Teenagers’ Quality of Life (T-QoL) is an age-specific measure to assess QoL of teenagers suffering from different skin diseases. A validated Spanish language version is lacking. We present the translation, cultural adaptation and validation of the T-QoL into Spanish.
MethodsA prospective study with 133 patients (between 12 and 19 years old), attended at the dermatology department of Toledo University Hospital, Spain (September 2019–May 2020), was carried out for the validation study. The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidelines were used for the translation and cultural adaptation. We evaluated the convergent validity with the Dermatology Life Quality Index (DLQI), the Children's Dermatology Life Quality Index (CDLQI) and with a Global Question (GQ) on self-assessed disease severity. We also analysed internal consistency and reliability of the T-QoL tool and confirmed its structure with a factor analysis.
ResultsGlobal T-QoL scores significantly correlated with the DLQI and the CDLQI (r=0.75) and with the GQ (r=0.63). The confirmatory factor analysis showed optimal fit for the bi-factor model and an adequate fit for the correlated three-factor model. Reliability indicators were high (Cronbach's α=0.89; Guttman's Lambda 6 index=0.91; Omega ω=0.91) and test–retest showed a high stability (ICC=0.85). The results were consistent with those found by the authors of the original test.
ConclusionOur Spanish version of the T-QoL tool is valid and reliable to assess QoL of Spanish-speaking adolescents with skin diseases.
Teenagers’ Quality of Life (T-QoL) es un cuestionario de calidad de vida específico para adolescentes con enfermedades cutáneas. Hasta el momento, no existe ningún método validado para este fin en español, por lo que presentamos la traducción, adaptación cultural y validación del T-QoL al español.
MétodoSe diseñó un estudio prospectivo con 133 pacientes (entre 12-19 años), atendidos en el Servicio de Dermatología del Hospital Universitario de Toledo, España (septiembre 2019-mayo 2020). Para la traducción y adaptación cultural se utilizaron las guías de la Sociedad Internacional de Farmacoeconomía e Investigación de Resultados (ISPOR). Se evaluó la validez convergente con el Índice de Calidad de Vida en Dermatología (DLQI), el Índice de Calidad de Vida en Dermatología Infantil (CDLQI) y con una Pregunta Global (GQ) sobre la gravedad de la enfermedad autoevaluada. También se analizó la consistencia interna y la fiabilidad de la herramienta T-QoL, y se confirmó su estructura con un análisis factorial.
ResultadosLas puntuaciones globales de T-QoL se correlacionaron significativamente con el DLQI y el CDLQI (r=0,75) y con la GQ (r=0,63). El análisis factorial mostró un ajuste óptimo para el modelo bifactorial y un ajuste adecuado para el modelo de 3 factores correlacionado. Los indicadores de fiabilidad fueron altos (α de Cronbach=0,89; índice Lambda 6 de Guttman=0,91; Omega ω=0,91) y el test-retest mostró una alta estabilidad (ICC=0,85). Los resultados fueron consistentes con los encontrados por los autores de la prueba original.
ConclusionesLa versión española del T-QoL es un cuestionario válido y fiable para evaluar la calidad de vida de adolescentes hispanohablantes con enfermedades cutáneas.
The psychological impacts on patients suffering from visible skin diseases (VSDs) includes depression, anxiety and low self-esteem.1,2 When VSDs are not addressed at an early age, they may result in significant detriments in overall emotional wellbeing, social functioning, and productivity.3
Adolescence has its own relevant topics such as physical maturation, body image, peer relationships, sexuality and autonomy must be considered to understand adolescents’ HRQoL.4 Several studies assessing the impact of VSDs in adolescence showed significant impact on psychological development.5–8 However, most of these studies used non-validated questionnaires.9–12
At present, there are three generic questionnaires available in Spanish to assess quality of life (QoL) in people with skin diseases: the Dermatology Life Quality Index (DLQI),13 the Skindex-29,14 and the Children's Dermatology Life Quality Index (CDLQI).15 Neither of these questionnaires delves into adolescent-specific issues. To address this, Basra et al. developed and validated the Teenagers’ Quality of Life (T-QoL©).16
To date, there is no published studies which evaluates the cultural and linguistic equivalence of the T-QoL for its use in other adolescent populations. Our aim was to translate, culturally adapt and psychometrically validate the T-QoL tool into Spanish.
MethodsParticipantsA prospective study was conducted to adolescents who attended the dermatology department of Toledo University Hospital between September 2019 and May 2020. Sampling was consecutive and no probabilistic. The sample size estimated was 5 patients per item but not less than 100 patients in total.17
Inclusion criteria were patients aged 12–19 years, suffering from a diagnosed skin disease, who were able to understand and read Spanish, and were able to give assent or written informed consent. Exclusion criteria included patients with significant co-morbidities that could impact on QoL. All personal information was kept confidential.
