In recent years, remarkable improvements in our understanding of atopic dermatitis (AD) have revolutionized treatment perspectives, but access to reliable data from clinical practice is essential.
Materials and methodThe Spanish Atopic Dermatitis Registry, BIOBADATOP, is a prospective, multicenter database that collects information on patients of all ages with AD requiring systemic therapy with conventional or novel drugs. We analyzed the registry to describe patient characteristics, diagnoses, treatments, and adverse events (AEs).
ResultsWe studied data entries for 258 patients who had received 347 systemic treatments for AD. Treatment was discontinued in 29.4% of cases, mostly due to a lack of effectiveness (in 10.7% of cases). A total of 132 AEs were described during follow-up. Eighty-six AEs (65%) were linked to a systemic treatment, most commonly dupilumab (39 AEs) and cyclosporine (38 AEs). The most common AEs were conjunctivitis (11 patients), headache (6), hypertrichosis (5), and nausea (4). There was 1 severe AE (acute mastoiditis) associated with cyclosporine.
ConclusionsInitial findings on AEs from the Spanish BIOBADATOP registry are limited by short follow-up times precluding comparisons or calculation of crude and adjusted incidence rates. At the time of our analysis, no severe AEs had been reported for novel systemic therapies. BIOBADATOP will help answer questions on the effectiveness and safety of conventional and novel systemic therapies in AD.
En los últimos años se ha producido una revolución en el conocimiento de la dermatitis atópica (DA) que ha revertido en un salto cualitativo en las expectativas terapéuticas. En este contexto, resulta fundamental disponer de datos de práctica clínica de calidad.
Material y métodoBIOBADATOP es el Registro Español de Dermatitis Atópica, un estudio observacional, prospectivo y multicéntrico, con una cohorte de pacientes de cualquier edad con DA que requieren el empleo de tratamiento sistémico (convencional o innovador). Se registraron los datos demográficos, de diagnóstico, los tratamientos y los acontecimientos adversos (AA).
ResultadosSe incluyeron 258 pacientes, con 347 tratamientos sistémicos iniciados para la DA. Se suspendieron el 29,4% de los tratamientos, principalmente por falta de eficacia (10,7%). Durante el período de seguimiento se registraron 132 AA. Del total, el 65% (86) se relacionaron con el tratamiento sistémico iniciado, siendo los más frecuentes dupilumab (39 AA) y ciclosporina (38 AA). Los AA más frecuentes fueron: conjuntivitis (11 pacientes), cefalea (6), hipertricosis (5) y náuseas (4). Se registró un AA grave (mastoiditis aguda) relacionado con ciclosporina.
ConclusionesEn este primer informe, la descripción de AA está limitada por los cortos períodos de seguimiento, que no permiten el cálculo de tasas de incidencias crudas ni ajustadas, y no se han realizado comparaciones. Hasta la fecha del análisis no se han registrado AA graves en relación con las nuevas terapias. BIOBADATOP permitirá generar conocimiento en términos de efectividad y de seguridad de los tratamientos sistémicos clásicos y de las nuevas terapias en DA.
Advances in our understanding of the pathogenesis of atopic dermatitis (AD) have led to the development of new therapeutic strategies targeting, for the first time in the history of this disease, key elements in the pathogenic pathway. Current treatments vary in their nature and mechanisms of action. Most of the available safety and efficacy data for new treatments are from clinical trials, whose findings led to the authorization of drugs such as dupilumab, baricitinib, upadacitinib, tralokinumab, and abrocitinib.1
While clinical trials are necessary, they are insufficient for determining the true effectiveness and safety of new treatments. There are several reasons for this. First, clinical trials usually study select groups of patients, excluding those with certain comorbidities and a higher risk of toxicity.2 Second, they have limited sample sizes and hence only detect the most common adverse events (AEs). Less common events (those with a frequency of less than 1:1000) are generally only detected in real-life clinical practice.
Registries are a useful source of data for pharmacovigilance purposes and evaluating long-term data on a broad set of patients, many of whom have comorbidities and are on other treatments. The Healthy Skin Foundation of the Spanish Academy of Dermatology and Venereology (AEDV) has created several registries, including BIOBADADERM (the Spanish Registry of Systemic Treatments in Psoriasis), which was launched by the AEDV Psoriasis Group in 20073 and has become an international reference point for information on psoriasis in real-world clinical settings.4,5
The Spanish Atopic Dermatitis Registry, BIOBADATOP, was started in 2020, with the inclusion of the first patients. BIOBADATOP is an initiative of the AEDV established in collaboration with 2 of the academy's working groups: the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) and the Spanish Pediatric Dermatology Group (GEDP). The registry was designed in tune with the European TREatment of severe Atopic eczema (TREAT) survey, an international enterprise aimed at standardizing the collection of observational data for patients receiving systemic treatment for AD.6
The BIOBADATOP registry is designed to collect high-quality, standardized data on the safety, effectiveness, and impact on quality of life of systemic AD treatments used in Spanish hospitals. This information can then be integrated with other national or international registries.
The main aims of the BIOBADATOP project are to:
- 1.
describe the short-term and long-term safety of systemic treatments for AD (including phototherapy) for pharmacovigilance purposes
- 2.
describe the short-term and long-term effectiveness of systemic treatments for AD (including phototherapy) to inform decision-making and guideline development
The secondary aims are to:
- 1.
describe the short- and long-term safety of topical treatments for AD
- 2.
describe the effectiveness of different models of AD care, including patient education
- 3.
describe comorbidities associated with AD
In this article, we describe the methodology used to create the BIOBADATOP registry and report on its initial findings.
Material and MethodsStudy DesignBIOBADATOP is a multicenter, observational, prospective, cohort study designed to collect information on patients of all ages with AD who are started on a systemic treatment.
