Biosynthetic porcine type I collagen dressing is useful to close ulcers and secondary intention healing of surgical cancer wounds.
ObjectiveTo identify factors associated with the healing time of such wounds.
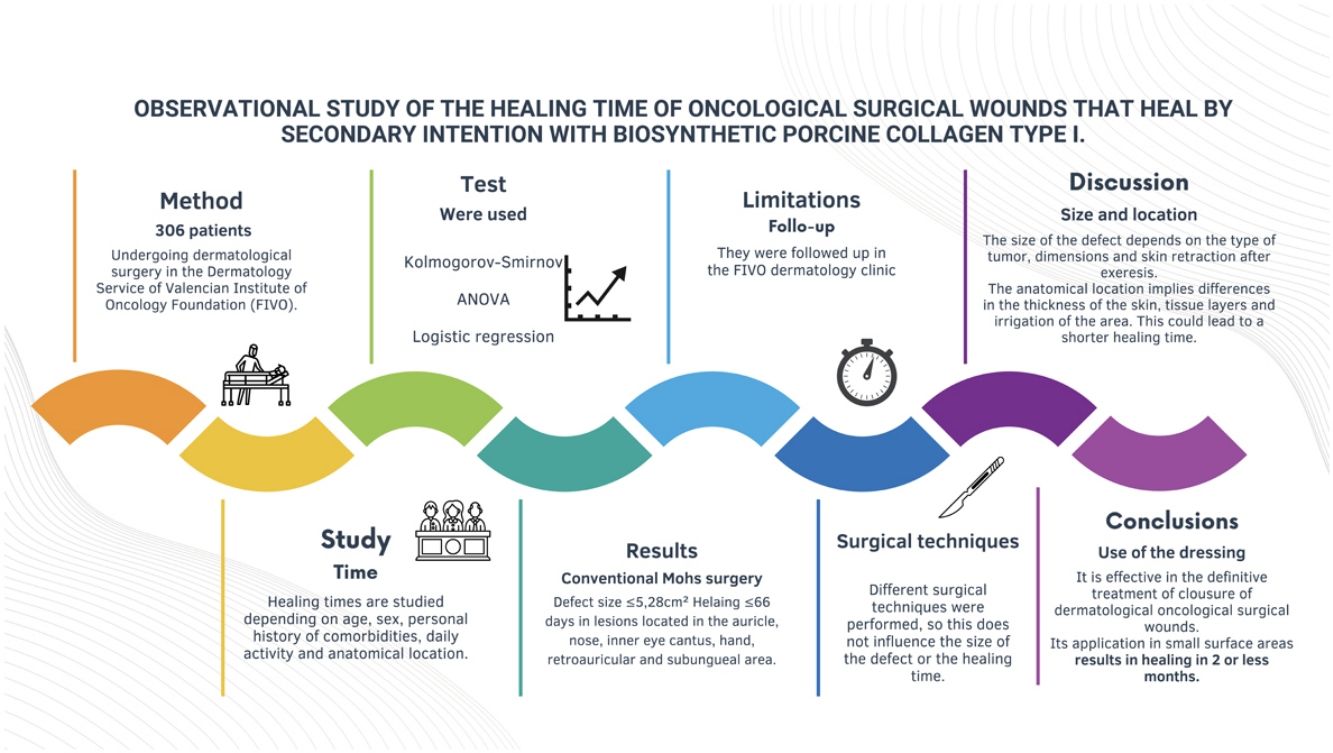
MethodsWe conducted a retrospective observational study of patients on oncological dermatological surgery in a cancer center. Healing time was studied based on age, sex, personal history of comorbidities, usual medication, anatomical location, type of surgery performed and size of the defect. Statistical analysis was performed using the ANOVA test and logistic regression.
ResultsA total of 306 patients were included with a median age of 73 years (62.4%, men). The statistically significantly characteristics associated with a healing time≤66 days were location—auricle, nose, inner canthus, hand, retroauricular region and nail bed—use of the Mohs technique and defect sizes≤5.28cm2.
LimitationsThis is a retrospective study from a single center.
ConclusionThe use of biosynthetic porcine type I collagen dressings for the definitive closure of dermatological surgical cancer wounds seems particularly suitable for small defects of the inner canthus of the eye, hand, auricle, nose, forehead, retroauricular region, and nail bed.
El parche biosintético de colágeno porcino tipoI se está utilizando en el cierre de úlceras y heridas quirúrgicas oncológicas que curan por segunda intención.
ObjetivoConocer los factores que se asocian al tiempo de cicatrización de este tipo de heridas.