InstrumentsT-QoL consists of 18 items scored using an ordinal scale from 0 to 2: Never, Occasionally or Always. The score can be reported as a total score, with minimum of 0 and maximum of 36, or as the scores of the three domains or subscales: Self-image (items 1–8), Physical well-being and future aspirations (items 9–12), and Psychological impact and relationships (items 13–18). Higher scores denote greater impairment of the QoL.
The Spanish validated versions of DLQI,18 designed for adults over 16 years, and CDLQI,19 designed for children between 4 and 16 years, were used as comparators. Both questionnaires consist of 10 items scored 0–3, yielding a maximum score of 30.
We also used a global question (GQ) on self-assessed disease severity on 0–10 scale, with 0 indicating clear skin and 10 most severe diseases.
ProcedureThe adaptation of the T-QoL into Spanish involved two main stages: translation and cultural adaptation, and validation.
For the first one, the guidelines made by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Task Force for Translation and Cultural Adaptation were used.20
Data analysisDifferences between test scores, GQ, age and gender groups were studied using the Wilcoxon rank sum test (Mann–Whitney U test) and Cohen's d index. Univariate normality was tested using Kolmogorov–Smirnov test21 and multivariate normality with Mardia test.22
Convergent validity was assessed using Spearman Rank correlation between T-QoL, DLQI, CDLQI and GQ. We hypothesised that convergent validity correlations between the T-QoL and the DLQI and CDLQI would be moderate-to-high and moderate-to-low with the GQ. Effect sizes were interpreted as negligible (r≤0.3), low (r=0.3–0.49), moderate (r=0.5–0.7) and high (r=0.71–0.9) and very high (r>0.9).23
The dimensionality of the test scores was studied by parallel analysis. We performed a confirmatory factor analysis (CFA) on a three-factor correlated model and on a bifactor model with three group factors. The magnitudes of the factor saturation and the item's variance explained by each model (R2) were also analysed.
Cronbach's α and Omega ω were used to assess the internal consistency of the tests. Scores ranged 0.7–0.9 were considered acceptable, and above 0.9 may indicate item redundancy.24–26 We also analysed the Spearman correlation matrix between items, the corrected item-rest of the test correlation, the average correlation inter-items and Guttman's Lambda 6 index (G6).27 Items were considered heterogeneous at G6 values <0.7, consistent close to 0.8, or redundant >0.9.28
Test–retest reliabilities were estimated for the three subscales and the total score using intraclass correlation coefficient (ICC). ICC scores <0.6 were considered insufficient, between 0.60 and 0.74 good, and >0.75 excellent.28 The tests’ temporal stability hypothesis seeks to find similar results between the scores of repeat test participants.
ResultsFirst phase: translation and cultural adaptationAfter reviewing the back-translations, it was proposed to change certain expressions whose meaning had different nuances depending on the language (Table 1). The resulting Spanish version of T-QoL was pretested in a pilot group of eight participants with skin disorders. All participants answered the questionnaire with a feasibility of 100% and a response time of less than 3min. In question 1 a synonym to the term “self-conscious” was included for a better understanding. The Spanish version of T-QoL was published on the Cardiff University website in October 2019 (see Supplementary Material): https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnaires.
Changes in the translation process.
| Part of the questionnaire | Original version | English translation of the forward translation | Back-translation |
|---|---|---|---|
| Explanatory paragraph | …the impact that your skin disease has on your Quality of Life at the moment | …the impact that your skin disease has on your Quality of Life currently | …the impact that your skin disease has on your Quality of Life at this time |
| All questions | Skin condition | Skin diseases | Skin problem |
| Question 1 | Does your skin condition make you feel self-conscious? | Are you aware of your skin disease? | Do you feel self-conscious about your skin problem? |
| Question 3 | Does your skin condition make you feel that you look different? | Does your skin disease make you feel different? | Do you feel you look different because of your skin problem? |
| Question 13 | Does your skin condition make you feel annoyed? | Does your skin disease make you feel upset? | Does your skin problem make you feel annoyed? |
| Question 16 | Do you receive any unfriendly comments from other people about your skin? | Do you receive unpleasant comments about your skin? | Do you receive unfriendly comments about your skin? |
The mean±SD age of the 133 patients was 16.1±1.9 years; 60.2% of patients were >16 years old and 51.1% were females. Diagnoses included acne (65.4%), eczematous dermatosis including psoriasis (10.5%), hyperhidrosis (5.3%), moles (3.8%), tinea versicolor (3.0%) and other (12.0%). A descriptive comparison of T-QoL mean scores at the two time it was administered showed no significant differences (T0: 8.3±6.1; T1: 9.2±6.6; p=0.4). These findings were congruent with the GQ.
