Nonmelanoma skin cancer is the most common malignancy in white individuals. The risk factors for squamous cell carcinoma, which belongs to the family of nonmelanoma skin cancers, have not been studied in Colombia.
ObjectiveTo determine the risk factors for squamous cell carcinoma in patients at a national referral center for skin diseases in Colombia.
Material and methodsWe conducted a case-control study that evaluated sociodemographic, epidemiological, and clinical factors among 332 individuals. Risk was calculated as odds ratio (ORs) using the multivariate conditional logistic regression analysis method.
ResultsThe following risk factors were identified: family history of skin cancer (OR, 6.55; 95% CI, 1.4-28.9), living in a rural area after the age of 30 years (OR, 3.13; 95% CI, 1.3-7.2), a lifetime working outdoors (OR, 2.98; 95% CI, 1.5-5.7), smoking more than 10 cigarettes a day (OR, 2.96; 95% CI, 1.3-6.5), actinic conjunctivitis (OR, 2.68; 95% CI, 1.2-5.9), poikiloderma of Civatte (OR, 3.29; 95% CI, 1.7-6.1), numerous facial actinic keratoses (OR, 9.23; 95% CI, 4.9-17.1), and numerous freckles (OR, 3.68; 95% CI, 1.3-10.1).
ConclusionsWe have documented clinical characteristics and personal history factors that should guide the physician in making decisions on the preventive and follow-up measures to be adopted for individuals at risk of squamous cell carcinoma. These findings may help guide policy for controlling the disease using local information.
El cáncer de piel no melanoma es la neoplasia maligna más común en sujetos de raza blanca. A este grupo pertenece el carcinoma espinocelular, para el cual no se han estudiado en nuestro medio los factores de riesgo.
ObjetivoEstablecer los factores de riesgo de carcinoma espinocelular en pacientes de un centro nacional de referencia de enfermedad dermatológica en Colombia.
Material y métodosSe realizó un estudio de casos y controles, que incluyó 332 sujetos. Se estudiaron factores sociodemográficos, epidemiológicos y clínicos. La estimación de riesgo empleó la razón de odds, la cual se estimó a través del método de análisis multivariado de regresión logística condicional.
ResultadosSe identificaron los siguientes factores de riesgo: antecedente familiar de cáncer de piel (OR: 6,55; IC 95%: 1,4-28,9), vivir en área rural después de los 30 años (OR: 3,13; IC 95%: 1,3-7,2), trabajos al aire libre a lo largo de la vida (OR: 2,98; IC 95%: 1,5-5,7), fumar más de 10 cigarrillos al día (OR: 2,96; IC 95%: 1,3-6,5), conjuntivitis actínica (OR: 2,68; IC 95%: 1,2-5,9), poiquilodermia de Civatte (OR: 3,29; IC 95%: 1,7-6,1), múltiples queratosis actínicas en la cara (OR: 9,23; IC 95%: 4,9-17,1) y múltiples efélides (OR: 3,68; IC 95%: 1,3-10,1).
ConclusionesSe documentaron factores asociados con la historia personal y las características clínicas, que deben orientar al médico en las medidas preventivas y de seguimiento que han de adoptarse. Estos hallazgos pueden contribuir a orientar la política de control de la enfermedad con información local.
Squamous cell carcinoma (SCC), like basal cell carcinoma, is a nonmelanoma skin cancer. It has been recognized as a public health problem, as nonmelanoma skin cancers are the most common malignant tumors in white individuals1 and they are also becoming increasingly prevalent in different parts of the world.2–4 In Colombia, Sánchez et al.5 reported an increase in the rate of nonmelanoma skin cancer from 23 to 41 cases per 100 000 population between 2003 and 2007. In the authors’ opinion, if this trend continues and if the risk factors remain unchanged, Columbia could face an incidence of 102 cases of nonmelanoma skin cancer per 100000 population by 2020, which would place a considerable burden on the health care system.
Exposure to UV radiation is a recognized factor in the development of SCC.6,7 However, there are multiple social, economic, cultural, economic, geographic, genotypic, and phenotypic factors that might modify the risk of SCC. Columbia is located in a part of the world with high levels of UV radiation exposure (equatorial region, latitude 0°).8 Furthermore, a large portion of the population lives in the Andes and works in agriculture, therefore spending long hours outdoors, exposed to the sun's rays. This geographic and socioeconomic context could modulate the true risk of SCC in the Colombian population.