MétodoSe diseñó un estudio observacional retrospectivo de los pacientes sometidos a cirugía dermatológica oncológica en el Servicio de Dermatología de la Fundación Instituto Valenciano de Oncología (FIVO). Se estudió el tiempo de cicatrización en función de la edad, el sexo, los antecedentes personales de comorbilidades, la medicación habitual, la localización anatómica, el tipo de cirugía realizado y el tamaño final del defecto, con la prueba ANOVA y modelos de regresión logística.
ResultadosSe incluyeron 306 pacientes, con una mediana de edad de 73años y un 62,4% de hombres. Las lesiones localizadas en el pabellón auricular, la nariz, el canto interno del ojo, la mano, el área retroauricular y la subungueal, así como la cirugía de Mohs y un tamaño del defecto quirúrgico menor o igual a 5,28cm2 se asociaron a un tiempo de cicatrización menor o igual a 66días.
LimitacionesEl diseño del estudio fue retrospectivo y de una única institución.
ConclusiónEl uso del apósito biosintético de colágeno porcino tipo I para el tratamiento definitivo del cierre de las heridas quirúrgicas dermatológicas oncológicas parece especialmente adecuado para defectos pequeños y localizados en el canto interno del ojo, la mano, el pabellón auricular, la nariz, la frente, el área retroauricular o la subungueal.
Secondary intention healing is a method to close surgical skin defects following cancer surgery. This technique offers some clinical advantages, particularly in areas where there is a high risk of recurrence, where secondary intention closure may be preferable to flap and graft closure as it facilitates the follow-up and early detection of tumor recurrence.1 In fact, performing a flap could mask undetected tumor growth. In addition, although grafts provide faster defect resolution and allow better assessment of the surgical area for detection of recurrence, they require leaving an additional scar in the donor area and they take more surgical time than placing a biosynthetic dressing.2,3
Secondary intention closure requires prolonged healing, which can cause patient discomfort and poor esthetics.4,5 Moreover, conditions that impair blood flow, such as advanced age or diabetes, can also slow healing. For example, diabetes affects the normal function of fibroblasts and epidermal cells, impairing angiogenesis and neovascularization and impacting the immune response, which can hinder the healing process.6 Other chronic conditions that require treatment with oral anticoagulants, including coronary heart disease and peripheral vascular disease can also delay healing.7
The porcine type I collagen dressing was first used back in 1979 to treat burns and donor sites. This semipermeable biosynthetic membrane is designed to temporarily replace the functions of the lost epidermis until these wounds are re-epithelialized.8 It consists of 2 layers: a non-biodegradable nylon mesh—whose outer coating prevents water loss and bacterial colonization—and a second silicone layer bound to the nylon mesh through porcine type I collagen. This dressing promotes the formation of granulation tissue in the wound bed. Its flexibility allows it to adapt to any part of the body surface even in difficult areas, including irregular areas such as the face. It can be secured to the skin surface with staples, sutures or adhesive.9–13 It also has the advantage of being transparent, which allows easier inspection of the wound bed and early detection of complications.
More recently, its use has been extended to cover defects following dermatological cancer surgery, mainly, as a temporary cover to promote granulation of the bed and ensure better nutrition of the graft placed in a second surgical act and, also, to accelerate definitive closure through secondary intention healing.
The benefits it brings to treating burns are widely demonstrated. Among others, it reduces healing time, the length of stay, pain, and need for analgesics.14–16 However, these effects have not been well studied in the context of dermatological cancer surgical wounds.
The aim of our study was to determine the mean healing time of cancer and dermatological surgical wounds closed by secondary intention healing using a biosynthetic porcine type I collagen dressing and identify the factors associated with a shorter healing time.
Patients and methodsWe conducted a retrospective observational study, including patients undergoing dermatological cancer surgery at Department of Dermatology of Instituto Valenciano de Oncología (IVO) (Valencia, Spain) from May 2008 to October 2018. Patients were eligible as long as they had undergone a secondary intention surgical wound closure using a biosynthetic porcine type I collagen dressing. A video illustrating the routine procedure used for dressing application has been included in the Supplementary data. The selection of the closure method was left at the surgeon's discretion depending on the risk of tumor recurrence and the patient's general condition. However, the final decision was made along with the patient, with whom the possible closure options were discussed beforehand.
