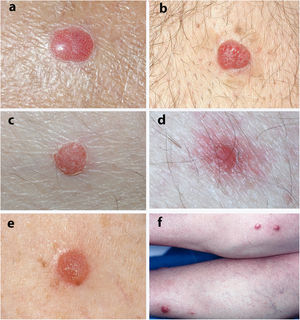
Fig. 2 shows the typical histologic characteristics of CCA.
A, Histological image at low magnification of clear cell acanthosis showing a well-defined area of psoriasiform epidermal hyperplasia, with acanthosis and keratinocytes with clear cytoplasm (hematoxylin–eosin, ×20). B, Detail of the previous lesion showing an abrupt border between clear cells and acrosyringeal cells (hematoxylin–eosin, ×100). C, Image showing clear cell acanthosis with papillomatosis and marked acanthosis with keratinocytes with a clear cytoplasm, as well as parakeratosis and exocytosis of neutrophils on the surface (hematoxylin–eosin, ×40).
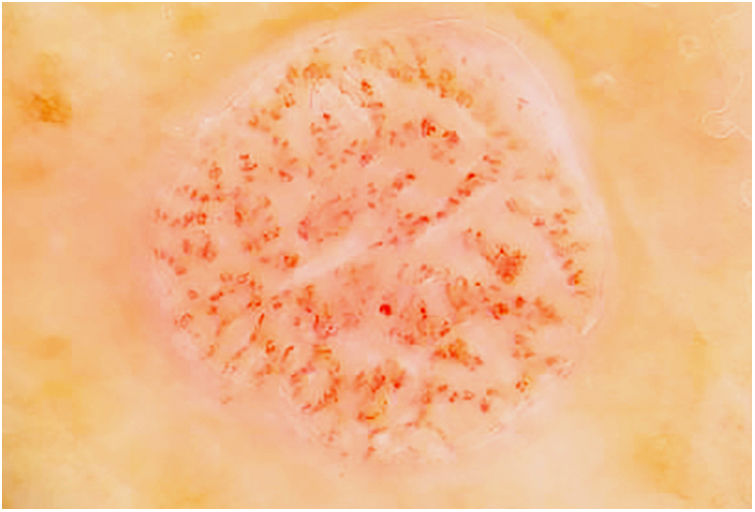
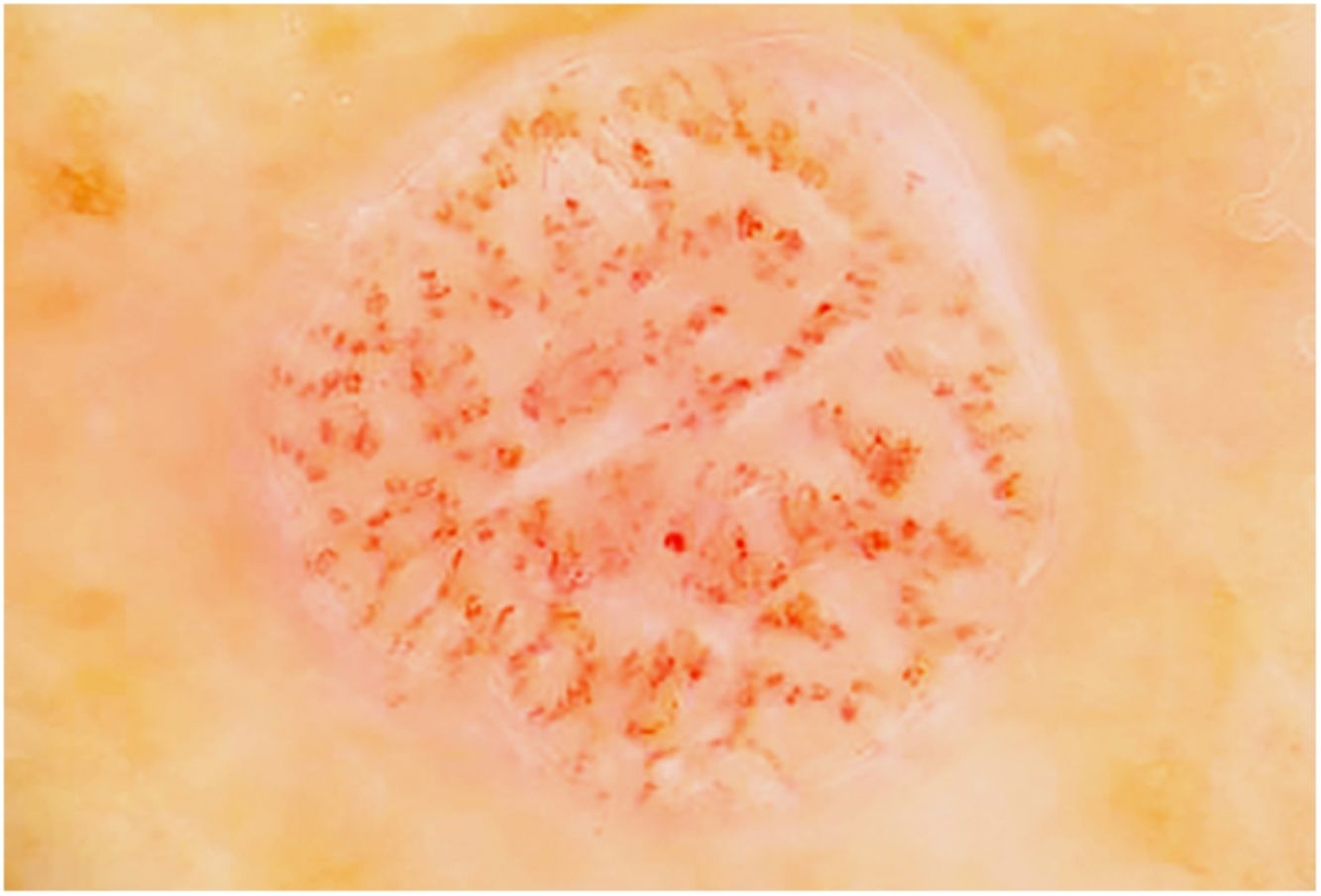
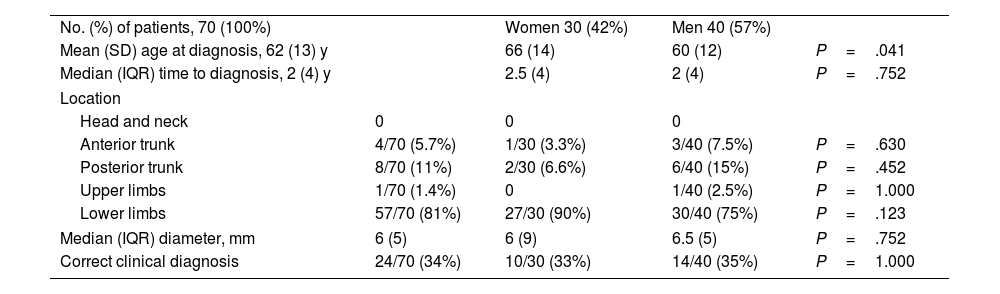
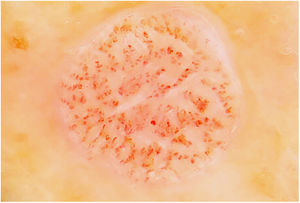
Fig. 3 shows a typical dermoscopic image of CCA.
Table 1 shows the results of a comparison of the clinical characteristics of CCA between men and women. The only statistically significant difference found was for age, i.e., men were significantly younger than women (60 vs. 66 years, P=.041).