Considering the lack of information on the risk factors for SCC in our country, we decided to perform a case-control study to identify the main risk factors for this cancer in a sample of patients from a national referral center for the treatment of skin disorders in Colombia.
Material and MethodsWe performed an analytical case-control study at the Centro Dermatológico Federico Lleras Acosta E.S.E. in which we consecutively enrolled all patients diagnosed with histologically confirmed SCC in 2010 and 2011. Each case included in the study was matched to a control of the same sex and a similar age (±3 years) to control for confounding factors. The controls were selected from among patients who consulted for skin diseases other than cancer and who had no lesions suggestive of skin cancer on physical examination. Patients with an underlying photodermatosis who had been prescribed routine protection against UV radiation were excluded.
The sample size was calculated based on the risk of SCC according to occupational sun exposure reported by Zanetti et al.9 (odds ratio [OR], 2.2; 95% CI, 1.1-4.1). Using the formula designed by Machin and Campbell,10,11 we calculated a sample size of 296 individuals (148 cases and 148 controls) for a statistical power of 80% and a 95% confidence level.
The questionnaire used to assess risk factors was drawn up following a systematic review of the literature and input from a panel of experts from the study center. It was also tested for reliability to ensure reproducibility. Questions with the highest levels of reliability were included in the final version, which addressed issues such as sociodemographic variables, place of residence, occupational and recreational exposure to UV radiation, personal and family history, and clinical aspects. The Fitzpatrick scale, standardized by the study investigators,12 was used to evaluate skin type.
The project was reviewed and approved by an independent ethics committee and conducted in accordance with national and international regulations. All participants in the study gave their informed consent to participate.
The statistical analysis included a general description of the study variables based on the scales used in each case. Bivariate analysis, using the McNemar χ2 test, was performed to explore associations between the variables and SCC, with calculation of ORs and corresponding 95% CIs in each case. A P value of less than .05 was considered statistically significant. We compared the means and medians of continuous variables, which were then reclassified as categorical variables to calculate the ORs.
Finally, we performed multivariate analysis with conditional logistic regression and included variables with statistically significant results, clinically relevant variables, and possible confounders.
The goodness of fit of the model was evaluated using the Hosmer-Lemeshow test. Analyses were performed using the Stata statistical package (version 10).
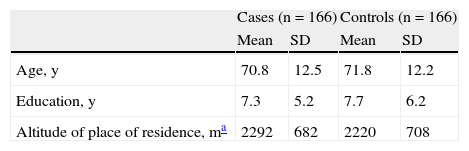
ResultsWe included 332 individuals (166 cases and 166 controls). The mean (SD) age of the 2 groups was 71 (12.3) years and the median age was 74 years (range, 32-94 years). No significant difference was observed between cases and controls with regards to age (P=.45). Women accounted for 69% of the sample and were equally distributed between the 2 groups (P=1.0). The 2 groups had a similar level of education (average of 7.3 years for cases and 7.7 years for controls, P=.5). No significant differences were observed between the groups for place of birth (P=.56). We also explored the relationship between risk of SCC and mean altitude above sea level of place of residence but found no differences between cases and controls (P=.9). The general characteristics of the cases and controls are shown in Table 1.
General Characteristics of Cases and Controls.
| Cases (n=166) | Controls (n=166) | |||
| Mean | SD | Mean | SD | |
| Age, y | 70.8 | 12.5 | 71.8 | 12.2 |
| Education, y | 7.3 | 5.2 | 7.7 | 6.2 |
| Altitude of place of residence, ma | 2292 | 682 | 2220 | 708 |
| No. | % | No. | % | |
| Female sex | 115 | 69 | 115 | 69 |
| Born in rural area | 88 | 53 | 70 | 42 |
| Skin type | ||||
| I | 5 | 3 | 1 | 0.6 |
| II | 87 | 52 | 54 | 33 |
| III | 56 | 34 | 70 | 42 |
| IV | 16 | 10 | 37 | 22 |
| V | 2 | 1 | 4 | 2.4 |
| VI | 0 | 0 | 0 | 0 |
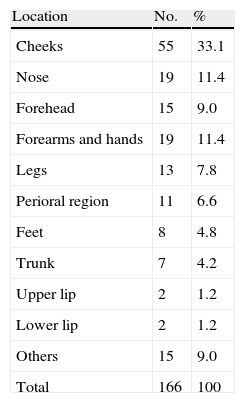
According to the histology reports, the most common tumor type was infiltrating SCC (58%, 97/166), followed by SCC in situ (31%, 52/166); other histologic types accounted for the remaining (11%, 17/166). The cheeks were the most common site affected (33.1%) (Table 2). The main diagnoses in the control group were tinea pedis (31%, 51/166), rosacea (25%, 41/166), seborrheic dermatitis (22%, 37/166), contact dermatitis (15%, 25/166), and psoriasis (7%, 12/166).