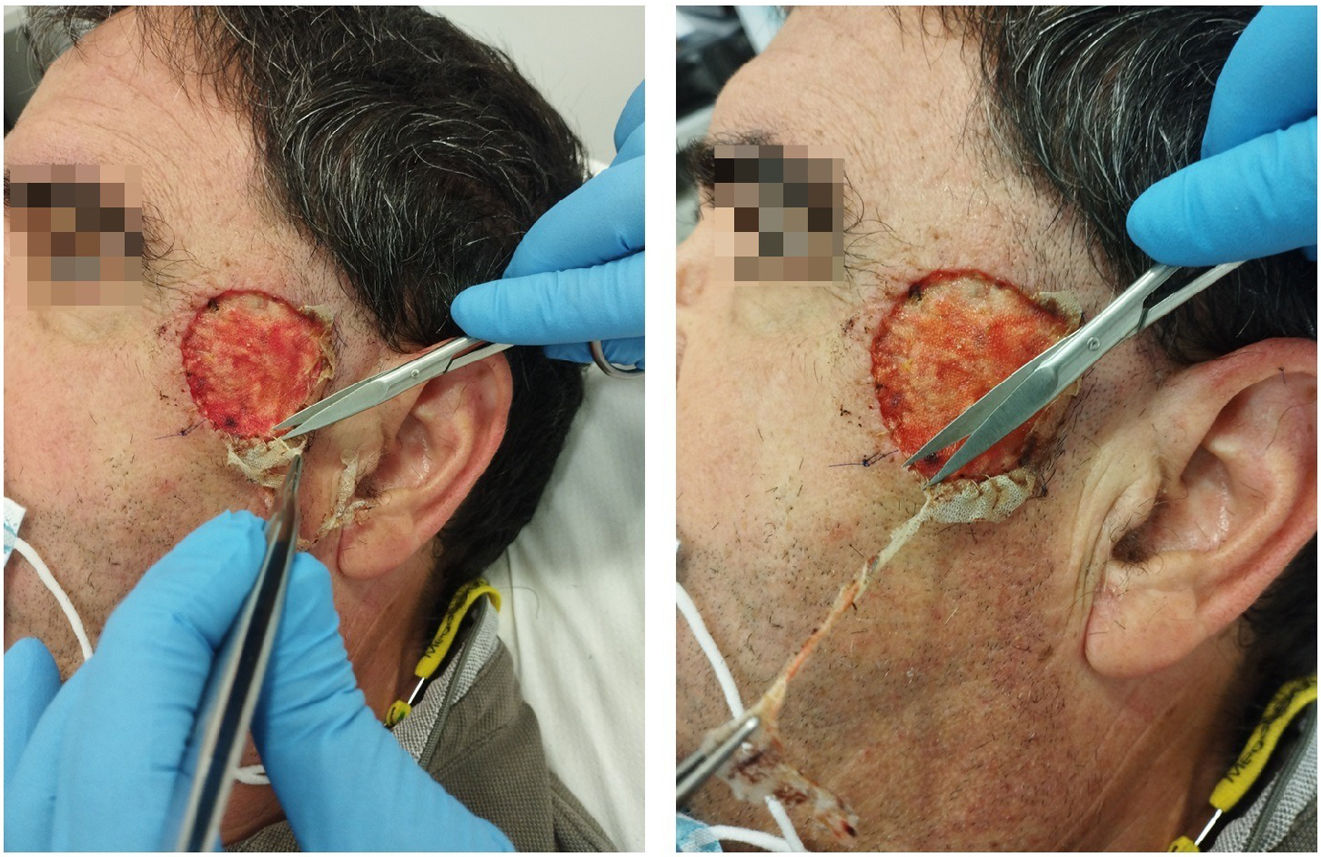
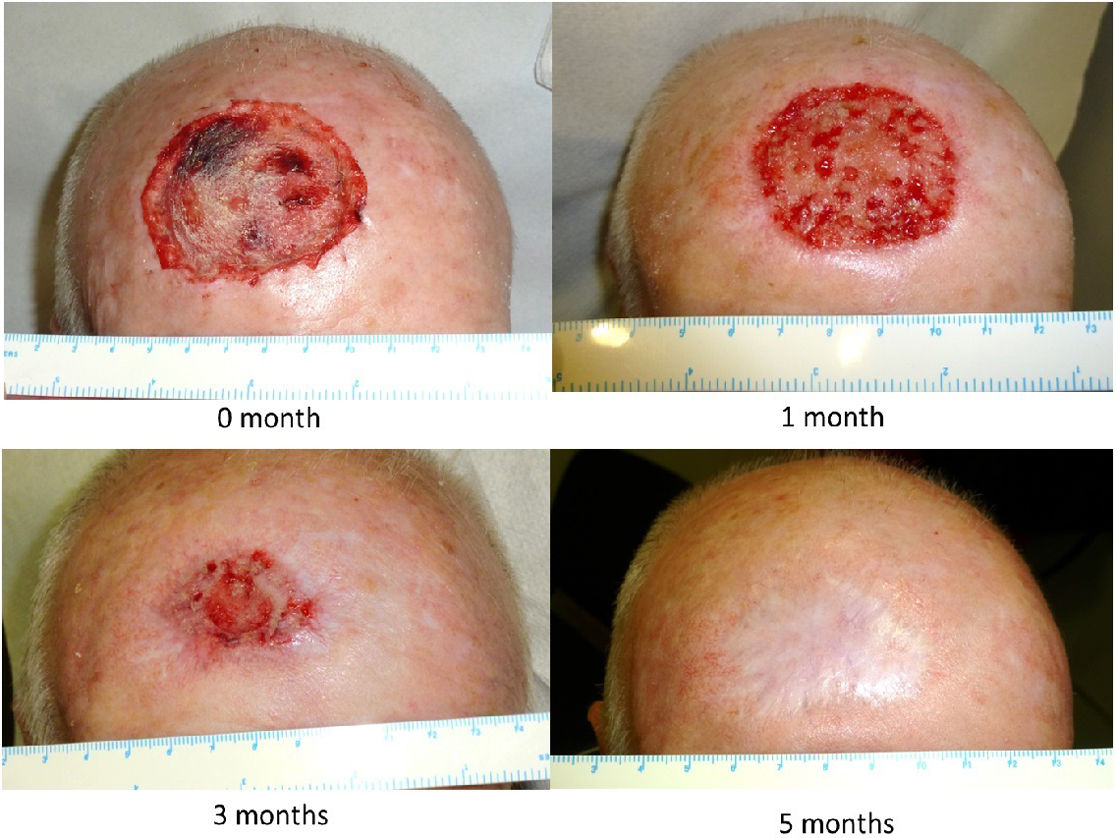
The dressing procedure from the Wound Care Unit consists of assessing the condition of the wound—these are usually clean wounds with no signs of infection, given the aseptic measures taken during the application of the Biobrane® biosynthetic primary dressing—irrigation with physiological saline solution, trimming the loose edges of the Biobrane® (to promote re-epithelialization from the edges of the wound) as shown in Fig. 1, and application of a secondary dressing, which may vary depending on the amount of wound exudate, condition of perilesional skin, frequency of dressing changes, patient preference and cost effectiveness. Afterwards, images are taken, and the size of the defect is measured. The progression of the scalp wound from the immediate postoperative to complete healing is illustrated in Fig. 2.
Patient data were obtained from the Wound Care Unit registry of the IVO Dermatology Department. All cases were prospectively collected, including pre- and postoperative iconography. Patients without a complete postoperative follow-up in our Wound Care Unit were excluded from the study. All patients signed the corresponding informed consent form, and the study was approved by the IVO Research Ethics Committee (registration #002-19).
