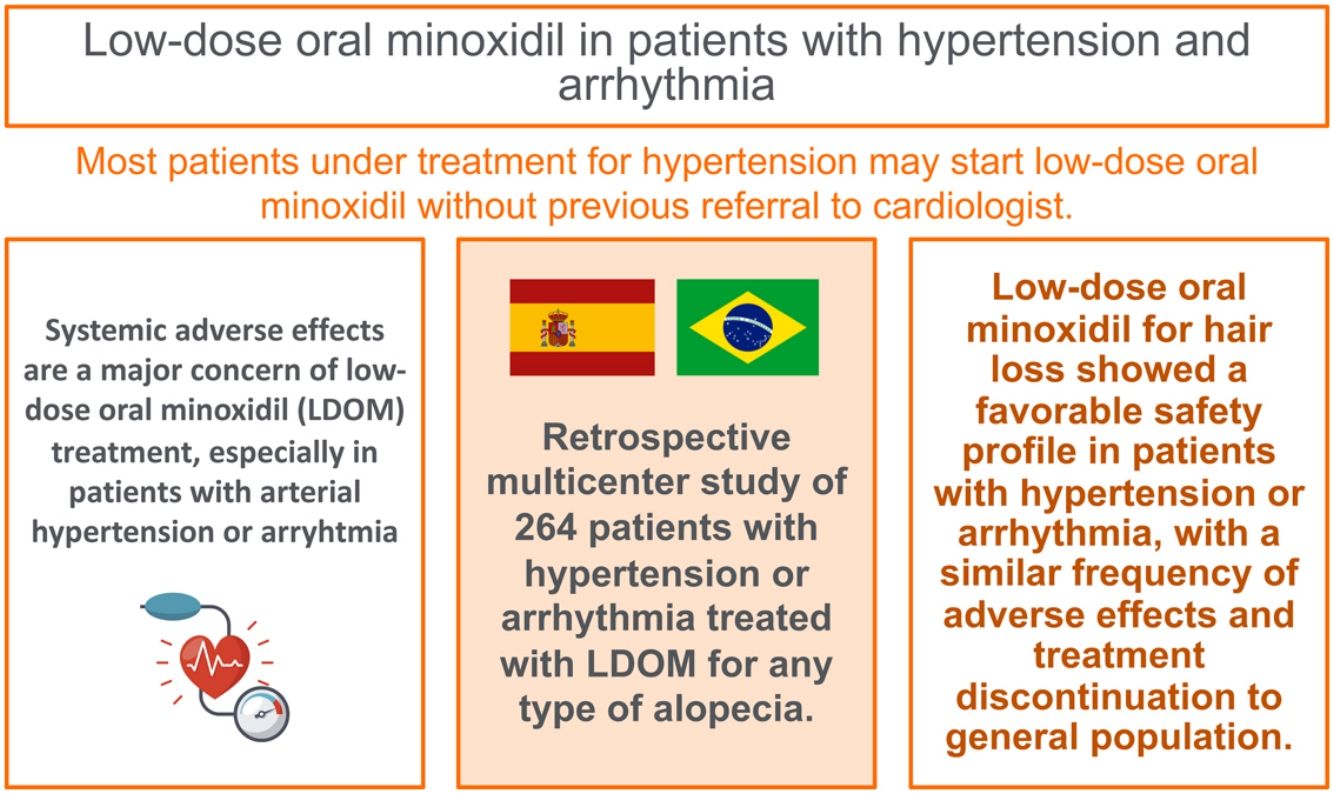
Systemic adverse effects (AE) are a major concern of low-dose oral minoxidil (LDOM) treatment, especially in patients with arterial hypertension or arrhythmia. The objective of this study was to evaluate the safety of LDOM in patients with hypertension or arrhythmia.
Patients and methodsRetrospective multicenter study of patients with hypertension or arrhythmia treated with LDOM for any type of alopecia.
ResultsA total of 254 patients with hypertension [176 women (69.3%) and 78 men (30.7%)] with a mean age of 56.9 years (range 19–82) were included. From them, the dose of LDOM was titrated in 128 patients, allowing the analysis of 382 doses. Patients were receiving a mean of 1.45 (range 0–5) antihypertensive drugs. Systemic AE were detected in 26 cases (6.8%) and included lightheadedness (3.1%), fluid retention (2.6%), general malaise (0.8%), tachycardia (0.8%) and headache (0.5%), leading to LDOM discontinuation in 6 cases (1.5%). Prior treatment with doxazosin (P<0.001), or with three or more antihypertensive drugs (P=0.012) was associated with a higher risk of discontinuation of LDOM.