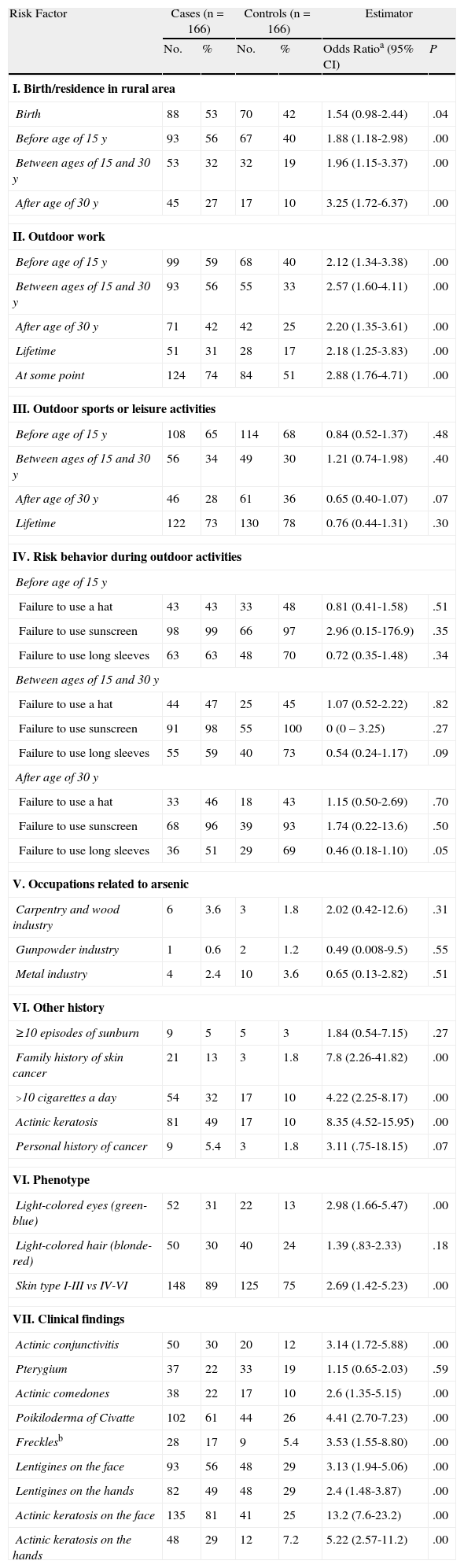
The unadjusted ORs for the main variables analyzed are shown in Table 3. Associations were found for rural residence and outdoor work. No significant between-group differences were observed for leisure or sporting activities.
Bivariate Analysis of Risk Factors for Squamous Cell Carcinoma.
| Risk Factor | Cases (n=166) | Controls (n=166) | Estimator | |||
| No. | % | No. | % | Odds Ratioa (95% CI) | P | |
| I. Birth/residence in rural area | ||||||
| Birth | 88 | 53 | 70 | 42 | 1.54 (0.98-2.44) | .04 |
| Before age of 15 y | 93 | 56 | 67 | 40 | 1.88 (1.18-2.98) | .00 |
| Between ages of 15 and 30 y | 53 | 32 | 32 | 19 | 1.96 (1.15-3.37) | .00 |
| After age of 30 y | 45 | 27 | 17 | 10 | 3.25 (1.72-6.37) | .00 |
| II. Outdoor work | ||||||
| Before age of 15 y | 99 | 59 | 68 | 40 | 2.12 (1.34-3.38) | .00 |
| Between ages of 15 and 30 y | 93 | 56 | 55 | 33 | 2.57 (1.60-4.11) | .00 |
| After age of 30 y | 71 | 42 | 42 | 25 | 2.20 (1.35-3.61) | .00 |
| Lifetime | 51 | 31 | 28 | 17 | 2.18 (1.25-3.83) | .00 |
| At some point | 124 | 74 | 84 | 51 | 2.88 (1.76-4.71) | .00 |
| III. Outdoor sports or leisure activities | ||||||
| Before age of 15 y | 108 | 65 | 114 | 68 | 0.84 (0.52-1.37) | .48 |
| Between ages of 15 and 30 y | 56 | 34 | 49 | 30 | 1.21 (0.74-1.98) | .40 |
| After age of 30 y | 46 | 28 | 61 | 36 | 0.65 (0.40-1.07) | .07 |
| Lifetime | 122 | 73 | 130 | 78 | 0.76 (0.44-1.31) | .30 |
| IV. Risk behavior during outdoor activities | ||||||
| Before age of 15 y | ||||||
| Failure to use a hat | 43 | 43 | 33 | 48 | 0.81 (0.41-1.58) | .51 |
| Failure to use sunscreen | 98 | 99 | 66 | 97 | 2.96 (0.15-176.9) | .35 |
| Failure to use long sleeves | 63 | 63 | 48 | 70 | 0.72 (0.35-1.48) | .34 |
| Between ages of 15 and 30 y | ||||||
| Failure to use a hat | 44 | 47 | 25 | 45 | 1.07 (0.52-2.22) | .82 |
| Failure to use sunscreen | 91 | 98 | 55 | 100 | 0 (0 – 3.25) | .27 |
| Failure to use long sleeves | 55 | 59 | 40 | 73 | 0.54 (0.24-1.17) | .09 |
| After age of 30 y | ||||||
| Failure to use a hat | 33 | 46 | 18 | 43 | 1.15 (0.50-2.69) | .70 |
