The Psoriasis Area and Severity Index (PASI) used for clinical assessment, and the Dermatology Life Quality Index (DLQI) for quality-of-life assessment, are the most referenced instruments.1
Although psoriasis severity would be expected to align with scores on HRQoL scales, the correlation found between these tools has been unpredictable.2 Therefore, we aimed to assess the sensitivity of the Colombian Spanish DLQI version in patients initiating biological therapy.
We performed an observational longitudinal study (with at least two measurements) based on retrospective data. Patients >16-years old with a clinical diagnosis of psoriasis/psoriatic arthropathy were seen between 2014 and 2022 at Medicarte-IPS in Medellin-Colombia. Patients without a clinical history or at least two DLQI/PASI measurements, were excluded. Sociodemographic, clinical, therapy and HRQoL variables were assessed.
A univariate analysis was performed to describe variables’ frequency. Responsiveness was assessed by calculating Glass’ delta and Cohen's d, between patients’ first measurement and each further DLQI measurements.3 Responsiveness was also evaluated according to psoriasis subtype, and baseline disease severity assessed by PASI. All analyses were performed in the R software/language version 4.1.1.
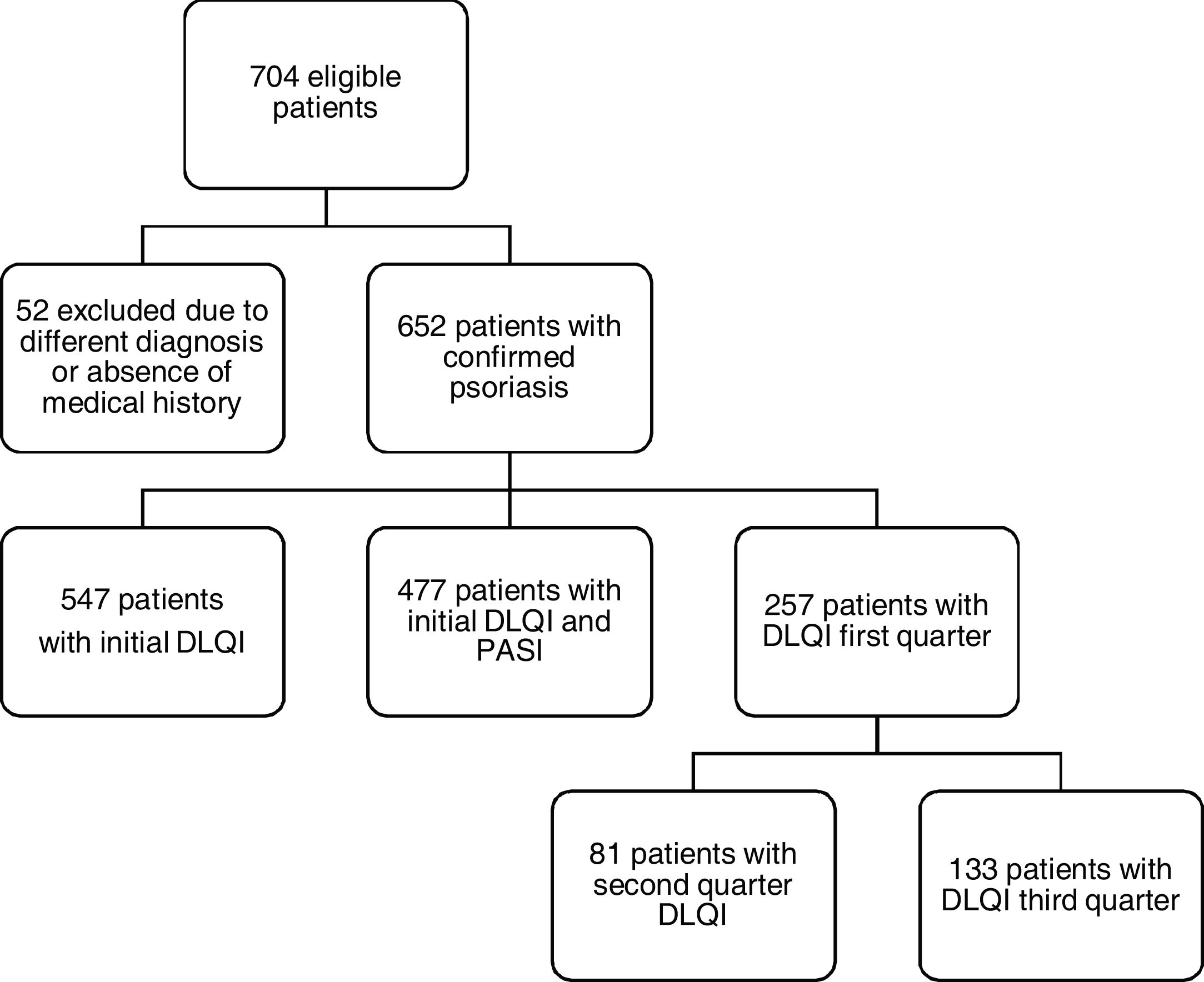
Of the 704 potentially eligible patients only 652 had a confirmed diagnosis of psoriasis, and among these, only 257 patients had information regarding initial and >2 measurements of DLQI/PASI scores (Fig. 1).
Participants mean age was 52.8 years (standard deviation (SD):14.4). Sociodemographic and clinical features are summarized in Supplement 1-Table A.
The main subtype was plaque-psoriasis (88.3%) and the most frequent comorbidities were overweight (43.1%), high blood pressure (27.6%) and obesity (22.9%) (Supplement 1-Table B). In addition, >50% of the patients received a tumour necrosis factor (TNF) inhibitor (57.8%), with adalimumab as the most used biologic (Supplement 1-Table C).