The dependent variable of the study was healing time, defined as the number of days elapsed between surgery and complete wound closure. This time was evaluated as a continuous variable and 2 categories were created based on the median healing time of the study population: ≤66>66 days. In the opinion of the research team, this cut-off point of nearly 2 months was considered acceptable to identify cases where cure by secondary intention was reasonable.
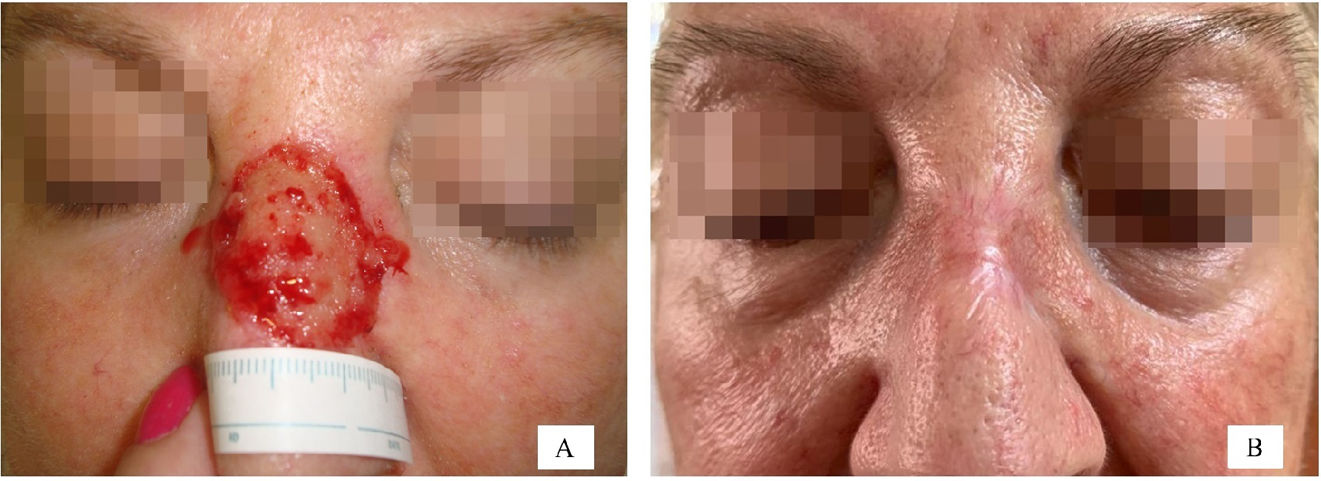
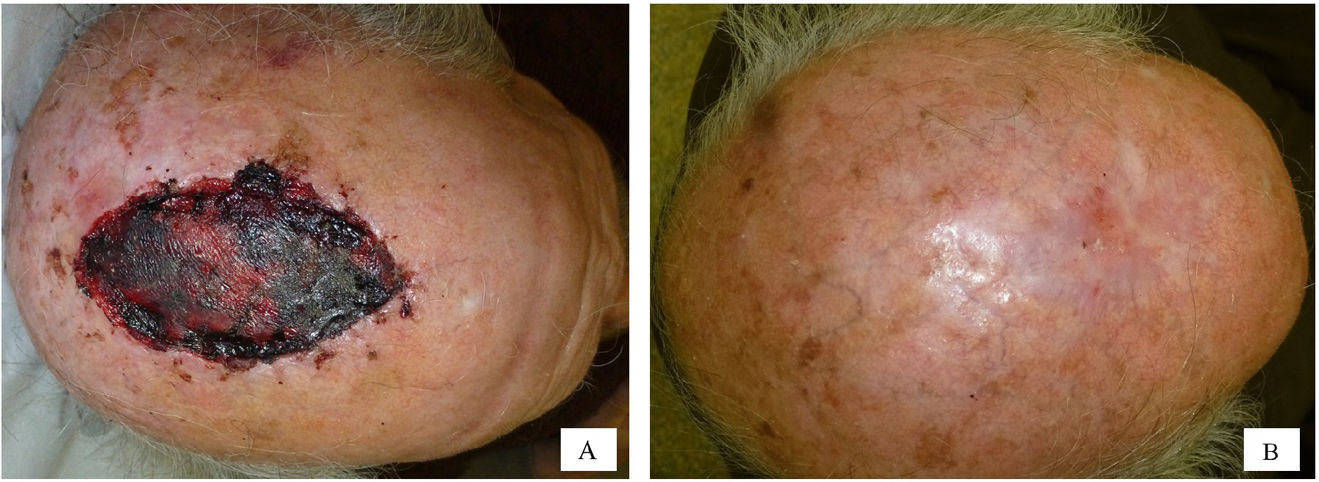
The following variables were collected: age categorized into quartiles (≤62 years, 63–73 years, 74–80 years, and >80 years), sex, presence or absence of chronic diseases—high blood pressure (HBP) and/or diabetes—use or non-use of anticoagulant drugs, and anatomical location of the surgical defect. A total of 21 different sites were included, and, then, categorized into 2 groups based on the results of a correspondence analysis (Supplementary data A). Group #1 included foot, temple, scalp, cheek, leg, forehead, and preauricular region. Group #2 included auricle, nose, inner canthus of the eye, hand, retroauricular region, and nailbed. Figs. 3 and 4 illustrate examples of healing wounds in 2 different locations—nose and scalp—with the corresponding pre- and postoperative images. The type of surgery was also recorded based on 2 modalities—conventional surgery/slow Mohs and conventional Mohs surgery—and the size of the surgical defect. For the analysis of this last variable, defects were assumed to have an elliptical shape, and the area was calculated by measuring the largest and smallest diameter of the defect. Thus, 2 categories were defined based on whether the surgical defect area was ≤5.28m2 or >5.28cm2. A cut-off point of 5.28cm2 was calculated using the classification and regression tree method (Supplementary data B). This cut-off point allowed for discrimination between a healing time≤66 and >66 days.
The normality of the continuous variable “healing time” distribution was evaluated using the Shapiro–Wilks test. The ANOVA test was used to compare means across groups. The associations between healing time and the studied features were determined by univariate and multivariate logistic regression models. The odds ratio (OR), 95% confidence intervals (CI) and the associated p-values were calculated. Statistical significance was considered for p-values<0.05. IBM SPSS version 23 (IBM SPSS, Inc.; Chicago, USA) was used to perform statistical tests.