Clinical Characteristics of 70 Patients With Clear Cell Acanthosis by Sex.
| No. (%) of patients, 70 (100%) | Women 30 (42%) | Men 40 (57%) | ||
| Mean (SD) age at diagnosis, 62 (13) y | 66 (14) | 60 (12) | P=.041 | |
| Median (IQR) time to diagnosis, 2 (4) y | 2.5 (4) | 2 (4) | P=.752 | |
| Location | ||||
| Head and neck | 0 | 0 | 0 | |
| Anterior trunk | 4/70 (5.7%) | 1/30 (3.3%) | 3/40 (7.5%) | P=.630 |
| Posterior trunk | 8/70 (11%) | 2/30 (6.6%) | 6/40 (15%) | P=.452 |
| Upper limbs | 1/70 (1.4%) | 0 | 1/40 (2.5%) | P=1.000 |
| Lower limbs | 57/70 (81%) | 27/30 (90%) | 30/40 (75%) | P=.123 |
| Median (IQR) diameter, mm | 6 (5) | 6 (9) | 6.5 (5) | P=.752 |
| Correct clinical diagnosis | 24/70 (34%) | 10/30 (33%) | 14/40 (35%) | P=1.000 |
Abbreviation: IQR, interquartile range.
In the comparative analysis of tumor characteristics according to whether the suspected diagnosis was correct, the only significant differences were between the percentage of cases diagnosed by a dermatologist and cases diagnosed by another specialist (P=.01).
DiscussionThe pathogenesis of CCA is unknown. Furthermore, it is unclear whether the lesion is a benign neoplasm or a reactive inflammatory skin disease.3,4,6 The factors pointing to a neoplastic origin include its well-defined borders, persistence, and the fact that it is almost always a solitary lesion.6 More recently, it was suggested that CCA could be a reactive inflammatory skin disease, since the expression of cytokines in immunohistochemistry is similar to that observed in inflammatory skin diseases such as psoriasis, lichen planus, and discoid lupus erythematosus.6–9 There have also been reports of CCA on pre-existing psoriatic plaques and cases of eruptive CCA with spontaneous regression in some lesions, thus further supporting this argument.10,11 There is no evidence pointing to a possible traumatic or toxic origin, and neither papillomavirus nor herpesvirus has been identified in the lesions.6 The absence of other significant skin lesions in our series at diagnosis of CCA favors a tumor over a reactive process. However, the patients we report were not followed up in the long term, and we do not know whether they subsequently developed lesions of skin diseases such as psoriasis.
According to reviews and text books, CCA affects both sexes equally, and most cases correspond to middle-aged persons, with incidence peaking between the ages of 50 and 60 years.2,12 However, published series show the mean age to be >60 years, with the lesion more commonly affecting men (1.2:1 and 1.3:1).4,5 In line with these series, we also observed a greater incidence in men (1.3:1) and a mean age higher than 60 years (63 years).
In clinical terms, CCA usually appears as a solitary papule or nodule that is dome-shaped, red or brown, and surrounded by a fine epidermal collarette.2,4,12 It frequently presents with pinpoint vessels on the surface and may bleed on even minimal injury.2,4,12 The surface may also be crusted or moist.12 CCA grows slowly over several years and varies between 3mm and 20mm in diameter.3,12 In the cases we report, the most frequent clinical manifestation was as a dome-shaped nodule with pinpoint vessels and a peripheral epidermal collarette; in these cases, the diagnosis of CCA was suspected based on clinical findings. Consistent with these data from the literature, the median time to diagnosis in our series was 2 years, and the median diameter was 6mm.
CCA is generally found on the lower limbs. However, in the longest series reported, by Morrison et al.5, it is noteworthy that only 53% were found on the lower limbs, whereas in that of Brownstein et al.4 and in the review by Degos and Civatte3 the corresponding figures were 95% and 81%, respectively. In our study too, the lesions were found on the lower limbs in more than 80% of the patients (81%).