Initial PASI mean was 5.58 (SD 8.79) and the means of subsequent measurements were 2.64, 2.46 and 2.19, respectively and initial DLQI mean was 5.33 (SD 6.75), with subsequent measurements of 3.10, 2.46, and 2.14, respectively (Table 1). The greatest impairment in HRQoL was found in pustular psoriasis (DLQI: 14.2 (SD 13.1)), followed by scalp and plantar/palmar involvement. A Cohen's d=0.37 (95% CI [0.24–0.50]) was found (Table 1 and Supplement 1-Fig. 2A), and for the second, third and last measurements, values were 0.38 95% CI [0.22–0.53], 0.45 95% CI [0.28, 0.63], 0.46 95% CI [0.26, 0.67], respectively (p<0.05). As for Glass’ delta, the values in those measurements were 0.40 95% CI [0.21–0.60], respectively (Table 2 and Supplement 1-Fig. 2B–D).
Measuring responsiveness with the DLQI.
| Responsiveness in the first quarter | ||||
|---|---|---|---|---|
| DLQI | Initial(N=257) | Quarter 1 (N=257) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 5.33 (6.75) | 3.10 (5.53) | 0.37 [0.24, 0.50] | 0.40 [0.21, 0.60] |
| Median [IR] | 2.00 [0, 8.00] | 1.00 [0, 3.00] | ||
| Responsiveness in the second quarter | ||||
|---|---|---|---|---|
| DLQI | Initial(N=173) | Quarter 2(N=173) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 4.55 (6.29) | 2.46 (4.94) | 0.38 [0.22, 0.53] | 0.42 [0.18, 0.67] |
| Median [IR] | 1.80 [0, 7.00] | 0 [0, 2.00] | ||
| Responsiveness in the third quarter | ||||
|---|---|---|---|---|
| DLQI | Initial(N=133) | Quarter 3(N=133) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 5.02 (6.93) | 2.14 (4.10) | 0.45 [0.28, 0.63] | 0.70 [0.36, 1.05] |
| Median [IR] | 2.00 [0, 7.00] | 0 [0, 2.00] | ||
| Responsiveness in the fourth quarter | ||||
|---|---|---|---|---|
| DLQI | Initial(N=101) | Quarter 4(N=101) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 4.91 (7.34) | 2.22 (4.65) | 0.46 [0.26, 0.67] | 0.58 [0.21, 0.95] |
| Median [IR] | 1.20 [0, 7.00] | 0 [0, 2.00] | ||
SD: standard deviation; IR: interquartile range (25th percentile–75th percentile).