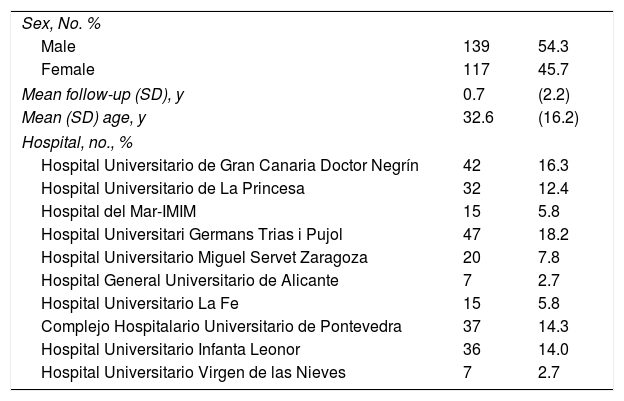
Participating HospitalsTo contribute to the BIOBADATOP registry, hospitals must have at least 1 dermatologist with interest and responsibilities in the treatment of moderate to severe AD. Hospitals can gradually join the project, which aims to maintain a sufficiently representative geographic scope. The hospitals in the project at the time of this analysis are shown in Table 1.
Demographic Description.
| Sex, No. % | ||
| Male | 139 | 54.3 |
| Female | 117 | 45.7 |
| Mean follow-up (SD), y | 0.7 | (2.2) |
| Mean (SD) age, y | 32.6 | (16.2) |
| Hospital, no., % | ||
| Hospital Universitario de Gran Canaria Doctor Negrín | 42 | 16.3 |
| Hospital Universitario de La Princesa | 32 | 12.4 |
| Hospital del Mar-IMIM | 15 | 5.8 |
| Hospital Universitari Germans Trias i Pujol | 47 | 18.2 |
| Hospital Universitario Miguel Servet Zaragoza | 20 | 7.8 |
| Hospital General Universitario de Alicante | 7 | 2.7 |
| Hospital Universitario La Fe | 15 | 5.8 |
| Complejo Hospitalario Universitario de Pontevedra | 37 | 14.3 |
| Hospital Universitario Infanta Leonor | 36 | 14.0 |
| Hospital Universitario Virgen de las Nieves | 7 | 2.7 |
The BIOBADATOP registry contains data on adult and pediatric patients with AD who are started on systemic immunomodulatory therapy in routine clinical practice. To be included, patients must meet the U.K. Working Party's Diagnostic Criteria for Atopic Dermatitis.7,8
Exposure and Follow-upData collected at the enrolment visit include demographic and diagnostic information, baseline disease severity assessed using the Eczema Area and Severity Index (EASI), the Dermatology Life Quality Index (DLQI), and the Patient-Oriented Eczema Measure (POEM), and information on diagnostic tests, comorbidities, and previous AD treatments.
Follow-up visits are carried out as per usual clinical practice. Data from visits closest to 3 and 6months after the start of each systemic treatment are recorded. Annual visits are recorded thereafter. A note is made of any changes to disease severity or treatment (main systemic treatment and concomitant treatments).
At each visit, all AEs the patient may have experienced since their last visit are recorded and coded using MedDRA (Medical Dictionary for Drug Regulatory Activities) terms (https://www.meddra.org).
Enrolled patients are expected to remain in the registry for an indefinite period of time. The duration envisaged for the BIOBADATOP project is at least 10 years.
Outcome MeasuresTable 2 summarizes the data collected to describe the study population and explore factors that might influence the occurrence of AEs.
Baseline and Follow-up Patient Data.
| Baseline visit |
| • Demographic data, including ethnic background |
| • Weight and height |
| • Family and personal history of atopic comorbidities |
| • Personal diagnosis of atopic dermatitis and treatment history |
| • Comorbidities from the Charlson Comorbidity Index |
| • Forms of atopic dermatitis: nummular eczema, palmoplantar eczema, prurigo nodularis |
| • Description of affected areas: face and eyelids, genitals, hands, erythroderma |
| • Total immunoglobulin E levels |
| • Disease severity on enrolment: EASI, pruritus VAS, DLQI, POEM |
| • Patient training or education programs (atopy schools) |
| • Previous screening tests (serology for HBV, HCV, HIV, and VZV) |
| • Previous treatments (including topical and systemic treatments and phototherapy) |
| Follow-up visits |
| • Changes in atopic dermatitis type, location, and/or severity |
| • Evaluation of main systemic treatment and/or concomitant treatments, phototherapy, and topical treatments |
| • If the main treatment has been changed: reasons for change and patient results on initiation of new treatment |
| • Reasons for treatment discontinuation if applicable |
| • Evaluation of adverse events since last visit |
| • Additional visits to a hospital or health care center related to atopic dermatitis or treatment |
| • Loss of productivity (days of activity impairment due to atopic dermatitis) |
| • Atopic dermatitis severity at time of visit: EASI, pruritus VAS, DLQI, POEM |
Abbreviations: DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; HCV, hepatitis C virus; HBV, hepatitis B virus; POEM, Patient-Oriented Eczema Measure; VAS, visual assessment scale; VZV, varicella zoster virus.
We performed a descriptive analysis of patients enrolled in the registry since its launch in March 2020 up to November 2022. Statistical analyses were performed in Stata (version 17.0 Statacorp). Demographic and clinical data are described using conventional statistics (mean [SD] and absolute and relative frequencies).
Sample Size EstimationBased on the experience with the BIOBADADERM registry, BIOBADATOP is expected to collect data on approximately 5700 persons-year (approximately 2500 patients in 10years). It is estimated that this sample will be sufficient to detect, with a statistical power of 80% and a bilateral significance level of 0.05, relative risks of between 1.5 and 2 for an AE incidence of 4 to 10 cases per 1000 person-years in the comparison group. The group will collaborate with other members of TREAT network to detect rare and late AEs.
Data Management and Quality ControlData are entered, following a standardized method, into an online software system (REDCap [Research Electronic Data Capture])9 hosted by the Healthy Skin Foundation. Each patient is assigned a unique identifier.
Ethical ConsiderationsThe study was approved by the research ethics committee of Aragón (PA18/051), the Spanish Agency for Medicines and Medical Devices (AEMPS), and all participating hospitals. It was conducted in compliance with the principles of the Declaration of Helsinki and current legislation. The BIOBADATOP project has also received the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) quality seal attesting to scientific independence and transparency. The ENCePP is coordinated by the European Medicines Agency.
ResultsThe first 2 patients were enrolled in BIOBADATOP in March 2020. This report describes entries recorded up to November 2022. At the time of analysis, 13 hospitals were contributing to the registry.