ConclusionsLDOM treatment showed a favorable safety profile in patients with hypertension or arrhythmia, similar to general population.
Los efectos adversos sistémicos son una de las principales limitaciones del uso de minoxidil oral a dosis bajas (MODB), especialmente en pacientes con hipertensión arterial o arritmias. El objetivo de este estudio fue evaluar la seguridad de MODB en estos pacientes.
Material y métodoEstudio retrospectivo multicéntrico con pacientes con antecedentes de hipertensión o arritmias tratados con MODB para cualquier tipo de alopecia.
ResultadosSe incluyó un total de 254 pacientes con hipertensión (176 mujeres [69,3%] y 78 hombres [30,7%]) con una edad media de 56,9 años (rango 19 – 82). La dosis de MODB se incrementó gradualmente en 128 pacientes, obteniendo un total de 382 dosis analizadas. Los sujetos estaban tomando de media 1,45 fármacos antihipertensivos (rango 0 – 5). Se detectaron EA sistémicos en 26 casos (6,8%), incluyendo mareo (3,1%), retención de líquidos (2,6%), malestar general (0,8%), taquicardia (0,8%) y cefalea (0,5%), requiriendo suspensión del MODB en seis casos (1,5%). Los pacientes en tratamiento con doxazosina (p < 0,001) o con tres o más antihipertensivos (p = 0,012) presentaron mayor riesgo de suspensión de MODB.
ConclusiónEl tratamiento con MODB mostró un perfil de seguridad favorable en pacientes con hipertensión o arritmias, similar al de la población general.
Minoxidil is an arteriolar vasodilator approved in the 1970s as a treatment of severe refractory hypertension, with standard doses ranging between 10 and 40mg daily (up to 100mg/day).1 Within last years, low-dose oral minoxidil (LDOM) has been increasingly used for the treatment of different types of alopecia, supported by numerous studies describing its effectiveness and favorable safety profile.2,3 One of the largest studies thoroughly described the frequency and chronology of adverse effects (AE) of LDOM treatment in 1404 patients.4 Hypertrichosis is a well-known and most common AE of LDOM, appearing in approximately 15% of patients.4,5 Although uncommon, systemic AE may occur and, in a minority of patients, lead to discontinuation of LDOM treatment. The majority of systemic AE are related with the cardiovascular and hemodynamic effect of minoxidil, including lightheadedness (dizziness, postural hypotension), fluid retention (lower limb or facial edema), tachycardia (palpitations), electrocardiogram alterations (mainly in T-wave), headache, insomnia, nightmares or increased appetite.1,4,6,7 These AE are generally well-tolerated and resolve after discontinuation or dose adjustment of LDOM. Serious AE have been reported with high doses of oral minoxidil when used for severe hypertension or due to compounding errors in hair loss treatment, including hypotensive syncope, pericarditis, pericardial effusion or myocardial infarction.1,8
Systemic AE represent the main concern of LDOM treatment in general population with hair loss, which are mostly healthy middle-aged adults. Considering that LDOM is used as an off-label therapy, these safety concerns become major when prescribing it in special situations such as patients with personal history of arterial hypertension (HT) or arrhythmia. It is still unknown whether LDOM could enhance the hypotensive effect of other antihypertensive drugs or increase the risk of systemic AE in these patients.
The objective of this study was to evaluate the safety of LDOM for the treatment of hair loss in patients with HT or arrhythmia.
MethodsThis retrospective, descriptive, multicenter study included adult patients with personal history of HT or arrhythmia who were under treatment with LDOM for hair loss of any cause for at least one month, at five centers from Brazil and Spain, from January 2018 to April 2022. This minimum treatment duration of one month was established considering the previously described AE's chronology of LDOM.4 Epidemiological, medical, and safety data were collected from patients’ medical records, including prior treatments for HT, AE, and the need to withdraw or adjust the dose of LDOM or antihypertensive treatments. In some patients, the dose of LDOM was titrated according to response and tolerability, and the above parameters were analyzed for each dose.