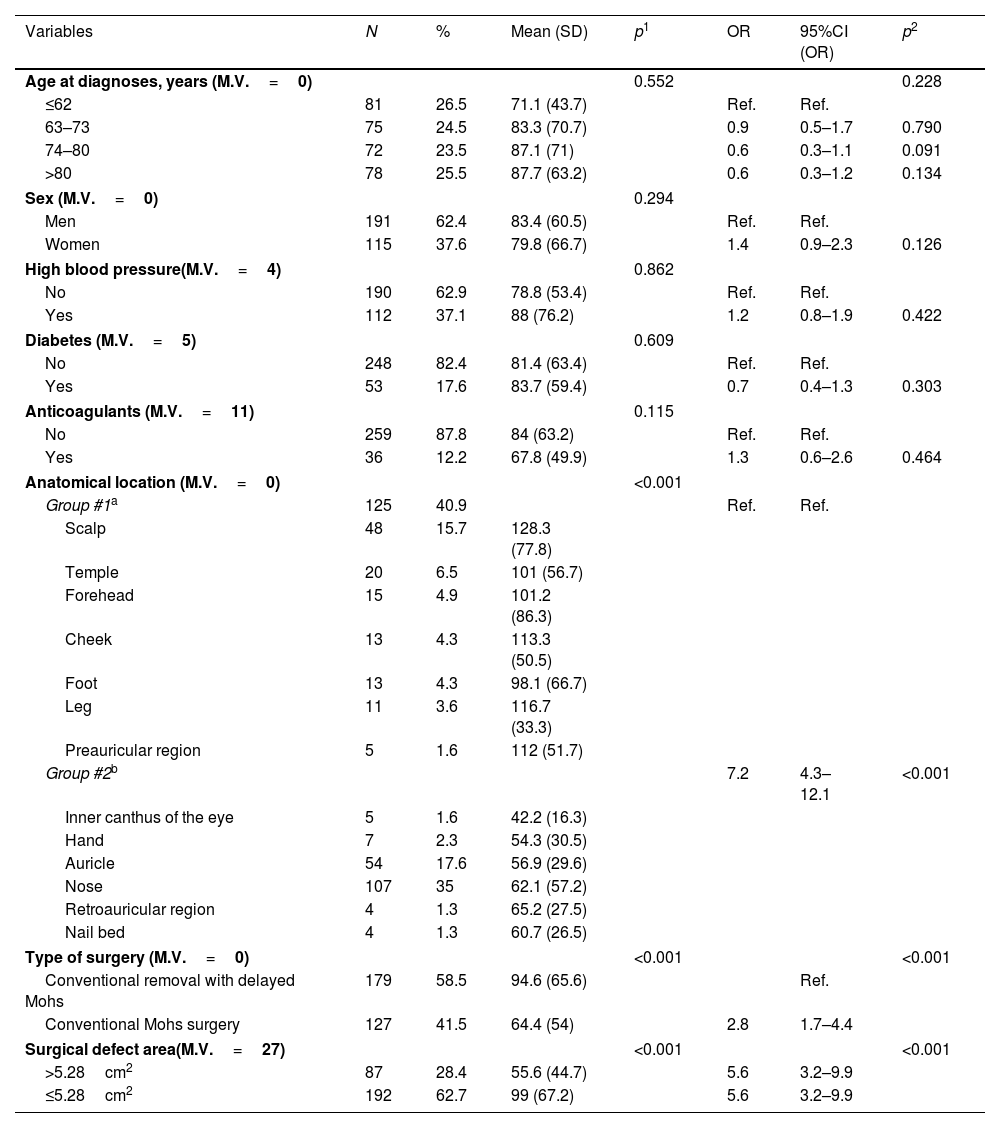
ResultsA total of 306 out of a total of 495 patients met the selection criteria (Supplementary data, Fig. A). The number of patients and their percentage based on the values of each variable studied is illustrated in Table 1. In conclusion, the median age was 73 years (IQR, 62–80 years; 62.4%, men). Regarding comorbidities, 37.1% had hypertension; 17.6%, diabetes; and 12.2% were on anticoagulants. Regarding location, 40.9% had the defect in group #1 (scalp, temple, forehead, cheek, foot, leg, or preauricular region), and 59.1% in group #2 (inner canthus of the eye, hand, auricle, nose, forehead, retroauricular region, and nail bed). Conventional Mohs surgery was performed in 41.5% of the patients, while conventional surgery or slow Mohs surgery in 58.5%. In 28.4% of the cases the defect size was ≤5.28cm2, and in 62.7%>5.28cm2.
Study of the variables associated with healing time≤66 days: descriptive analysis and results of the mean comparison using the ANOVA test.