Main Baseline CharacteristicsAs of November 2022, BIOBADATOP contained data on 258 patients (139 [54.3%] males). Mean (SD) age on enrolment was 32.6 (16.2) years. Mean follow-up was 9 months.
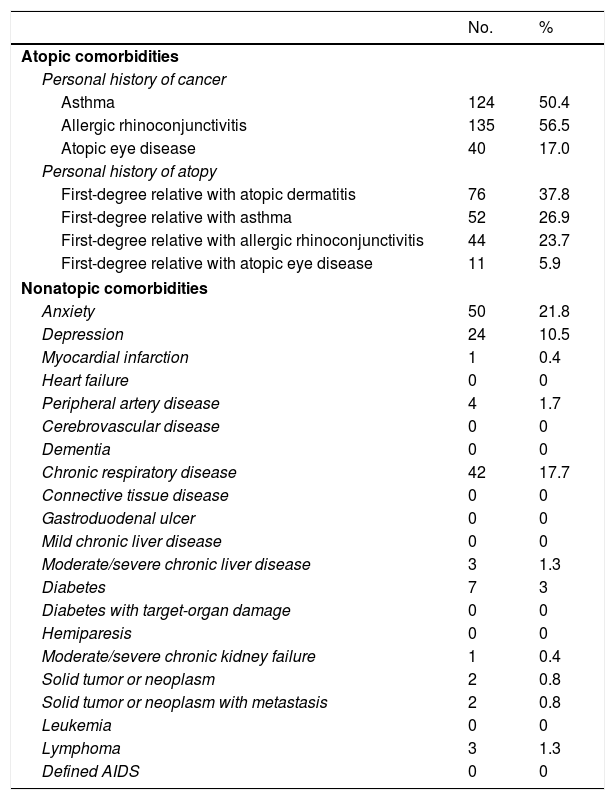
The main comorbidities are shown in Table 3. The most common conditions were asthma (50.4%) and allergic rhinoconjunctivitis (56.5%). Just 17% of patients had an atopic eye disease. Other notable comorbidities were anxiety (21.8%), chronic respiratory disease (17.7%), and depression (10.5%).
Atopic and Nonatopic Comorbidities in Enrolled Patients.
| No. | % | |
|---|---|---|
| Atopic comorbidities | ||
| Personal history of cancer | ||
| Asthma | 124 | 50.4 |
| Allergic rhinoconjunctivitis | 135 | 56.5 |
| Atopic eye disease | 40 | 17.0 |
| Personal history of atopy | ||
| First-degree relative with atopic dermatitis | 76 | 37.8 |
| First-degree relative with asthma | 52 | 26.9 |
| First-degree relative with allergic rhinoconjunctivitis | 44 | 23.7 |
| First-degree relative with atopic eye disease | 11 | 5.9 |
| Nonatopic comorbidities | ||
| Anxiety | 50 | 21.8 |
| Depression | 24 | 10.5 |
| Myocardial infarction | 1 | 0.4 |
| Heart failure | 0 | 0 |
| Peripheral artery disease | 4 | 1.7 |
| Cerebrovascular disease | 0 | 0 |