No statistical differences were observed between children younger adolescents (12–15.9 years) and older adolescents (16–19 years) at any times when the test was completed (T0: T-QoLchildrenyounger 7.2±5.2; T-QoLadolescentsolder 9.1±6.5; p=0.14; T1: T-QoLchildrenyounger 9.4±6.6; T-QoLadolescentsolder 9.4±6.6; p=0.8). Only in domain 3 scores were higher among those over 16 years (p<0.05, d=−0.362). T-QoL mean score was significantly higher in females than in males globally (T-QoLfemales 10.3±6.4; T-QoLmales 6.3±5.0; p<0.001; d=−0.693), in domains 1 and 3 and in the two moments where the test was administered. We also found gender differences in the GQ, CDLQI and DLQI assessments (GQfemales 4.9±2.6, GQmales 3.7±2.6, p<0.05, d=−0.475; CDLQI/DLQIfemales 4.0±3.6, CDLQI/DLQImales 2.7±3.0, p<0.05, d=−0.385).
The distribution of item scores was also reflected in the mean domain scores with domain 1: Self-image had the highest MS (5.2; range 0–16) followed by domain 3: Psychosocial impact and relationships, (2.1, range 0–12) and domain 2: Physical well-being and future aspirations (1.2, range 0–8).
Convergent validityThe Kolmogorov–Smirnov test was significant for the T-QoL total score (D=0.12875; p<0.001), the DLQI/CLQI (D=0.209; p<0.001) and GQ (D=0.107; p=0.020). Therefore, normality cannot be assured for any of the total scores.
The correlations of the T-QoL total scores with the DLQI and CLQI were r=0.75 and r=0.63 with the GQ, suggesting that overall results seem to converge moderately between the different measurement tools.
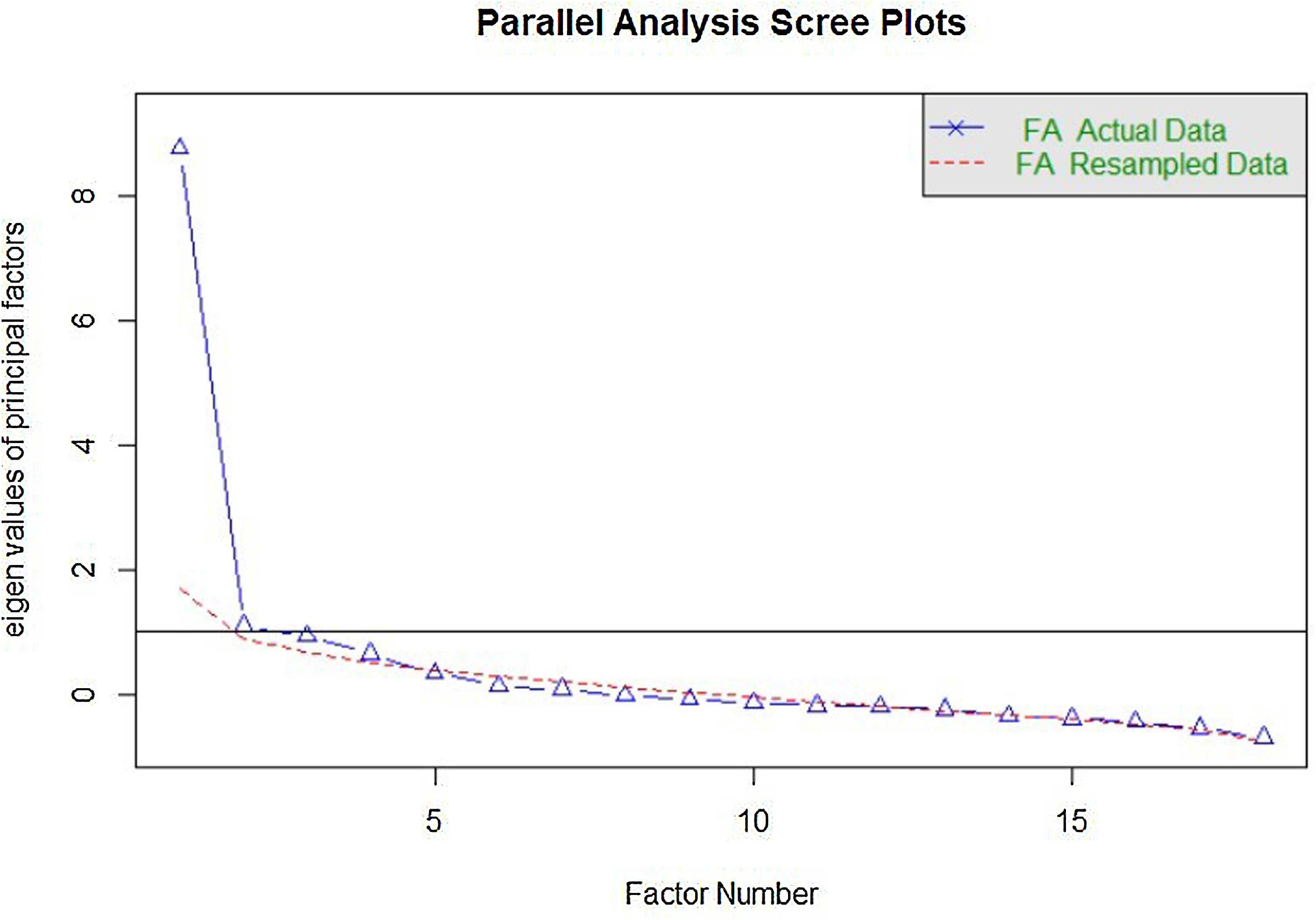
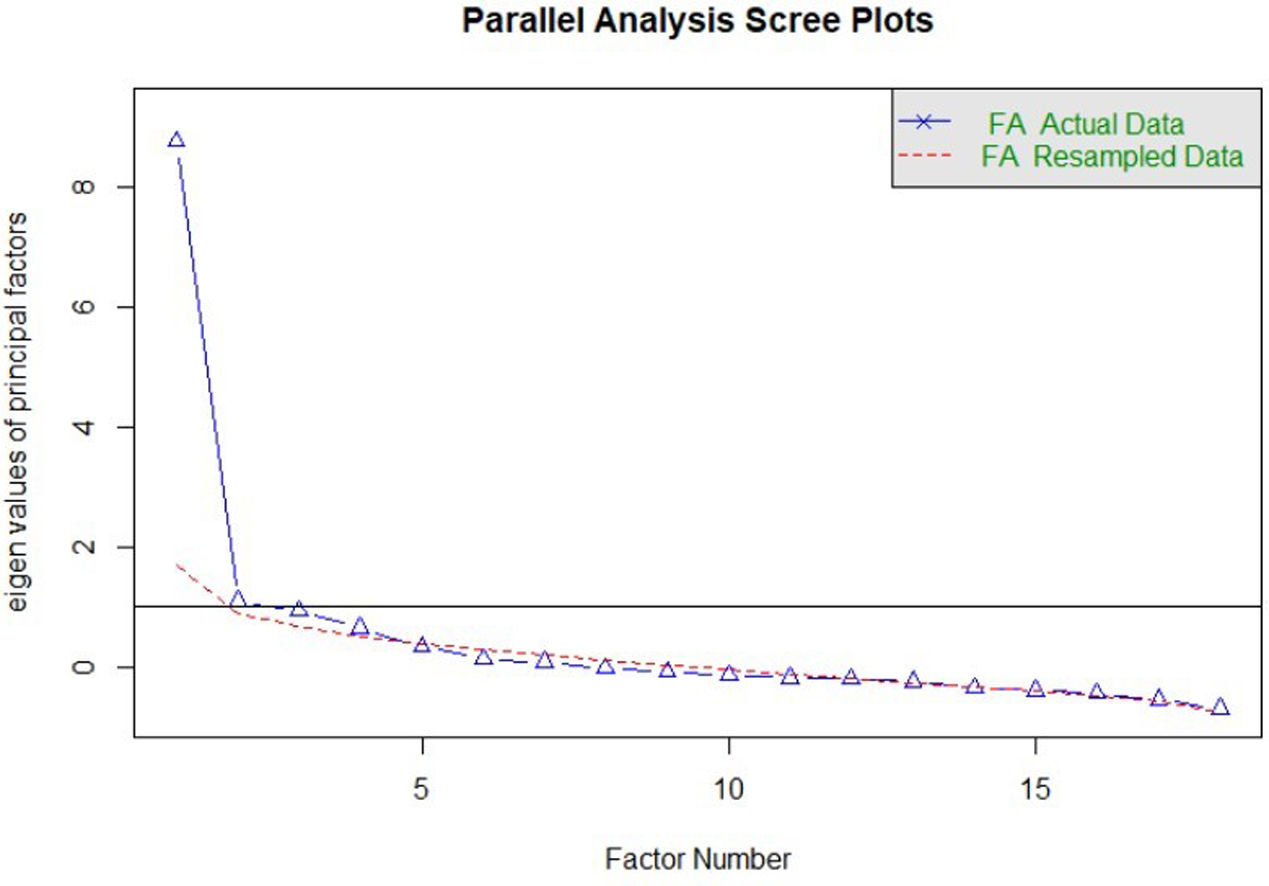
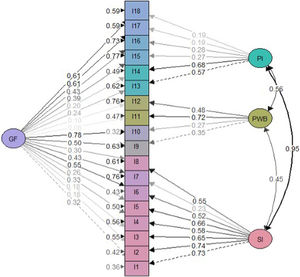
Construct validityDimensionality studyThe parallel analysis performed recommends the extraction of four factors whose empirical eigenvalues were higher than the mean of the simulated ones and their 95th percentile (Fig. 1). The first factor seemed to be dominant, while the other three contain secondary eigenvalues that explain less variance than the first factor.