Statistical analysis was performed with IBM SPSS Statistics (Version 25.0: IBM Corp., 2017). Continuous variables are expressed as mean (range). Logistic regression was used to identify the variables associated with an increased risk of systemic AEs, withdraw or adjustment of LDOM or antihypertensive treatments, which was considered statistically significant if the P-value was<0.05.
ResultsHypertensionA total of 254 patients [176 women (69.3%) and 78 men (30.7%)] with a mean age of 56.9 years (range 19–82) were included. From them, the dose of LDOM was titrated at various incremental doses as tolerated in 82 patients (32.2%), allowing the analysis of 382 different doses. The mean dose of LDOM was 1.59mg (range 0.2–10) and the mean duration was 10.9 months (1–51). LDOM was the only systemic therapy for hair loss in 124 patients (48.8%). In the remaining patients, the most frequent concomitant drug was oral dutasteride. The most common indication for LDOM treatment was androgenetic alopecia (68.9%), followed by frontal fibrosing alopecia (10.2%), telogen effluvium (4.3%), lichen planopilaris (3.5%), fibrosing alopecia in a pattern distribution (3.1%), alopecia areata (2.4%), and other less common types of alopecia.
Prior to LDOM treatment, patients were receiving a mean of 1.4 antihypertensive drugs (range 0–5). Only 5 patients (1.3%) were not under treatment for HT. The most frequent drug class was angiotensin II receptor blockers (ARBs) in 190 patients (49.7%), followed by thiazides (28.5%), beta-blockers (28%), and others (Table 1).
Description of the cohort of patients with hypertension treated with low-dose oral minoxidil (LDOM), and the subgroups who required dose adjustment and discontinuation of LDOM.
| N | Require dose adjustment | P-value | Require discontinuation | P-value | |
|---|---|---|---|---|---|
| Totala | 382 | 14/382 (3.6%) | 6/382 (1.5%) | ||
| Gender | 0.367 | 0.352 | |||
| Women | 277 (72.5%) | 12/277 | 3/277 | ||
| Men | 105 (27.5%) | 2/105 | 3/105 | ||
| Age, mean (range), years | 56.9 (19–82) | 58.79 years (45–70) | 0.64 | 61.33 years (49–70) | 0.24 |
| Minoxidil (LDOM) | |||||
| Dose, mean (range), mg | 1.59 (0.2–10) | 1.46mg (0.42–5) | 0.92 | 2.29mg (0.5–5) | 0.13 |
| Duration, mean (range), months | 10.9 (1–51) | 3.7 (1–7) | 0.51 | 5.5 (1–12) | 0.14 |
| Compound LDOM | 353 (92.4%) | 13/353 | 0.94 | 5/353 | 0.39 |
| LDOM monotherapy | 187 (49%) | 8/187 | 0.53 | 3/187 | 0.95 |
| Adverse effects (AE) | 84 (21.9%) | 14/84 | <0.001 | 6/84 | <0.001 |
| >1 AE | 7 (1.8%) | 2/7 | 0.024 | 3/7 | <0.001 |
| Hypertrichosis | 61 (15.9%) | 6/61 | 0.003 | 1/61b | 0.651 |
| Intense shedding | 1 (0.2%) | 1/1 | |||
| Systemic AE | 26 (6.8%) | 8/26 | <0.001 | 5/26 | <0.001 |
| Lightheadedness | 12 (3.1%) | 4/12 | <0.001 | 3/12 | <0.001 |
| Fluid retention | 10 (2.6%) | 1/10 | 0.315 | 2/10 | 0.009 |
| General malaise | 3 (0.8%) | 1/3 | 0.106 | 2/3 | 0.001 |
| Tachycardia | 3 (0.8%) | 2/3 | 0.004 | 1/3b | 0.047 |
| Headache | 2 (0.5%) | 1/2 | 0.07 | 0/2 | |
| Antihypertensive drug, mean number (range) | 1.45 (0–5) | 1.79 (1–5) | 0.14 | 1.83 (1–3) | 0.20 |
| 0 drugs | 5 (1.3%) | 0/5 | 0.66 | 0/5 | 0.77 |
| 1 drug | 225 (58.9%) | 7/225 | 0.45 | 3/225 | 0.62 |
| 2 drugs | 125 (32.7%) | 5/125 | 0.54 | 1/125 | 0.69 |
| 3 or more drug | 27 (7.1%) | 2/27 | 0.29 | 2/27 | 0.012 |
| ARBs | 190 (49.7%) | 5/190 | 0.41 | 4/190 | 0.44 |
| Thiazides | 109 (28.5%) | 5/109 | 0.36 | 2/109 | 0.54 |
| Beta-blockers | 107 (28%) | 7/107 | 0.06 | 1/107 | 0.46 |
| ACE inhibitors | 75 (19.6%) | 2/75 | 0.45 | 1/75 | 0.66 |
| Ca channel blockers | 63 (16.4%) | 2/63 | 0.58 | 1/63 | 0.66 |
| Spironolactone | 25 (6.5%) | 2/25 | 0.23 | 0/25 | 0.51 |
| Hydralazine | 6 (1.4%) | 1/6 | 0.08 | 0/6 | 0.75 |
| Doxazosin | 3 (0.7%) | 1/3 | 0.006 | 2/3 | <0.001 |
ACE: angiotensin-converting enzyme; AE: adverse effects; ARBs: angiotensin II receptor blockers; LDOM: low-dose oral minoxidil; P value considered statistically significant if <0.05 (bold).