| Failure to use sunscreen | 68 | 96 | 39 | 93 | 1.74 (0.22-13.6) | .50 |
| Failure to use long sleeves | 36 | 51 | 29 | 69 | 0.46 (0.18-1.10) | .05 |
| V. Occupations related to arsenic | ||||||
| Carpentry and wood industry | 6 | 3.6 | 3 | 1.8 | 2.02 (0.42-12.6) | .31 |
| Gunpowder industry | 1 | 0.6 | 2 | 1.2 | 0.49 (0.008-9.5) | .55 |
| Metal industry | 4 | 2.4 | 10 | 3.6 | 0.65 (0.13-2.82) | .51 |
| VI. Other history | ||||||
| ≥10 episodes of sunburn | 9 | 5 | 5 | 3 | 1.84 (0.54-7.15) | .27 |
| Family history of skin cancer | 21 | 13 | 3 | 1.8 | 7.8 (2.26-41.82) | .00 |
| >10 cigarettes a day | 54 | 32 | 17 | 10 | 4.22 (2.25-8.17) | .00 |
| Actinic keratosis | 81 | 49 | 17 | 10 | 8.35 (4.52-15.95) | .00 |
| Personal history of cancer | 9 | 5.4 | 3 | 1.8 | 3.11 (.75-18.15) | .07 |
| VI. Phenotype | ||||||
| Light-colored eyes (green-blue) | 52 | 31 | 22 | 13 | 2.98 (1.66-5.47) | .00 |
| Light-colored hair (blonde-red) | 50 | 30 | 40 | 24 | 1.39 (.83-2.33) | .18 |
| Skin type I-III vs IV-VI | 148 | 89 | 125 | 75 | 2.69 (1.42-5.23) | .00 |
| VII. Clinical findings | ||||||
| Actinic conjunctivitis | 50 | 30 | 20 | 12 | 3.14 (1.72-5.88) | .00 |
| Pterygium | 37 | 22 | 33 | 19 | 1.15 (0.65-2.03) | .59 |
| Actinic comedones | 38 | 22 | 17 | 10 | 2.6 (1.35-5.15) | .00 |
| Poikiloderma of Civatte | 102 | 61 | 44 | 26 | 4.41 (2.70-7.23) | .00 |
| Frecklesb | 28 | 17 | 9 | 5.4 | 3.53 (1.55-8.80) | .00 |
| Lentigines on the face | 93 | 56 | 48 | 29 | 3.13 (1.94-5.06) | .00 |
| Lentigines on the hands | 82 | 49 | 48 | 29 | 2.4 (1.48-3.87) | .00 |
| Actinic keratosis on the face | 135 | 81 | 41 | 25 | 13.2 (7.6-23.2) | .00 |
| Actinic keratosis on the hands | 48 | 29 | 12 | 7.2 | 5.22 (2.57-11.2) | .00 |
aUnadjusted odds ratio.
bReference categories: none-few vs several-many.
The use of sun protection measures was remarkably low, with percentages ranging between 0% and 7%, with no significant differences between cases and controls. There were also no differences when we analyzed occupations involving risk due to arsenic exposure (carpentry trade and wood, gunpowder, or metal industries).