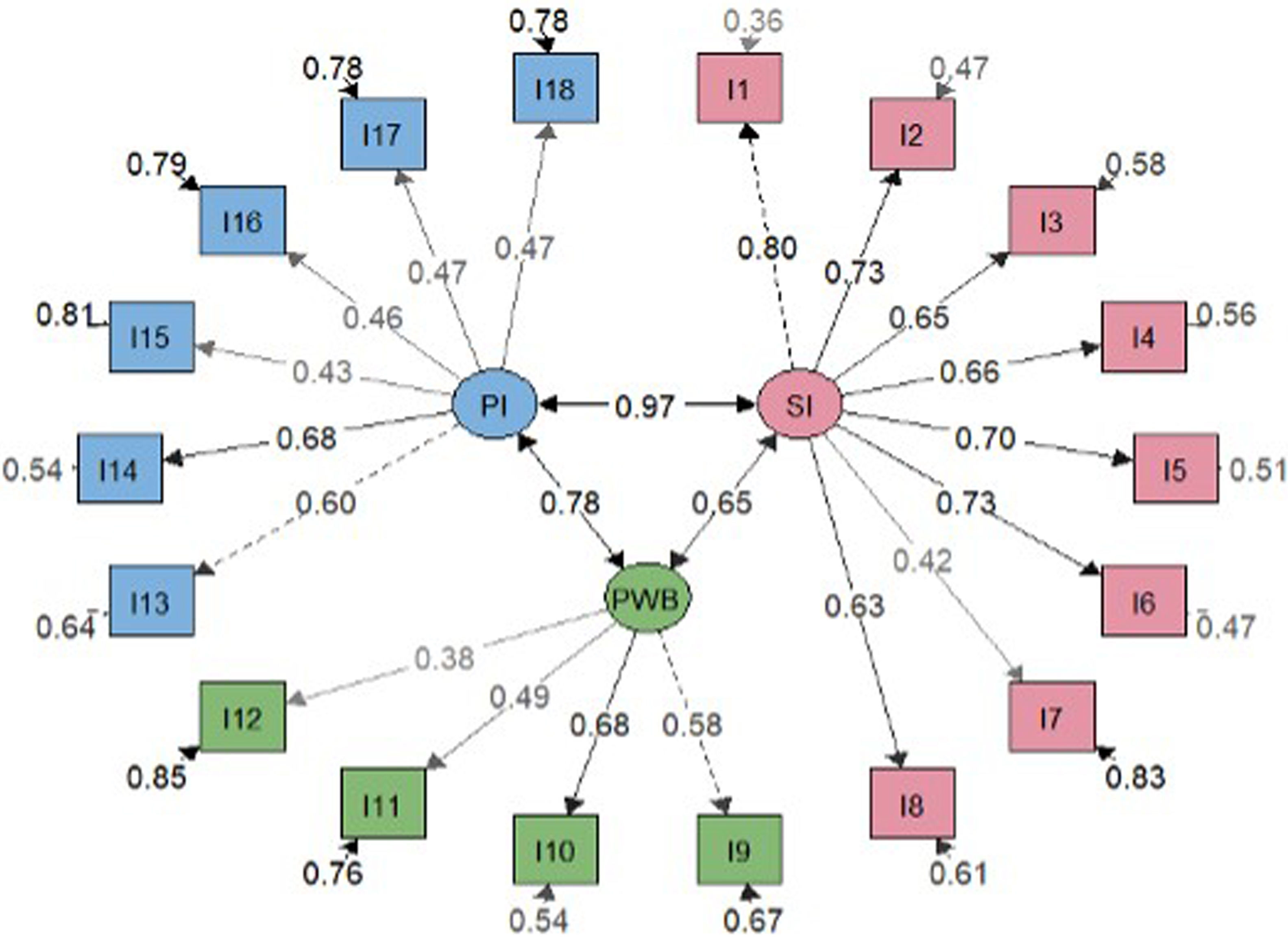
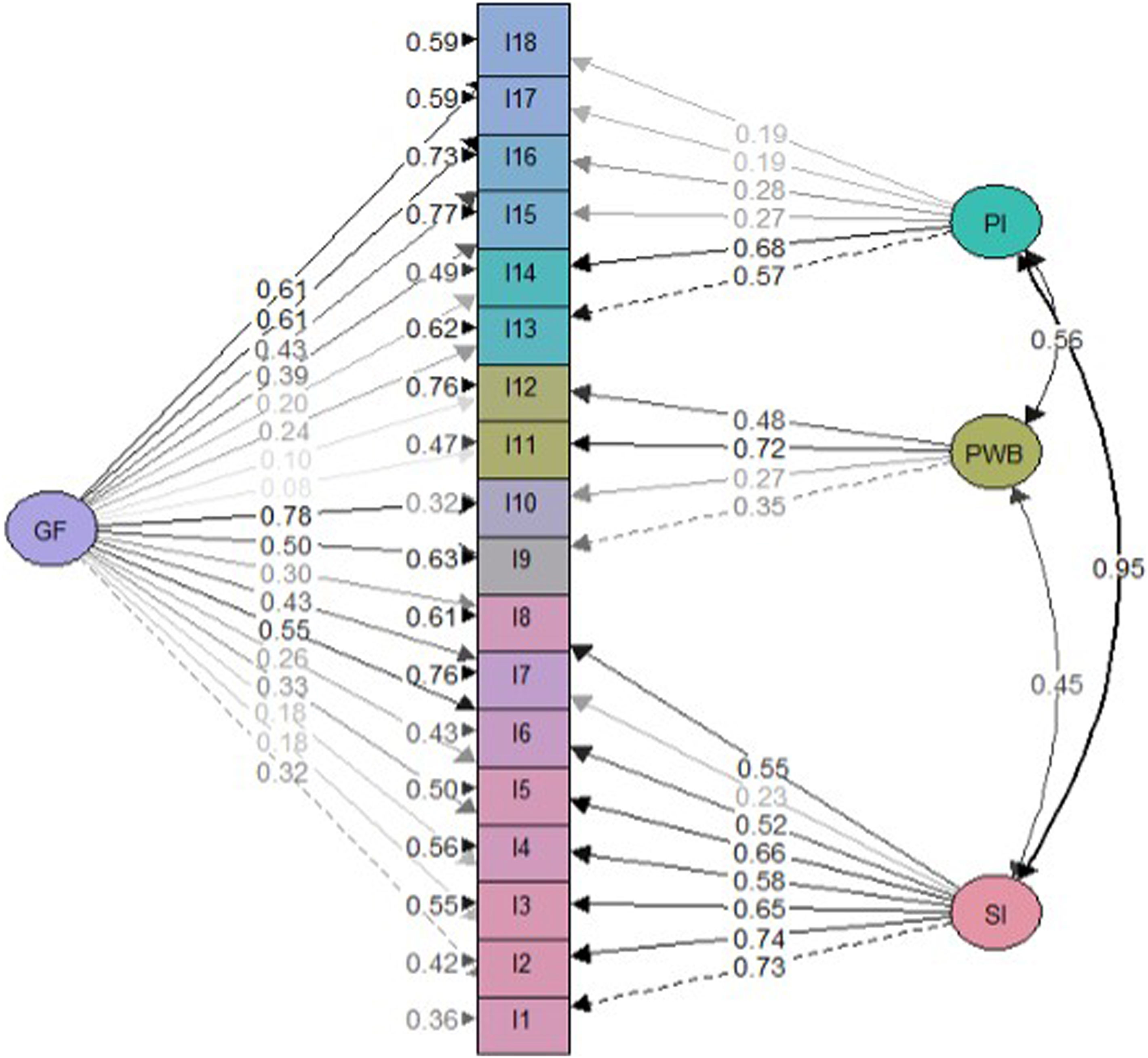
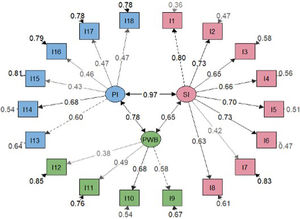
Factor analysisOverall model fit statistics suggested a better fit for the bifactor model than the correlated three-factor model (Table 2). Less significant saturations were obtained in the bifactor model compared with the general factor one (Figs. 2 and 3). Notwithstanding, factor loadings in the bifactor model did not reach values below 0.20 except for items 17 and 18 for the third group factor and items 2, 3, 11 and 12 for the general factor. All saturations and error variances were positive in both models. The only item that did not saturate significantly in any of the two models was item 12. The results of R2 show that the bifactor model explained more variance for each item including a general factor. The items of domain 1 (except item 7) and items 13, 14 were the best explained items in both models. Otherwise, items 7, 12, 15 and 16 had the lowest R2 value (<0.30) in the 3 models.
Model comparation.
| Model | Chi square | Df | CFI | TLI | RMSEA | SRMRu | SRMRu/R2 |
|---|---|---|---|---|---|---|---|
| CFA 3 correlated factors | 323.38** | 132 | 0.979 | 0.975 | 0.020 | 0.069 | 0.108 |
| CFA bifactor 3 group factors | 218.23** | 114 | 0.991 | 0.987 | 0.014 | 0.043 | 0.067 |
Note: CFA: confirmatory factor analysis; CFI: Comparative Fit Index; TLI: Tucker–Lewis Index; Df: degrees of freedom; RMSEA: root mean squared error of approximation; SRMRu: unbiased standardized root mean squared residual.
A model fits satisfactorily if: CFI >0.95, RMSEA <0.05 and SRMRu <0.08.