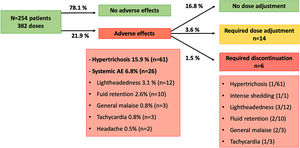
AE were detected in 71 patients (27.9%) with 84 doses (21.9%). The most common AE was hypertrichosis in 49 patients (19.2%) with 61 doses (15.9%), given that 7 patients (2.7%) developed hypertrichosis with several doses. Systemic AE were identified in 23 patients (9%) with 26 doses (6.8%) and included lightheadedness (n=12; 3.1%), fluid retention (n=10; 2.6%), general malaise (n=3; 0.8%), tachycardia (n=3; 0.8%) and headache (n=2; 0.5%). Five patients (1.9%) developed more than one AE: hypertrichosis plus lightheadedness (n=2), hypertrichosis plus tachycardia (n=1), lightheadedness plus general malaise (n=1), and lightheadedness plus general malaise plus fluid retention (n=1, who required LDOM discontinuation).
All of the AE improved with dose adjustment or discontinuation of the drug, and no life-threatening AE were observed. Dose of LDOM was decreased in 14 cases (3.6%), from whom 8 cases were due to systemic AE, and 6 cases due to hypertrichosis. LDOM discontinuation was required in 6 cases (1.5%) due to intense hair shedding (n=1), fluid retention (n=1), lightheadedness (n=1), lightheadedness plus general malaise (n=1), lightheadedness plus general malaise plus fluid retention (n=1), and tachycardia plus hypertrichosis (n=1; this patient stopped LDOM because of tachycardia, then restarted treatment with no systemic AE, and stopped again after 1 year because of hypertrichosis).
The most frequent AE leading to LDOM adjustment or withdrawal was lightheadedness (n=7; P<0.001). Hypertrichosis was associated with a higher risk of dose adjustment (P=0.003) but not with discontinuation (P=0.65). Age, gender, and dose of LDOM were no associated with the risk of systemic AE nor with adjustment of LDOM. Other factors associated with LDOM adjustment and withdrawal are shown in Table 1.
Patients who received 3 or more antihypertensive drugs had a higher risk of developing more than 1 AE (P<0.001), lightheadedness (P<0.001), general malaise (P<0.001), and of requiring LDOM discontinuation (P=0.012). Also, prior treatment with doxazosin was associated with a higher risk of lightheadedness (P<0.001), general malaise (P<0.001), fluid retention (P=0.01) and necessity of both dose adjustment (P=0.006) and withdrawal of LDOM (P<0.001).
Regarding antihypertensive therapy, only two patients (0.7%) required an adjustment or discontinuation of their prior treatment. One 67-year-old woman treated with minoxidil 0.5mg and spironolactone 50mg daily, developed symptomatic hypotension four days after changing from ramipril to olmesartan (by her general practitioner). Olmesartan was suspended and HT was controlled with minoxidil and spironolactone. The other patient was a 71-year-old woman whose prior treatment with losartan was suspended because of a good control of her HT with minoxidil 2.5mg daily. The remaining patients did not report changes or impairment of their HT during LDOM treatment.