Significant associations were observed between SCC and a family history of skin cancer (OR, 7.8, P=.0001, smoking more than 10 cigarettes a day (OR, 4.22; P=.0000), and a history of actinic keratosis (OR, 8.35; P=.0000).
Skin types I to III (OR, 2.69; P=.0010) and light-colored eyes (blue, gray, or green) were also associated with an increased odds of developing SCC (OR, 2.98; P=.0001).
The most important clinical findings on physical examination were actinic conjunctivitis (OR, 3.14; P=.0001), actinic comedones (OR, 2.6; P=.0019), poikiloderma of Civatte (OR, 4.41; P=.0000), freckles (OR: 3.53; P=.0009), lentigines on the face (OR, 3.13; P=.0000) or hands (OR, 2.4; P=.0001), and actinic keratosis on the face (OR, 13.2; P=.0000) or hands (OR, 5.22; P=.0000).
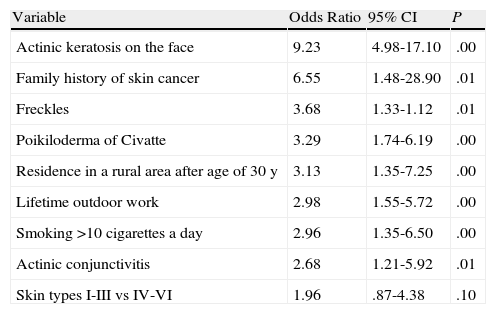
Using the results from the bivariate analysis, we generated a logistic regression model, including significant variables (P<.05) from the bivariate analysis and potential confounders. The model included demographic, occupational, and clinical variables. We found a significant association between SCC and residence in a rural area (especially after the age of 30 years), outdoor work, a family history of skin cancer, a history of smoking more than 10 cigarettes a day, clinical findings of actinic conjunctivitis, poikiloderma of Civatte, and several or multiple actinic keratosis lesions and freckles on the face. The details of the model are shown in Table 4.
Multivariate Logistic Regression Analysis of Risk Factors for Squamous Cell Carcinomaa.
| Variable | Odds Ratio | 95% CI | P |
| Actinic keratosis on the face | 9.23 | 4.98-17.10 | .00 |
| Family history of skin cancer | 6.55 | 1.48-28.90 | .01 |
| Freckles | 3.68 | 1.33-1.12 | .01 |
| Poikiloderma of Civatte | 3.29 | 1.74-6.19 | .00 |
| Residence in a rural area after age of 30 y | 3.13 | 1.35-7.25 | .00 |
| Lifetime outdoor work | 2.98 | 1.55-5.72 | .00 |
| Smoking >10 cigarettes a day | 2.96 | 1.35-6.50 | .00 |
| Actinic conjunctivitis | 2.68 | 1.21-5.92 | .01 |
| Skin types I-III vs IV-VI | 1.96 | .87-4.38 | .10 |
In view of the rapid increase in skin cancer rates in Columbia5 and the need to develop national skin cancer awareness and prevention campaign,5,13 we decided to open a line of research into skin cancer at the Centro Dermatológico Federico Lleras Acosta de Colombia.
This article, which is one of the outcomes of our research to date, is the first study of the risk factors for SCC in Colombian patients and has generated a series of empirical data that may contribute to the design of a national skin cancer prevention program based on local evidence.
While increasing altitude has been associated with a considerable increase in the doses of UV radiation to which the skin is exposed,8 we found no significant differences between cases and controls with regard to the altitude of their place of residence (P=.9). This lack of association may be because the hospital at which the controls were selected, despite being a national referral center, receives a large proportion of patients from the capital, which is situated at an altitude of 2600 m above sea level.
We also found no significant differences between cases and controls for level of education (P=.77), possibly because the majority of patients seen at the hospital are from a middle socioeconomic background (levels 2 and 3), which might have resulted in a natural matching by educational level, thereby possibly not allowing detection of any true association between SCC and education.
As mentioned earlier, it is widely accepted that UV radiation is the main risk factor for the development of SCC,6,7,9 and while it is logical to assume that individuals who work outdoors are at higher risk than the general population, our findings are important in that they confirm this. Thus, even after controlling for potential confounders (Table 3), we saw that people who work outdoors are almost 3 times as likely as those who do not to develop SCC (adjusted OR [aOR], 2.98; 95% CI, 1.55-5.72). These results are in agreement with data from a recent systematic review by Schmitt et al.6 showing a pooled OR of 1.77 (95% CI, 1.40-2.22) for SCC occurrence and occupational UV radiation exposure.