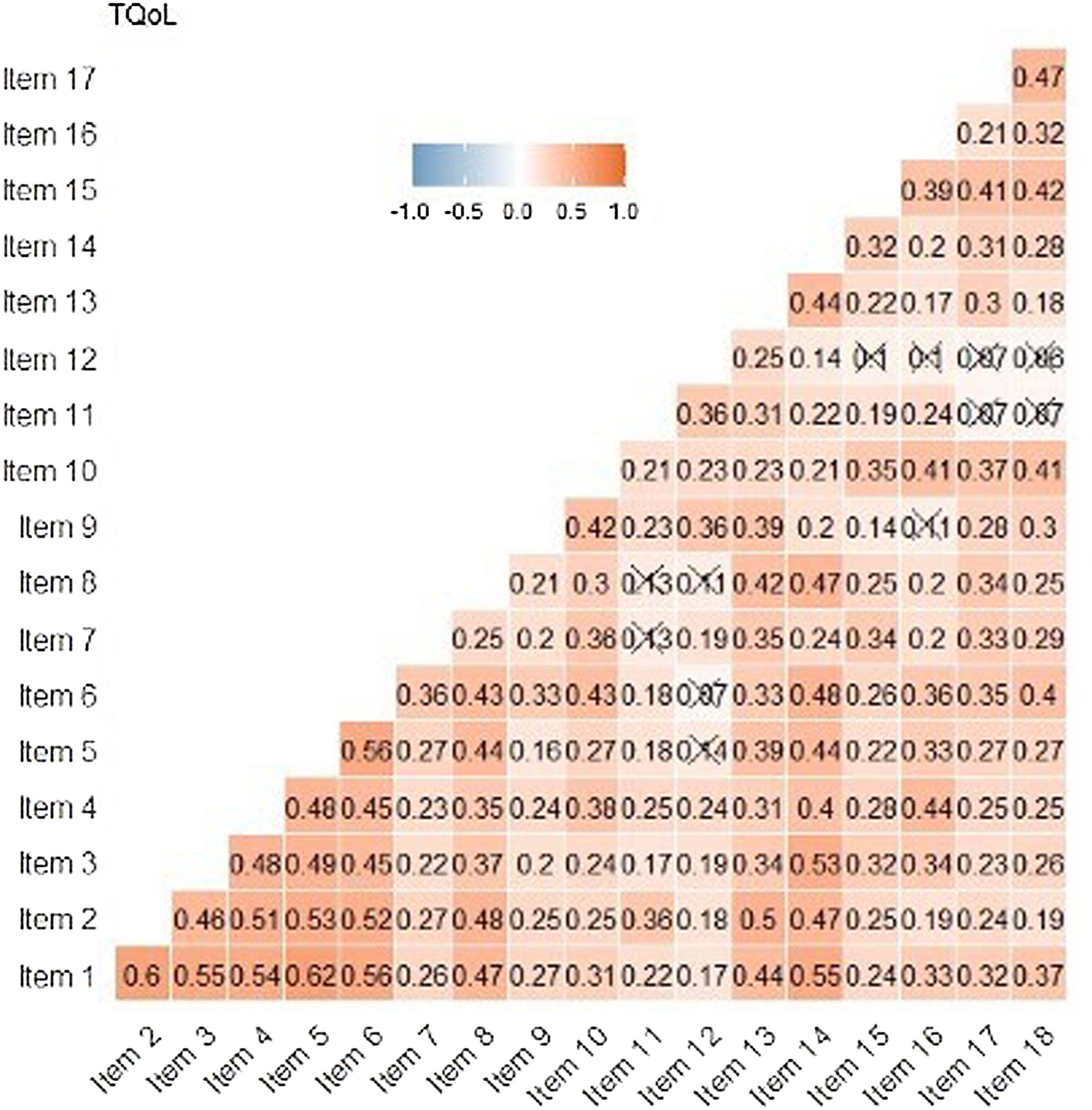
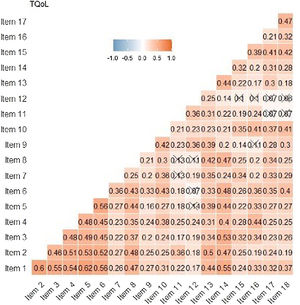
Reliability indicators reveal pronouncedly high values such as Cronbach's α=0.89, G6=0.91 and Omega ω=0.91. These results show that the items seem to be closely related to each other. In the Spearman correlation matrix between items, questions 1–6 correlate highly with each other (correlation coefficient >0.3). Items 11 and 12 have less significant correlations with other items (Fig. 4). In the item-rest of the test correlation, item 12 is the only with correlation <0.30, being the least correlated with the rest of the test.
For the test–retest, 47 patients (35% of 133) completed the second set of questionnaires after a mean of 12.8 days. ICC was highest for the total T-QoL mean score=0.85 (95% CI=0.77–0.91) followed by domain 1=0.81 (95% CI=0.71–0.88), domain 3=0.80 (95% CI=0.69–0.87) and domain 2=0.75 (95% CI=0.62–0.84).
DiscussionWe carried out the most demanding translation based on the standards accepted by the ISPOR consensus guidelines.20 The most problematic issues pertained to the “self-conscious” item. As for the Spanish version of DLQI18 and CDLQI,19 we added a synonym, resulting in the following question: “¿Te sientes cohibido/a o cortado/a por tu problema de la piel?”.
From the first descriptive analyses, no significant differences in T-QoL scores were observed when repeated one week later. These results are a good indicator of reliability from a descriptive perspective. Comparison with the DLQI, CDLQI and GQ also showed no significant differences, which is a preliminary indicator of good convergent validity.