| Dementia | 0 | 0 |
| Chronic respiratory disease | 42 | 17.7 |
| Connective tissue disease | 0 | 0 |
| Gastroduodenal ulcer | 0 | 0 |
| Mild chronic liver disease | 0 | 0 |
| Moderate/severe chronic liver disease | 3 | 1.3 |
| Diabetes | 7 | 3 |
| Diabetes with target-organ damage | 0 | 0 |
| Hemiparesis | 0 | 0 |
| Moderate/severe chronic kidney failure | 1 | 0.4 |
| Solid tumor or neoplasm | 2 | 0.8 |
| Solid tumor or neoplasm with metastasis | 2 | 0.8 |
| Leukemia | 0 | 0 |
| Lymphoma | 3 | 1.3 |
| Defined AIDS | 0 | 0 |
The most common clinical presentations were dermatitis involving extensor surfaces (93.3%), the face and eyelids (84.6%), and the hands (67.1%). There was also genital involvement (31.6%), erythroderma (covering>70% of the body surface area) (17.2%), palmoplantar eczema (12.1%), nummular eczema (12.6%), and prurigo nodularis (≥5 palpable nodules) (11.6%).
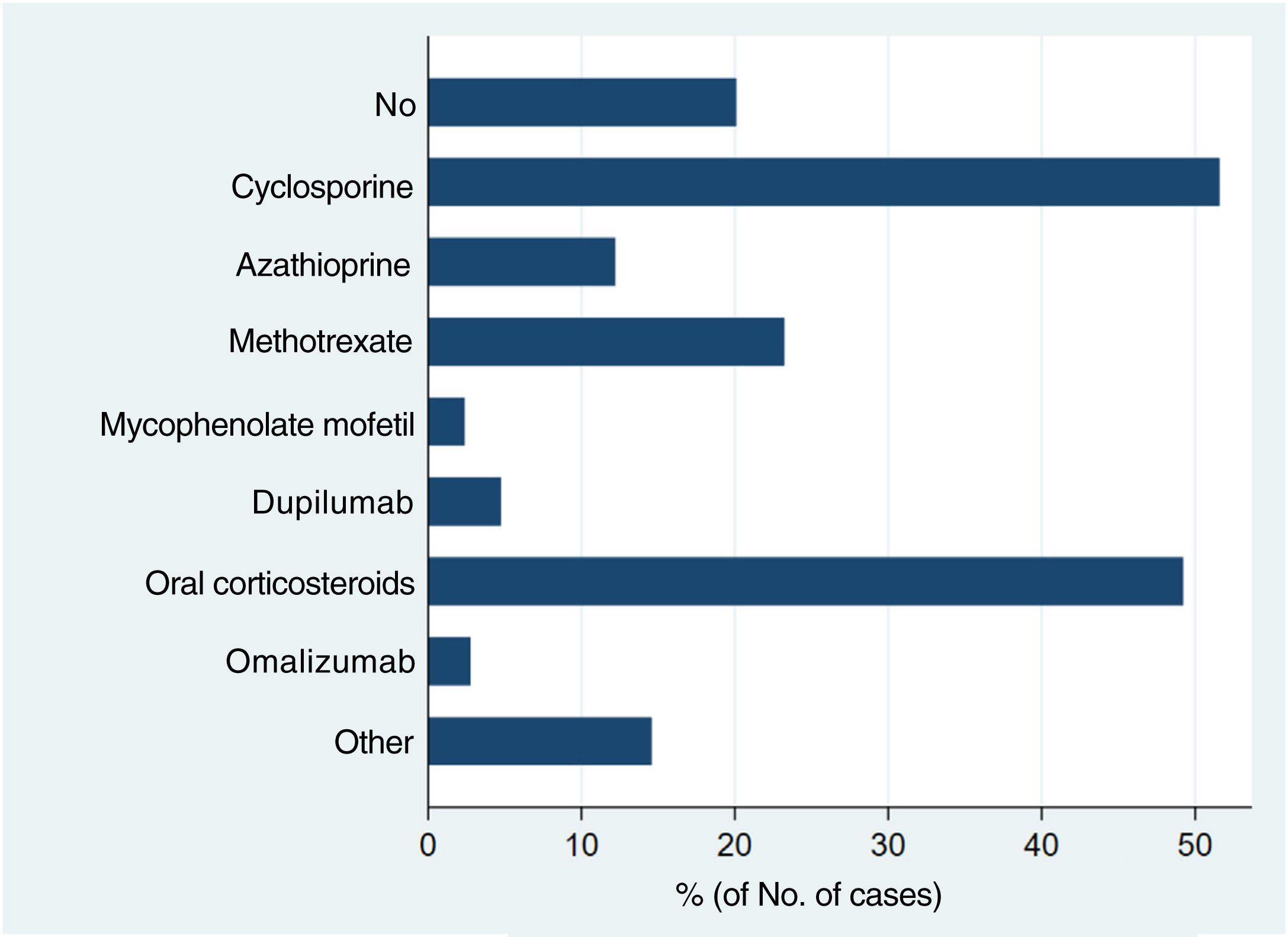
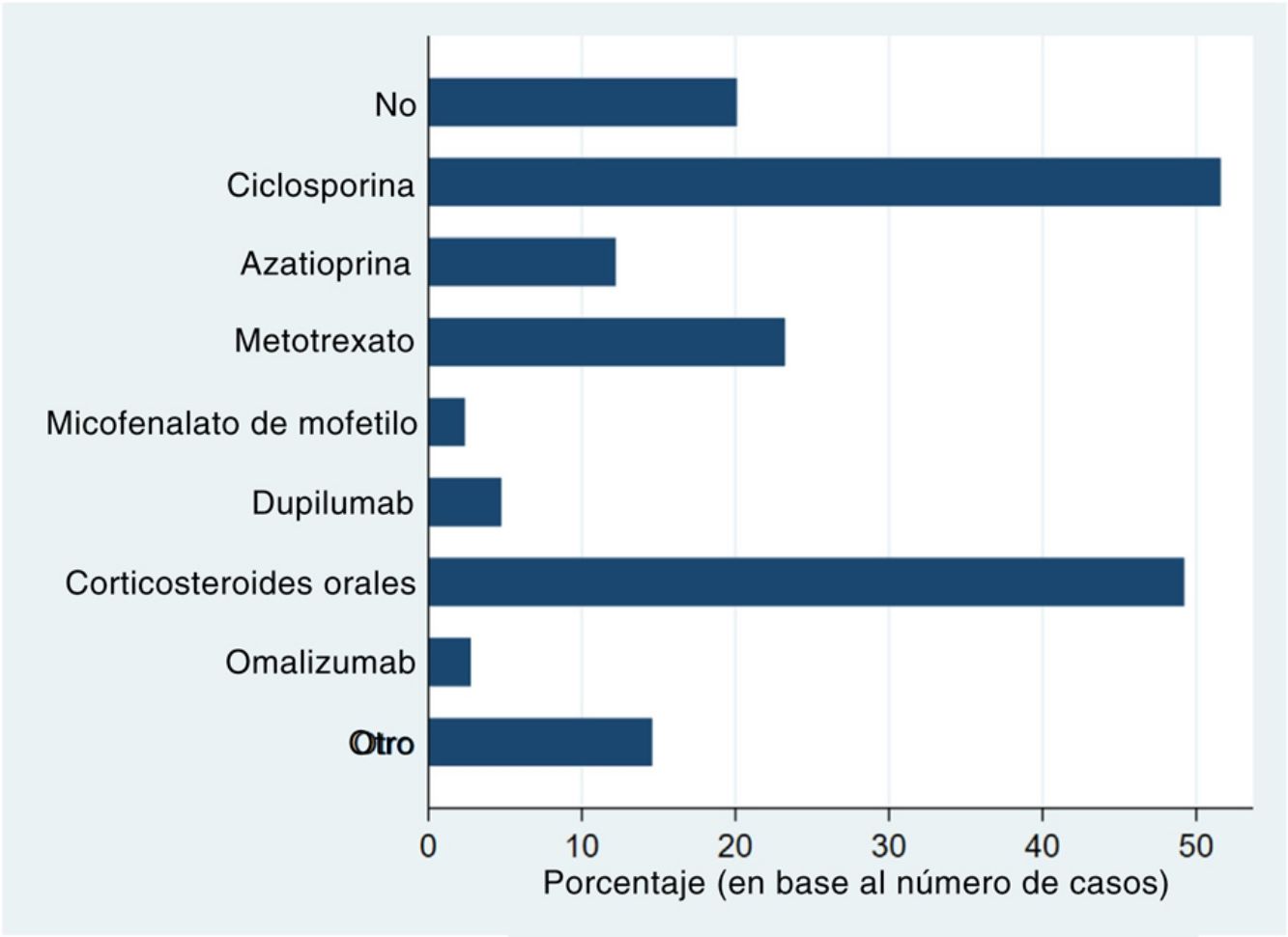
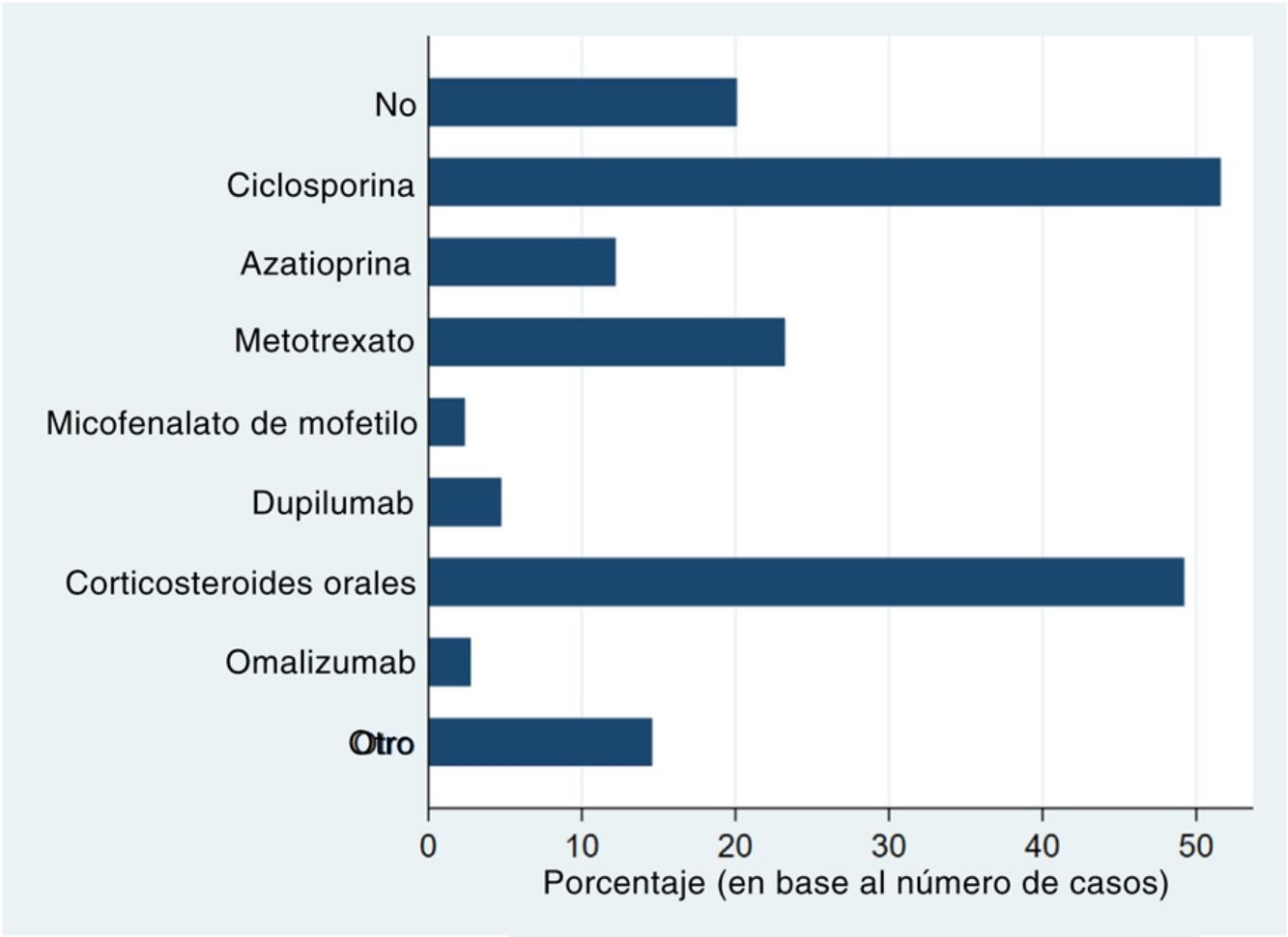
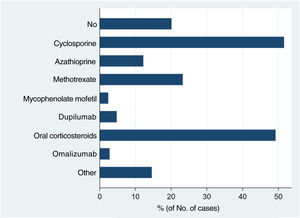
Systemic treatments received prior to inclusion in the BIOBADATOP registry are shown in Fig. 1. Twenty percent of patients had not been previously treated with a systemic agent. The most widely used previous systemic treatments were cyclosporine (> 50% of patients) and oral corticosteroids (49%), followed at quite a distance by methotrexate, at just over 20%.