The lesions are usually solitary, although multiple CCAs have been reported (38 cases up to 2016).13 Only 1 of our patients presented multiple lesions (8 on the lower limbs). CCA has also been reported on the oral mucosa,14 although not in our series.
While the clinical and dermoscopic appearance may be distinctive, CCA was not diagnosed based on clinical findings in the few large case series reported.6 Clinical diagnosis had a sensitivity of 2.7% in one study,5 5.4% in another,4 and 10% in a review of 104 cases.3 In our study, a diagnosis of CCA was proposed in 24 of 70 cases (34%); however, if we restrict cases to those treated by dermatologists, the diagnosis was suspected based on clinical data in 40% of cases. These differences may arise because some series are old, and the condition was not as well recognized as at present. More widespread application of dermoscopy in recent years may have also played a role. Furthermore, in the comparison of tumor characteristics according to whether the suspected diagnosis was confirmed, we only detected significant differences between the percentage of cases diagnosed by a dermatologist and cases diagnosed by other specialists, thus suggesting that most nondermatologists are not aware of the condition.
In dermoscopy, CCA is characterized by the presence of dotted blood vessels, erythematous globules, or glomerular vessels arranged in a reticular pattern or pearl necklace–like pattern.15,16 Either of these patterns differentiates CCA from other lesions that also contain glomerular or dotted vessels, such as psoriasis, pityriasis lichenoides, and Bowen syndrome.15,16 In some cases, we can also observe areas of bleeding, orange-colored crusts, and a peripheral collarette of translucent scales.15
Histologically, CCA is characterized by a well-defined area of psoriasiform epidermal hyperplasia with an abrupt transition between the lesional epidermis and the adjacent healthy epidermis.4,17 It comprises enlarged keratinocytes that are pale in color owing to the intracellular accumulation of glycogen that stains positive with periodic acid–Schiff and is sensitive to diastase.4,17 The basal cell layer of the epidermis, the infundibulum, and the acrosyringium are not affected.17 We can also observe spongiosis and exocytosis of numerous polymorphonuclear leukocytes that can form parakeratotic microabscesses.2,4 Mitosis is rare, and cellular pleomorphism is not observed.4 The papillary dermis is edematous and the superficial veins and capillaries are increased in number.2 Dilated vessels in the superficial dermis could be due to venous stasis, since they are not observed in lesions not found on the lower limbs.4 Immunohistochemistry reveals release of the enzyme phosphorylase in keratinocytes, which is necessary for degradation of glycogen.17 Pigmented CCA is characterized by a higher number of intraepidermal melanocytes with melanin inside the lesion and melanophages in the dermis.9 In the present series, we observed 3 clinically pigmented lesions, for which the clinical diagnosis proposed was melanocytic nevus or seborrheic keratosis. Some of the characteristics of CCA may overlap with those of psoriasiform keratosis, such as the presence of neutrophils in the stratum corneum and suprapapillary thinning. However, in CCA, we can observe an abrupt border between normal keratocytes and glycogenated keratinocytes that enables them to be differentiated clearly.18
Once diagnosis is confirmed, it may be appropriate not to administer any treatment.2 Removal with minimal margins is curative. Curettage and electrocautery can also be performed, as can laser treatment and cryotherapy.2,12 In the present series, 42 patients underwent surgical excision; the remainder underwent electrocautery or cryotherapy after histologic confirmation.
The limitations of the present study are its observational and retrospective design, the low number of patients, and the fact that not all patients were seen by a dermatologist.
In summary, CCA are found on the lower limbs in 80% of cases. The typical clinical appearance is that of a dome-shaped erythematous lesion with pinpoint vessels and an epidermal collarette. In our study, the diagnosis was current in 40% of patients seen by a dermatologist. The percentage of clinically diagnosed CCA has improved compared with older series, probably because of better clinical knowledge of the disease and more widespread use of dermoscopy.
FundingThe authors declare that no funding was received for the present study.
Conflicts of InterestThe authors declare that they have no conflicts of interest.