The similar test behaviour between children older and younger adolescents is a good approximation of reliability for both age groups. Differences were only observed in domain 3, in which scores were higher in participants over 16 years. A possible explanation is that domain 3, related to psychological impact and relationships, is more relevant for older adolescents. Females generally scored higher than males in T-QoL and in comparators tests, suggesting the T-QoL seems reliable in the gender analysis. In both the Spanish and English samples,16 females seem to show a greater interest in questions related to image (domain 1) and relationships (domain 3). Nevertheless, questions about physical well-being and future aspirations seem to be of similar interest to both genders.
The distribution of item scores was also consistent with the results of Basra et al.16 For both, domain 1 had the highest scores followed by domain 3 and domain 2. Other similarities with the original version were acne, eczematous dermatosis and psoriasis being the three commonest conditions included.
In the convergent validity assessment, we observed a high correlation (r=0.75) between the T-QoL and the DLQI and CDLQI tools. Similar results were observed in the original version, where T-QoL results were equivalent with CDLQI (r=0.75) and DLQI (r=0.74) tools. We also found a moderate correlation with GQ evaluation, which is higher than in the original version (r=0.5).
The results from the CFA support that a total score can be calculated despite the multidimensionality of T-QoL. It should be noted that the original authors extracted three factors based on the eigenvalues founded using Kaiser's rule, not recommended according to Ruiz & San Martín29 and in this study four factors have been extracted using the parallel analysis technique.
Although the bifactor model obtains a more positive fit than the three-factor correlated model, the cautions that previous literature highlights about bifactor models should be taken into account, especially when the sample size is not very large. However, unlike the original version, this time the saturations of the general factor were not found to be always higher than those of the group factors. This does not seem to be an indication of overestimation of the saturations in the general factor by absorbing variance of the group factor,30,31 although the implications of not having specified cross loadings should be subject of attention in future studies.
For internal consistency reliability, the Cronbach's α values of the Spanish version of the T-QoL were equal to the original version (Cronbach's α=0.89 for total scale score). Additional estimators were also studied with excellent levels (ω=0.91, G6=0.91). High values are sometimes a symptom of redundancy or low specificity24–26; however, we cannot assure that the results of internal consistency indexes indicate unidimensionality. The value of ICC to the total T-QoL mean score was slightly lower than the results in the original version (0.85 vs 0.91), but >0.75 demonstrates excellent stability of the measure.
Both, inter-item and item-rest of the test correlation, item 12 (concerning sleep) was the less correlated. In the factor analysis the same item was the only that did not saturate significantly in any of the three models, whose revision seems more than advisable.
The value of this study goes beyond the methodology followed and the results obtained in the validation process. An attempt has been made to expand the number of reliability and validity test indicators and to highlight some recommendations from the literature of the bifactor models of CFA, so that future research will continue to question the methodological decisions to validate the factor structure of this test. In routine clinical practice, the Spanish version of T-QoL will improve the patient-physician relationship, as it addresses the emotional aspects of the patient's conditions.32 Responses to the questionnaire also provide valuable information to facilitate multimodal management and the collaboration of dermatologists with psychologists and psychiatrists when necessary.33
Given the current lack of translations of the T-QoL, our adaptation to Spanish will allow its use to be extended to new countries, fostering the development of international multicenter studies. Nevertheless, we believe that it would be advisable to conduct a pilot study of the tool to ensure its validity in a cultural context other than Spain before its large-scale application.
We also believe that another value of this tool could be for future cost-utility analyses, particularly relevant in the field of dermatology.34
Limitations of the studyThe total number of patients attending the dermatology consultation was not recorded, nor was the number of adolescents who refused to participate during recruitment. Validation may be hampered by response bias, common to most questionnaire-based studies, although consistency and agreement with other widely used instruments was positively tested.
In the test–retest there was a decrease in sample size, from 133 to 47 (−35%). However, authors decided to proceed with the analysis, as health care conditions drastically changed during the lockdown period (March–June 2020) of the COVID-19 pandemic. This decrease also occurred in the validation of the original version: from 203 to 61 (−30%).16
In conclusion, we have successfully translated, adapted and validated a Spanish language version of the T-QoL for adolescent patients with dermatological diseases. To our knowledge, this is the first version of the T-QoL to be published in a language other than English.
Its implementation in countries and populations belonging to a Spanish language culture will make it possible to expand research horizons and enhance our knowledge of those adolescents with skin diseases, thus contributing to improving their QoL.
Conflict of interestDr. González-Cantero has served as a consultant for Abbie, Janssen, Novartis, Almirall, Celgene and Leo Pharma receiving grants/other payments, outside the submitted work. All other authors report no conflicts of interest.
We would like to thank all participants, as well as the colleagues of Toledo University Hospital dermatology department for help recruiting participants to our study. Furthermore, we want to thank Dr. Andrew Finlay, Dr. Agustín Buendía-Eisman, Omar Alonso-Naranjo and Francisco Estupiñan-Romero for reviewing our manuscript.