Phototherapy, mostly with narrowband UVB light, had been used in 44.3% of cases. The vast majority of patients (97.4%) had been treated with topical corticosteroids; 44.3% had received topical calcineurin inhibitors.
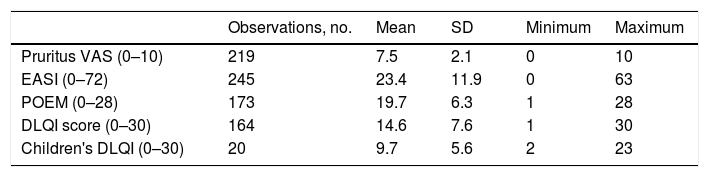
Baseline and Follow-up FindingsMean (SD) AD severity according to the EASI was 23.4 (11.9; range, 0–63) at baseline. The mean (SD) quality of life scores on enrolment were 19.7 (6.3) for POEM, 14.6 (7.6) for DLQI in patients>15years, and 9.7 (5.6) in patients aged 15years. The baseline scores for the main outcomes of interest are summarized in Table 4.
Baseline Scores for Main Outcome Measures.
| Observations, no. | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Pruritus VAS (0–10) | 219 | 7.5 | 2.1 | 0 | 10 |
| EASI (0–72) | 245 | 23.4 | 11.9 | 0 | 63 |
| POEM (0–28) | 173 | 19.7 | 6.3 | 1 | 28 |
| DLQI score (0–30) | 164 | 14.6 | 7.6 | 1 | 30 |
| Children's DLQI (0–30) | 20 | 9.7 | 5.6 | 2 | 23 |
Abbreviations: DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; POEM, Patient-Oriented Eczema Measure; VAS, visual assessment scale.