| Variables | N | % | Mean (SD) | p1 | OR | 95%CI (OR) | p2 |
|---|---|---|---|---|---|---|---|
| Age at diagnoses, years (M.V.=0) | 0.552 | 0.228 | |||||
| ≤62 | 81 | 26.5 | 71.1 (43.7) | Ref. | Ref. | ||
| 63–73 | 75 | 24.5 | 83.3 (70.7) | 0.9 | 0.5–1.7 | 0.790 | |
| 74–80 | 72 | 23.5 | 87.1 (71) | 0.6 | 0.3–1.1 | 0.091 | |
| >80 | 78 | 25.5 | 87.7 (63.2) | 0.6 | 0.3–1.2 | 0.134 | |
| Sex (M.V.=0) | 0.294 | ||||||
| Men | 191 | 62.4 | 83.4 (60.5) | Ref. | Ref. | ||
| Women | 115 | 37.6 | 79.8 (66.7) | 1.4 | 0.9–2.3 | 0.126 | |
| High blood pressure(M.V.=4) | 0.862 | ||||||
| No | 190 | 62.9 | 78.8 (53.4) | Ref. | Ref. | ||
| Yes | 112 | 37.1 | 88 (76.2) | 1.2 | 0.8–1.9 | 0.422 | |
| Diabetes (M.V.=5) | 0.609 | ||||||
| No | 248 | 82.4 | 81.4 (63.4) | Ref. | Ref. | ||
| Yes | 53 | 17.6 | 83.7 (59.4) | 0.7 | 0.4–1.3 | 0.303 | |
| Anticoagulants (M.V.=11) | 0.115 | ||||||
| No | 259 | 87.8 | 84 (63.2) | Ref. | Ref. | ||
| Yes | 36 | 12.2 | 67.8 (49.9) | 1.3 | 0.6–2.6 | 0.464 | |
| Anatomical location (M.V.=0) | <0.001 | ||||||
| Group #1a | 125 | 40.9 | Ref. | Ref. | |||
| Scalp | 48 | 15.7 | 128.3 (77.8) | ||||
| Temple | 20 | 6.5 | 101 (56.7) | ||||
| Forehead | 15 | 4.9 | 101.2 (86.3) | ||||
| Cheek | 13 | 4.3 | 113.3 (50.5) | ||||
| Foot | 13 | 4.3 | 98.1 (66.7) | ||||
| Leg | 11 | 3.6 | 116.7 (33.3) | ||||
| Preauricular region | 5 | 1.6 | 112 (51.7) | ||||
| Group #2b | 7.2 | 4.3–12.1 | <0.001 | ||||
| Inner canthus of the eye | 5 | 1.6 | 42.2 (16.3) | ||||
| Hand | 7 | 2.3 | 54.3 (30.5) | ||||
| Auricle | 54 | 17.6 | 56.9 (29.6) | ||||
| Nose | 107 | 35 | 62.1 (57.2) | ||||
| Retroauricular region | 4 | 1.3 | 65.2 (27.5) | ||||
| Nail bed | 4 | 1.3 | 60.7 (26.5) | ||||
| Type of surgery (M.V.=0) | <0.001 | <0.001 | |||||
| Conventional removal with delayed Mohs | 179 | 58.5 | 94.6 (65.6) | Ref. | |||
| Conventional Mohs surgery | 127 | 41.5 | 64.4 (54) | 2.8 | 1.7–4.4 | ||
| Surgical defect area(M.V.=27) | <0.001 | <0.001 | |||||
| >5.28cm2 | 87 | 28.4 | 55.6 (44.7) | 5.6 | 3.2–9.9 | ||
| ≤5.28cm2 | 192 | 62.7 | 99 (67.2) | 5.6 | 3.2–9.9 | ||
Bivariate logistic regression. Descriptive analysis of the variables and results of the mean comparison using the non-parametric Mann–Whitney U test. ANOVA. Analysis of the variables associated with healing time≤66 days. Bivariate logistic regression.
The epidemiological and clinical characteristics associated with healing time and the comparison of means by ANOVA test are shown in Table 1. The variables associated with a healing time≤66 days were a defect size≤5.28cm2, conventional Mohs surgery, and location in group #2. No differences in healing time were found based on age, sex, or the presence of comorbidities, including hypertension, diabetes, and treatment with oral anticoagulants (Table 1).
Although not an objective of this study, the number of complications associated with the use of biosynthetic porcine type I collagen dressings was recorded. In conclusion, there were 15 cases of hypergranulation (4.9%), 175 cases of integration of the dressing into the tissue (57.2%), 13 detachments (4.2%), 1 serous collection (0.3%) and 6 infections (2%), in which Cloceleritorebacter, Bacteroides eggrthii and Pseudomonas were isolated. Furthermore, 2 cases required re-evaluation by the plastic surgery unit (0.7%). One patient had the defect on the nose and required plastic surgery for remodeling and cartilage grafting due to nasolabial flap retraction, which raises the edges of the wings. The other patient had the defect on the cheek and required surgery for a functional complication (lower eyelid ectropion).
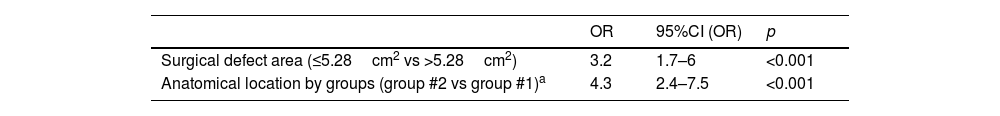
After multivariate analysis, defect size≤5.28cm2 and defect location in group #2 remained associated with shorter healing time (Table 2).