ArrhythmiaA total of 10 patients (8 women and 2 men) with a median age of 61 years (range 37–74) were included. From them, the dose of LDOM was titrated in three patients, allowing the analysis of 15 doses. The median dose of LDOM was 0.75mg (range 0.25–5) and the median duration was 5 months (range 1–11). The most frequent indication for LDOM treatment was androgenetic alopecia (n=6), followed by chronic telogen effluvium (n=2), and frontal fibrosing alopecia (n=2). Nine patients had personal history of arrhythmia, namely supraventricular extrasystole (n=3), atrial fibrillation (n=1), sinus tachycardia (n=1), cardiac syncope (n=1), and unknown arrhythmia (n=3, missing data). From them, four patients were under medical treatment with beta-blockers (n=3) or amiodarone (n=1), two patients had undergone a cardiac ablation, one patient had a pacemaker, and two patients were not receiving any treatment. The remaining patient had a mechanical aortic valve due to an aortic aneurysm repaired by Bentall procedure. From these 10 patients, a consultation with cardiologist was made in five of them before starting LDOM treatment.
Systemic AE were reported in only one patient with supraventricular extrasystole in treatment with carvedilol who developed periorbital and pedal edema with LDOM 1mg, which resolved after adjusting the dose to 0.25mg. No patients reported palpitations, worsening of their prior arrhythmia or any other cardiological AE, and none of them required a modification of their prior medical treatment.
DiscussionTo our knowledge, this represents the first study of the safety of LDOM in patients with HT or arrhythmia. Numerous studies have shown its favorable safety profile in general population,2–4,6 and recent studies have investigated the use of LDOM in special situations such as pediatric,9 adolescent,10 and elderly population,11 but there are scarce data of its use in patients with HT or arrhythmia. The global prevalence of HT in adults is 34%,12 so it seems important to expand knowledge about the management and safety issues of LDOM treatment in these patients.
In our cohort of hypertensive patients treated with LDOM, the global frequency and type of AE were consistent to those found in recent studies.2–4 AE were detected in 21.9% of the cases, and hypertrichosis was the most common one (15.9%), similarly to the data found by Vaño-Galvan et al.4 However, we did find a higher frequency of cardiovascular AE (lightheadedness and fluid retention), and more patients presenting more than 1 AE (Table 2).
Description of frequency of adverse effects and discontinuation of low-dose oral minoxidil (LDOM) found in hypertensive patients (current study) and those found in general population (Vaño-Galvan's study).
| Current study | 2021, Vaño-Galvan et al. | |
|---|---|---|
| Doses, n | 382 | 2469 |
| Age, mean | 56.9 years (19–82) | 43 years (8–86) |
| LDOM dose, mean | 1.59mg (0.2–10) | 1.63mg (0.03–15) |
| Adverse effects | 21.9% | 20.6% |
| Hypertrichosis | 15.9% | 15.1% |
| Systemic AE | 6.8% | 5.5% |
| Lightheadedness | 3.1% | 1.7% |
| Fluid retention (periorbital or ankle) | 2.6% | 1.6% |
| Tachycardia | 0.8% | 0.9% |
| General malaise | 0.8% | – |
| Headache | 0.5% | 0.4% |
| More than 1 AE | 1.9% | 0.9% |
| LDOM withdrawal | 1.5% | 1.7% |
| Hypertricosis | 0.2% | 0.5% |
| Intense hair shedding | 0.2% | 0.08% |
| Systemic AE | 1.3% | 1.2% |
Lightheadedness and tachycardia were significantly associated with a higher risk of both adjustment and suspension of LDOM (Table 1). Hypertrichosis was associated with dose adjustment but not with suspension, supporting that this AE is usually well-tolerated. Patients who were treated with three or more antihypertensive drugs had also more risk of lightheadedness and suspension of LDOM. Among all antihypertensive drugs, doxazosin led to a higher risk of lightheadedness, fluid retention and suspension of LDOM. This could be explained by the fact that doxazosin is an alpha-adrenergic blocker that also causes arteriolar vasodilation,13 which might produce a synergistic effect with minoxidil. Although it might be plausible that other vasodilators, such as hydralazine or calcium channel blockers, could increase this synergistic effect with minoxidil, we did not find a statistically significant association.