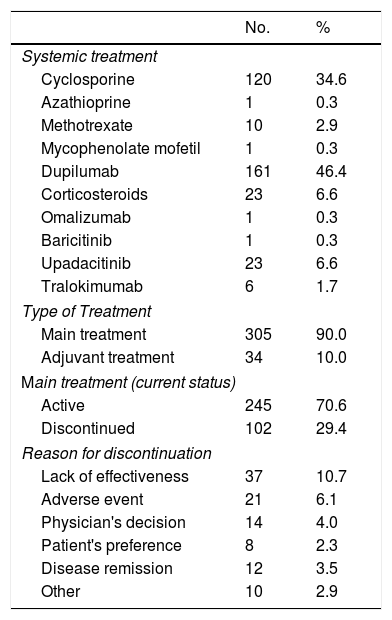
A total of 347 systemic therapies were started between March 2020 and November 2022 (Table 5). Practically all the treatments (90%) were primary treatments. The discontinuation rate for newly initiated treatments was 29.4%, and the main reason was a lack of effectiveness (10.7%).
New Systemic Treatments Initiated (Baseline or BIOBADATOP Visit).
| No. | % | |
|---|---|---|
| Systemic treatment | ||
| Cyclosporine | 120 | 34.6 |
| Azathioprine | 1 | 0.3 |
| Methotrexate | 10 | 2.9 |
| Mycophenolate mofetil | 1 | 0.3 |
| Dupilumab | 161 | 46.4 |
| Corticosteroids | 23 | 6.6 |
| Omalizumab | 1 | 0.3 |
| Baricitinib | 1 | 0.3 |
| Upadacitinib | 23 | 6.6 |
| Tralokimumab | 6 | 1.7 |
| Type of Treatment | ||
| Main treatment | 305 | 90.0 |
| Adjuvant treatment | 34 | 10.0 |
| Main treatment (current status) | ||
| Active | 245 | 70.6 |
| Discontinued | 102 | 29.4 |
| Reason for discontinuation | ||
| Lack of effectiveness | 37 | 10.7 |
| Adverse event | 21 | 6.1 |
| Physician's decision | 14 | 4.0 |
| Patient's preference | 8 | 2.3 |
| Disease remission | 12 | 3.5 |
| Other | 10 | 2.9 |
Of the 258 patients enrolled in the database, 232 (89.9%) were still under active treatment with a systemic drug at the time of analysis, 15 (5.8%) had been taken off systemic treatment, and 11 (4.3%) had been lost to follow-up.
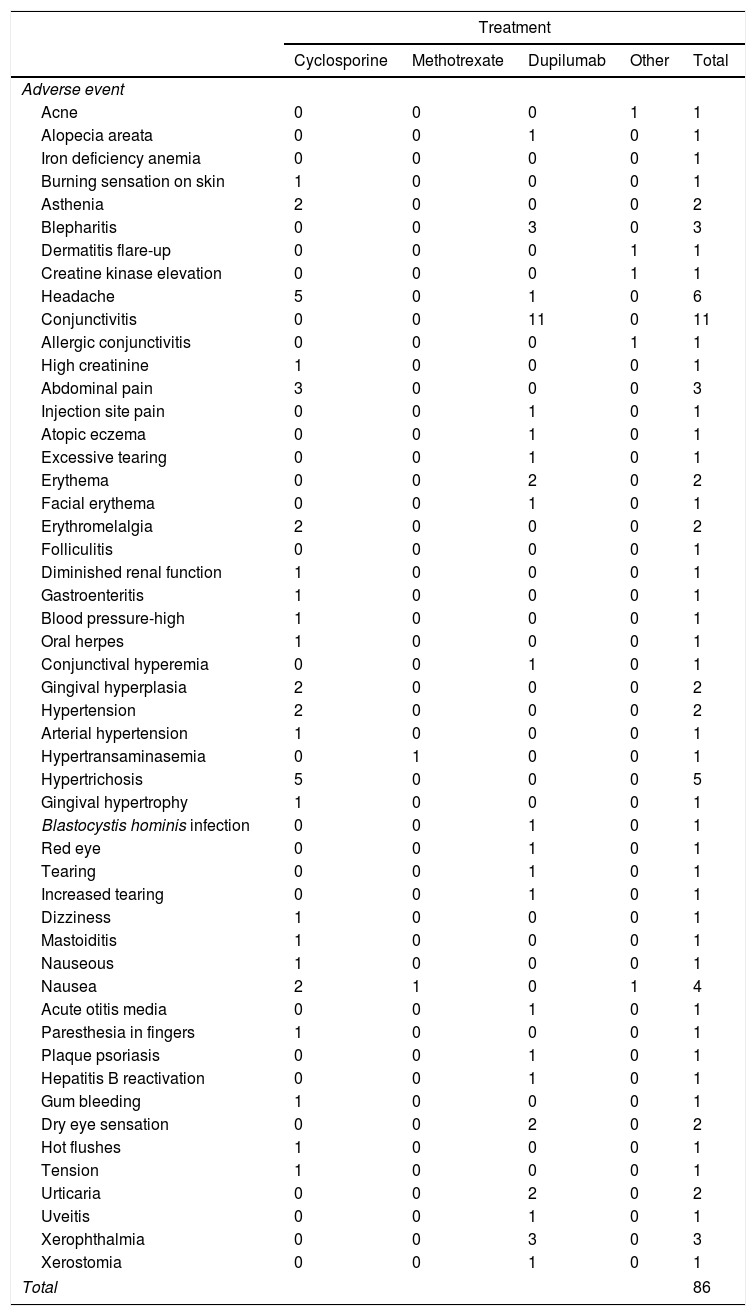
Description of AEsIn total, 132 AEs were registered during follow-up; 7 of these (5.3%) were considered serious. Of the 132 documented AEs, 65% (86) were linked to the new systemic treatment. The most common drugs implicated were dupilumab (39 AEs) and cyclosporine (38). The most common AEs were conjunctivitis (11 cases), headache (6), hypertrichosis (5), and nausea (4). We were unable to calculate incidence rates according to MedDRA terms because of the low frequency of cases at the time of analysis. The AEs recorded are shown by drug in Table 6. There was just 1 serious event—acute mastoiditis requiring hospital admission—in a patient treated with cyclosporine. The patient recovered completely and the drug was permanently discontinued.