In our series, 80% of cases worked in the agriculture and livestock sector. These results fuel the debate on whether or not skin cancer should be considered an occupational disease, a development that would have important implications in terms of treatment and prevention. By comparing residence in rural and urban areas at different ages, we were able to detect differences between risk at different life stages. In the multivariate analysis, we found that living in a rural area after the age of 30 years was associated with a higher odds of developing SCC (adjusted OR, 3.13; 95% CI, 1.35-7.25). Living in a rural area has been associated with occupational exposure to UV radiation. However, in our model (and population), both rural residence and occupational UV exposure emerged as candidates for intervention as rural workers are vulnerable in more ways than through simple exposure to UV radiation. People who live in rural environments are subject to high levels of sun exposure, but additionally they receive little information on preventive health care, have to travel long distances to see a health specialist, and may often live in areas with unreliable communication and transport networks.
Similar findings have been reported by other authors, who have described that people residing in rural areas are less likely to use sun protection measures, despite their exposure to higher levels of UV radiation. This could be related to factors such as education, access to communication, income, race, and others.14–17 Such findings are particularly important in Colombia as they highlight the need to design prevention strategies and campaigns that reach those living in rural areas, where there is a high risk of SCC occurrence.
It is important to promote educational strategies encouraging the population to use alternative preventive measures and UV radiation blockers, particularly if we consider that 81% of SCCs develop on the face and other sun-exposed areas. Personal protection measures and strategies aimed at avoiding sun exposure at peak hours of radiation are effective in reducing skin cancer rates.15,18,19 Our findings show that, in the best of cases, under 7% of cases and controls used sunscreen during the different life stages analyzed. This might have several explanations, including economic factors (e.g., prohibitive cost of sunscreen products for individuals at risk). Other important factors that should be considered are false beliefs and a lack of preventive education, among others. There are, however, other, simple protective measures that are not so strongly influenced by financial considerations, such as wearing a hat or long sleeves, but under 60% of cases and controls reported taking such measures. There is thus clearly a need to design comprehensive strategies that encourage the use of physical protection methods and to increase access to sunscreens among at-risk populations, as it has been demonstrated that these products play an important role in preventing skin cancer.20,21 We found a family history of skin cancer to be a risk factor for the development of SCC, just as it is for BCC.22 Different genes have been implicated in the development of SCC,23 but considering the variability that has been observed, we believe it would be worthwhile performing genetic studies in the Colombian population. Skin type is associated with genetics. Coinciding with reports for other populations,24–26 in our bivariate analysis we observed that SCC was associated with skin types I to III (OR, 2.69), although this association lost its significance in the multivariate analysis. Considering that Colombia is a tropical country with high levels of UV radiation throughout the year, it is possible that fair-skinned individuals might have experienced sunburn during the earlier years of their lives and as a result decided to avoid the sun, thereby reducing the associated risk.
Another important finding in our study is that related to signs of sun damage (e.g., lentigines, poikiloderma, freckles, and actinic keratosis) detected during physical examination. These findings could be particularly important in terms of primary and secondary prevention policies, as educational strategies aimed at helping health care providers (and primary care physicians in particular) to identify these signs during routine visits would allow them to more closely monitor their patients and would also favor early detection of SCC. The risk associated with actinic keratosis is particularly high and therefore early detection and treatment of this condition should be a health care priority.
Finally, smoking prevention interventions should also be developed. While the association between smoking and SCC is a subject of debate,27 in our series, smokers had a 2.96 increased odds of developing SCC compared to nonsmokers after adjustment for confounding factors. It should also be noted that smoking has been associated with SCC not only of the skin, but also of the mucosa, which carries a higher risk of metastasis and therefore worse prognosis.28
In conclusion, we have identified 2 major categories of risk of SCC in our study population: a personal history of exposure to different risk factors and signs of sun damage, detectable on physical examination. Our findings could help clinicians implement preventive and follow-up measures in this population and also help inform policies for SCC control based on local evidence.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
FundingThe study was conducted with research investment funds from the Centro Dermatológico Federico Lleras Acosta, E.S.E., in Bogotá, Colombia.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank the Centro Dermatológico Federico Lleras Acosta, E.S.E., Bogotá, Colombia.
Please cite this article as: Sánchez G, Nova J. Factores de riesgo de carcinoma espinocelular, un estudio del Centro Nacional de Dermatología de Colombia. Actas Dermosifiliogr. 2013;104:672–8.