Lightheadedness (also referred as dizziness or postural hypotension) was the most frequent systemic AE and the most common cause of both adjustment and discontinuation of LDOM (Fig. 1). It was generally mild and reported more frequently in summer and in the morning. There were no cases of serious hypotension or impairment of blood pressure (BP) control. In this regard, two recent studies investigated the variation of BP in male patients treated with 5mg LDOM using 24h ambulatory BP monitoring. They found a slight reduction in BP in the 2h after intake of LDOM14 and at week 24.7 Few additional studies have assessed BP during treatment with LDOM at doses between 0.45 and 5mg, obtaining similar results.15–17 This is consistent with 1970s studies with 2.5mg, 5mg and 10mg minoxidil which found a minimal or no hypotensive effect in normotensive patients.18
Fluid retention (mainly pedal edema; only 2 cases of facial edema) was detected in our study in 2.6% of the patients, which is a slightly higher frequency than previous studies in general population,3,4 but much lower than treatment with standard doses (7%).1 We did not find a lower frequency in patients taking diuretics as antihypertensive (thiazides or spironolactone).
Tachycardia was only detected in 0.8% of the patients with hypertension, all of them requiring adjustment or suspension of LDOM. Tachycardia is the result of reflex sympathetic activation induced by the vasodilatory effect of oral minoxidil, and it is traditionally described as a frequent AE with standard doses.1 However, studies with LDOM have shown that palpitations are rather uncommon and transient, usually appearing within the first intakes of LDOM.4,7,15,17
EKG changes are another common AE with standard doses of minoxidil, with changes in direction and magnitude of the T-waves in up to 60% of patients, mainly due to a coronary steal mechanism associated with the reduction of blood pressure in the coronary arteries. They are asymptomatic, and usually disappear despite continuation of the treatment.1 Only a few studies have performed EKG in patients with LDOM. One study from Thailand found asymptomatic T-wave changes in 10–20% of the patients (non-ischemic T wave inversion in one lead).15 Sanabria et al. found a non-significant increasing in the occurrence of supraventricular and ventricular extrasystole after 24 weeks of treatment with 5mg LDOM, with no remarkable alterations in ventricular repolarization in the 24-h Holter monitoring.7
Interestingly, from our cohort of patients with arrhythmia, no patients reported palpitations or worsening of their prior disease. We did not perform routine EKG in these patients, but five of them were referred to their Cardiologist who approved treatment with LDOM. However, extreme caution should be taken in patients with coronary disease or chronic heart failure since tachycardia may limit myocardium oxygenation (Fig. 2).
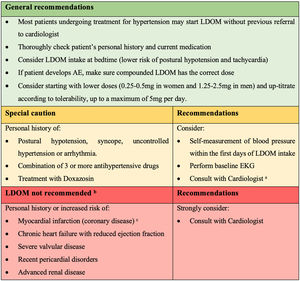
Recommendations for the management of LDOM therapy in patients with hypertension or arrhythmia (based on authors’ clinical experience and results of this study). a Cardiologist's comment: dermatologist may consider decreasing the dose or changing the hour intake of prior antihypertensive drug if the patient develops lightheadedness. b Contraindications according to minoxidil product monograph. c Cardiologist's comment: LDOM could be used after a myocardial infarction only once a stable post-infarction state has been achieved (12 months after). Do not use if non-revascularized angina/ischemic event.
Consistently with previous studies of LDOM, we did not detect any serious cardiovascular AE such as syncope, ischemic heart disease, pericarditis, or pericardial effusion. These AE were described with standard doses of minoxidil, but they occurred mainly in patients with underlying diseases such as systemic lupus erythematous, advanced renal disease or congestive heart failure, and are extremely rare with LDOM.19,20
Based on our results, the clinical experience of the authors in LDOM treatment, and the collaboration of a cardiologist in this study (MR), most patients undergoing treatment for HT may start LDOM without previous referral to cardiologist. However, we suggest some recommendations for the management of LDOM therapy in patients with HT or arrhythmia (Fig. 2).
The limitations of this study were the retrospective design, lack of a control group and lack of objective measurement of blood pressure and EKG.
In conclusion, LDOM treatment showed a favorable safety profile in patients with HT or arrhythmia, similar to general population. Most common systemic AE were lightheadedness and fluid retention, which improved after adjustment or withdrawal of LDOM. Combination of LDOM with 3 or more antihypertensive drugs or with doxazosin was associated with a higher risk of these systemic AE.
Conflict of interestThe authors declare that they have no conflict of interest.