Relevant Adverse Events Associated With Initiation of New Systemic Treatment.
| Treatment | |||||
|---|---|---|---|---|---|
| Cyclosporine | Methotrexate | Dupilumab | Other | Total | |
| Adverse event | |||||
| Acne | 0 | 0 | 0 | 1 | 1 |
| Alopecia areata | 0 | 0 | 1 | 0 | 1 |
| Iron deficiency anemia | 0 | 0 | 0 | 0 | 1 |
| Burning sensation on skin | 1 | 0 | 0 | 0 | 1 |
| Asthenia | 2 | 0 | 0 | 0 | 2 |
| Blepharitis | 0 | 0 | 3 | 0 | 3 |
| Dermatitis flare-up | 0 | 0 | 0 | 1 | 1 |
| Creatine kinase elevation | 0 | 0 | 0 | 1 | 1 |
| Headache | 5 | 0 | 1 | 0 | 6 |
| Conjunctivitis | 0 | 0 | 11 | 0 | 11 |
| Allergic conjunctivitis | 0 | 0 | 0 | 1 | 1 |
| High creatinine | 1 | 0 | 0 | 0 | 1 |
| Abdominal pain | 3 | 0 | 0 | 0 | 3 |
| Injection site pain | 0 | 0 | 1 | 0 | 1 |
| Atopic eczema | 0 | 0 | 1 | 0 | 1 |
| Excessive tearing | 0 | 0 | 1 | 0 | 1 |
| Erythema | 0 | 0 | 2 | 0 | 2 |
| Facial erythema | 0 | 0 | 1 | 0 | 1 |
| Erythromelalgia | 2 | 0 | 0 | 0 | 2 |
| Folliculitis | 0 | 0 | 0 | 0 | 1 |
| Diminished renal function | 1 | 0 | 0 | 0 | 1 |
| Gastroenteritis | 1 | 0 | 0 | 0 | 1 |
| Blood pressure-high | 1 | 0 | 0 | 0 | 1 |
| Oral herpes | 1 | 0 | 0 | 0 | 1 |
| Conjunctival hyperemia | 0 | 0 | 1 | 0 | 1 |
| Gingival hyperplasia | 2 | 0 | 0 | 0 | 2 |
| Hypertension | 2 | 0 | 0 | 0 | 2 |
| Arterial hypertension | 1 | 0 | 0 | 0 | 1 |
| Hypertransaminasemia | 0 | 1 | 0 | 0 | 1 |
| Hypertrichosis | 5 | 0 | 0 | 0 | 5 |
| Gingival hypertrophy | 1 | 0 | 0 | 0 | 1 |
| Blastocystis hominis infection | 0 | 0 | 1 | 0 | 1 |
| Red eye | 0 | 0 | 1 | 0 | 1 |
| Tearing | 0 | 0 | 1 | 0 | 1 |
| Increased tearing | 0 | 0 | 1 | 0 | 1 |
| Dizziness | 1 | 0 | 0 | 0 | 1 |
| Mastoiditis | 1 | 0 | 0 | 0 | 1 |
| Nauseous | 1 | 0 | 0 | 0 | 1 |
| Nausea | 2 | 1 | 0 | 1 | 4 |
| Acute otitis media | 0 | 0 | 1 | 0 | 1 |
| Paresthesia in fingers | 1 | 0 | 0 | 0 | 1 |
| Plaque psoriasis | 0 | 0 | 1 | 0 | 1 |
| Hepatitis B reactivation | 0 | 0 | 1 | 0 | 1 |
| Gum bleeding | 1 | 0 | 0 | 0 | 1 |
| Dry eye sensation | 0 | 0 | 2 | 0 | 2 |
| Hot flushes | 1 | 0 | 0 | 0 | 1 |
| Tension | 1 | 0 | 0 | 0 | 1 |
| Urticaria | 0 | 0 | 2 | 0 | 2 |
| Uveitis | 0 | 0 | 1 | 0 | 1 |
| Xerophthalmia | 0 | 0 | 3 | 0 | 3 |
| Xerostomia | 0 | 0 | 1 | 0 | 1 |
| Total | 86 | ||||
The AEDV BIOBADATOP registry was created to collect clinical-epidemiological, pharmacovigilance, and effectiveness data on patients with moderate to severe AD under treatment with conventional or novel systemic therapies at a turning point in the history of the treatment of this disease. In this first report, we describe the current situation captured by the registry and the main characteristics of the patients enrolled.
Most patients prescribed a systemic treatment for AD in our setting have severe disease, in terms of not just clinical manifestations but also itching severity and impact on quality of life. While these are key aspects that influence decisions by both physicians and patients, they are not part of the reimbursement criteria for new drugs. Despite the clinical heterogeneity of AD, almost all the patients in the BIOBADATOP registry had dermatitis affecting the extensor surfaces. In addition, many patients had lesions in locations that have a greater impact on everyday life and can be challenging to treat, such as the face, eyelids, genitals, and hands. A large percentage of patients had atopic comorbidities such as asthma. Other comorbidities, notably mood disorders, were also common.
Dupilumab was approved by the European Medicines Agency in September 2017. Authorization was subsequently granted for baricitinib, followed by tralokinumab and upadacitinib. Dupilumab is the most widely used systemic AD treatment used by the hospitals participating in the BIOBADATOP project. This is, at least in part, because it was approved earlier, meaning that clinicians are more familiar with its use, and there is a greater understanding of longer-term effects in real-world settings. Choice of drug, however, can also be influenced by other factors, such as access to authorization or first-line treatment policies in different health care regions.
Although the BIOBADATOP registry covers both pediatric and adult patients, the patients included were on average aged between 30 and 40. This is probably because most of the researchers that contribute to the registry are heads of units that mainly treat adults with moderate to severe AD. Pediatric units are less well represented. In addition, new systemic therapies for AD were approved later, and at different time points, for children.
This first report on the BIOBADATOP registry is limited by the short follow-up times, which precluded comparisons and an accurate measure of incidence rates. We were only able to describe frequencies, which do not reflect time of exposure to given drugs. Most of the AEs reported were associated with the most widely used drugs: dupilumab and cyclosporine. Despite this limitation, no serious AEs had been reported for any of the novel systemic treatments at the time of this analysis. There was 1 serious infection (mastoiditis), linked to cyclosporine. Other nonserious but relatively common AEs were headache, hypertrichosis, and gum bleeding.