Multivariate analysis of the variables associated with healing time≤66 days.
| OR | 95%CI (OR) | p | |
|---|---|---|---|
| Surgical defect area (≤5.28cm2 vs >5.28cm2) | 3.2 | 1.7–6 | <0.001 |
| Anatomical location by groups (group #2 vs group #1)a | 4.3 | 2.4–7.5 | <0.001 |
Location:
The excision of some skin tumors leaves defects whose reconstruction can be a surgical challenge due to their size, location and risk of recurrence, making flap coverage questionable. In this study we have shown that the use of a biosynthetic porcine collagen type I dressing helps to achieve a relatively fast healing time, particularly in small defects located in certain anatomical regions.
The final size of the defect in dermato-oncological surgery depends on the type of tumor, its dimensions and skin retraction after tumor removal. We found, like other studies and as expected, that a small surgical defect—considered as such if its area was ≤5.28cm2—was associated with a faster healing time.4 In these cases, the use of a biosynthetic porcine collagen type I dressing allowed a healing time<66 days in 41.6% of cases.
Anatomical location results in differences in vascularization, thickness and tissue layers (skin, subcutaneous cellular tissue, aponeurosis, perichondrium, cartilage, periosteum or bone).17 These differences seem to be crucial for adequate healing in <66 days, especially when defects were reported in group #2, as they lack a thick layer of subcutaneous cellular tissue. In these locations, granulation tissue quickly appears with help of the biosynthetic porcine type I collagen dressing. Moreover, skin in these areas does not undergo significant retraction after tumor removal, so the final defect depends mainly on the type and size of the tumor. Furthermore, because these anatomical locations are obvious, tumors are usually diagnosed earlier and are smaller in size. In particular, the skin on the forehead may have a slightly thicker layer of subcutaneous cellular tissue and may be subject to some retraction. However, other inherent characteristics of this site, such as the distinct vascularization pattern, the visibility of the area, or the potential exposure of the periosteum, may also be important to confer faster healing.17 On the other hand, the auricle, the nose—in its inferior aspect—and retroauricular region have cartilaginous support. The greater presence of perichondrial collagen in these anatomical locations may result in faster and more efficient healing.
Other authors have described the efficacy of biosynthetic dressing in the management of surgical wounds with exposed vital structures, thus highlighting the role of the dressing in protecting vascular structures and anastomoses prior to definitive closure. However, they do not describe the healing time required for closure.11 Two former studies showed that this dressing appeared to be effective in the treatment of scalp defects>5cm2 where periosteal resection was also performed. However, this type of surgical wound was reported to take longer to heal (between 95 and 135 days—a mean of 115 days).1,2
In the present study, clinical features of the patients such as an advanced age, hypertension, diabetes, or oral anticoagulants did not impact healing time. The biological component of the dressing might promote neovascularization and the formation of fine granulation tissue in the bed of even poorly vascularized wounds.8 This relevant finding requires further study since it suggests that the dressing could be a good alternative for all patients regardless of their comorbidities.
Some studies of burn patients treated with biosynthetic dressings have shown that the use of these biosynthetic dressings within the first 12h after sustaining the injury significantly reduces re-epithelialization time.13–17 In dermatological cancer surgery, this dressing is applied immediately after the surgery, regardless of the location of the defect. In addition, the clean surgical wound bed promotes optimal adherence, which could explain the good results obtained in our study, which is consistent with the findings made by other authors.16
Furthermore, it has been possible to demonstrate that the biosynthetic porcine type I collagen dressing is safe since it has a low rate of complications. The integration of the dressing was the only complication observed in a relatively high percentage of patients. However, this could be the case because in dermatological cancer surgical wounds, to achieve complete healing or an optimal wound bed to receive the graft, the dressing was left in place longer than the time recommended by the manufacturer. The manufacturer recommends removing the dressing after 7–14 days. The price of a 10cm×10cm Biobrane® sheet is €70. Since the dressing can be reused, the approximate cost of the part used to cover defects smaller than 5.28cm2 is under €10.
The main limitation of this study is the retrospective design with patients from a single center.
In conclusion, the biosynthetic porcine type I collagen dressing is a simple, inexpensive and effective tool in the definitive treatment of dermatological cancer surgical wound closure in secondary intention healing. Its use seems to be particularly useful in small defects or in some sites, such as the inner canthus of the eye, the hand, the auricle, the nose, the forehead, the retroauricular region or the subungual bed, where healing time is nearly ≤2 months.
Funding sourcesThis research has not received specific aid from public sector agencies, the commercial sector or non-profit entities.
Conflict of interestNone.