Of all the novel systemic treatments used by Spanish hospitals in the BIOBADATOP registry, dupilumab was the medication with the most available data. The most common AEs were conjunctivitis and other AEs affecting the surface of the eye, such as blepharitis and xerophthalmia. There was just 1 case of facial erythema. These findings are consistent with recent real-world data on drug safety.10,11 Our findings did not reveal any new safety alerts.
The incidence of certain key AEs, such as cancer and other potentially serious AEs, is too low to be accurately captured in national registries, but this limitation can be overcome by combining data from different countries. BIOBADATOP is directly linked to the TREAT project and uses a standardized European protocol, similar to that followed by the United Kingdom, the Netherlands, Germany, and other European countries. Combining data from different registries should provide the necessary statistical power to calculate incidence rates for less common AEs. Germany (TREATgermany)12 and Denmark (SCRATCH)13 have both reported findings from their national registries, and the TREAT Registry Taskforce published a recent status update on the 8 databases in the network, including BIOBADATOP. The update covered 4702 patients enrolled up to May 2022.14
The treatment of AD is undergoing a major transformation.15 Until very recently, patients with disease refractory to topical treatment and/or phototherapy were largely treated with cyclosporine or off-label immunosuppressants, such as methotrexate and azathioprine. This is reflected in the data on previous treatments showing the use of at 1 conventional immunosuppressive treatment and a high use of corticosteroids. Although clinical trial data suggest that novel systemic therapies for AD have an adequate safety profile, their effects in the broader population must be studied.
The BIOBADATOP registry will generate useful data for pharmacovigilance purposes and for evaluating treatment effectiveness in real-world clinical settings, contributing to a greater understanding of moderate to severe AD and better management of this disease.
FundingThe BIOBADATOP project is led by the Healthy Skin Foundation of the Spanish Academy of Dermatology and Venereology (AEDV) and receives funding from the pharmaceutical companies Sanofi, AbbVie, Pfizer, and LEO Pharma. None of these companies had any involvement in study design or conduct; data collection, management, analysis, or interpretation; manuscript preparation, revision, or approval; or the decision to submit this manuscript for publication.
Conflicts of InterestM. Munera-Campos has received fees for consultancy services, presentations, and other related activities from AbbVie, LEO Pharma, Janssen, Sanofi, and Galderma and has served as a principal or co-investigator on clinical trials sponsored by Lilly, LEO Pharma, Novartis, Janssen, Sanofi, Pfizer, AbbVie, Almirall, UCB, and Galderma. P. Chicharro has provided consultancy services for and participated in presentations and clinical trials organized by Janssen Pharmaceuticals, Almirall, Sanofi Genzyme, Lilly, AbbVie, Novartis, LEO Pharma, and Pfizer-Wyeth. A. González Quesada has provided consultancy services and participated in presentations and clinical trials for AbbVie, Pfizer, Novartis, Sanofi, Boeringher, Bristol-Meyer, LEO Pharma, and Jansen. Á. Flórez Menéndez has participated in clinical trials, given presentations, and provided consultancy services for AbbVie, Almirall, Amgen, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi, and UCB Pharma. P. de la Cueva Dobao has served as a consultant and/or researcher and/or speaker for AbbVie, Almirall, BMS, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, and UCB. A. M. Gimenez-Arnau has served as a medical advisor for Uriach Pharma/Neucor, Genentech, Novartis, FAES, GSK, Sanofi-Regeneron, Amgen, Thermo Fisher Scientific, Almirall, Celldex, and LEO Pharma. Y. Gilaberte-Calzada has served as a consultant for Isdin and Roche Posay, and Galderma, given presentations for Almirall, Sanofi, Avene, Rilastil, Lylly, Uriage, Novartis, and Cantabria Labs, and participated in research projects for Almirall, Sanofi, Pfizer, AbbVie, and LEO Pharma. M. Rodríguez Serna has served as a consultant for Sanofi, Pfizer, LEO Pharma, Novartis, and Abbie. M. Elosua-González has served as a researcher and/or speaker for AbbVie, Lilly, Galderma, LEO Pharma, Pfizer, UCB Pharma, and Sanofi Genzyme. E. del Alcázar has served as a speaker and/or principal or co-investigator on clinical trials for Amgen, Almirall, Janssen, Lilly, LEO Pharma, Novartis, UCB, and AbbVie. A. Batalla has participated in training activities and attended courses and conferences for AbbVie, Celgene, Faes Farma, Isdin, Janssen, LEO Pharma, Lethipharma, Lilly, Mylan, Novartis, Pierre Fabre, and Sanofi; served as a co-investigator on clinical trials sponsored by AbbVie, Celgene, LEO Pharma, Lilly, Novartis, Pfizer, and Sanofi; and provided consultancy services for AbbVie and Sanofi. H. Jin Suh Oh has received training fees and provided consultancy services and/or participated in clinical trials for AbbVie, Celgene, and Faes Farma. C. Mauleón Fernández has participated in clinical trials on atopic dermatitis for LEO Pharma and Sanofi. L. Curto Barredo has received speaker's and consultancy fees from Novartis, Sanofi, AbbVie, Lilly, LEO pharma, and Menarini and has worked as a principal and/or co-investigator in research projects/clinical trials for Sanofi, Amgen, Almirall, Lilly, LEO Pharma, Novartis, AbbVie, and Pfizer. G. Roustan Gullón has received consultancy and training fees from Sanofi, AbbVie, Lilly, Pfizer, and LEO Pharma. A. Rosell Díaz has received speaker's fees from Sanofi and LEO Pharma and worked as a co-investigator in studies for Sanofi and Pfizer. I. García-Doval has received financial support for attending conferences from AbbVie, MSD, Pfizer, and Sanofi. José Manuel Carrascosa has served as a principal/co-investigator and/or received speaker's fees, and/or served on expert or steering committees for AbbVie, Novartis, Janssen, Lilly, Sandoz, Amgen, Almirall, BMS, Boehringer Ingelheim, Biogen, UCB. The other authors declare no conflicts of interest.
We would like to thank all the BIOBADATOP researchers for their dedication and diligence in recording detailed information on adverse events and other relevant data in routine practice and other clinical settings, where both time and financial resources linked to other dermatology department activities are typically allocated to these tasks.