Responsiveness according to psoriasis severity.
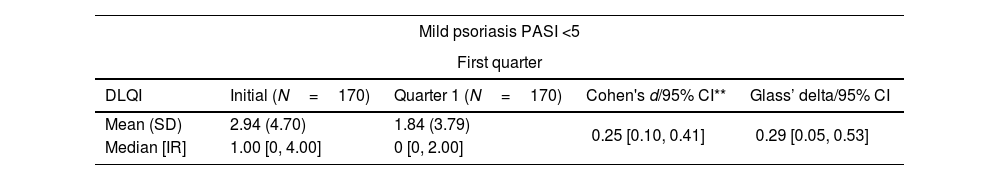
| Mild psoriasis PASI <5 | ||||
|---|---|---|---|---|
| First quarter | ||||
| DLQI | Initial (N=170) | Quarter 1 (N=170) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 2.94 (4.70) | 1.84 (3.79) | 0.25 [0.10, 0.41] | 0.29 [0.05, 0.53] |
| Median [IR] | 1.00 [0, 4.00] | 0 [0, 2.00] | ||
| Second quarter | ||||
|---|---|---|---|---|
| DLQI | Initial(N=121) | Quarter 2 (N=121) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 2.61 (4.43) | 1.45 (3.17) | 0.29 [0.10, 0.47 | 0.29 [0.10, 0.47 |
| Median [IR] | 1.00 [0, 4.00] | 0 [0, 1.00] | ||
| Third quarter | ||||
|---|---|---|---|---|
| DLQI | Initial (N=95) | Quarter 3 (N=95) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 2.77 (4.66) | 1.66 (3.75) | 0.24 [0.03, 0.44] | 0.30 [−0.03, 0.62] |
| Median [IR] | 1.00 [0, 4.00] | 0 [0, 1.00] | ||
| Forth quarter | ||||
|---|---|---|---|---|
| DLQI | Initial (N=74) | Quarter 4 (N=74) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 2.95 (5.61) | 1.15 (2.67) | 0.32 [0.08, 0.55] | 0.68 [0.13, 1.21] |
| Median [IR] | 1.00 [0, 3.75] | 0 [0, 1.00] | ||
| Moderate psoriasis PASI ≥5–10 | ||||
|---|---|---|---|---|
| First quarter | ||||
| DLQI | Initial (N=44) | Quarter 1 (N=44) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 7.49 (5.78) | 4.77 (6.30) | 0.43 [0.12, 0.74] | 0.43 [0.02, 0.84] |
| Median [IR] | 6.50 [1.98, 12.3] | 2.00 [0, 6.25] | ||
| Second quarter | ||||
|---|---|---|---|---|
| DLQI | Initial(N=31) | Quarter 2 (N=31) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 7.15 (5.53) | 3.45 (5.63) | 0.78 [0.28, 1.31] | 1.07 [0.29, 1.82] |
| Median [IR] | 5.00 [1.95, 11.5] | 1.00 [0, 4.50] | ||
| Third quarter | ||||
|---|---|---|---|---|
| DLQI | Initial (N=20) | Quarter 3 (N=20) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 7.74 (5.63) | 2.75 (4.67) | 0.24 [0.03, 0.44] | 0.30 [−0.03, 0.62] |
| Median [IR] | 8.00 [2.73, 12.5] | 2.00 [0, 3.00] | ||
| Forth quarter | ||||
|---|---|---|---|---|
| DLQI | Initial (N=16) | Quarter 4 (N=16) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 7.17 (6.14) | 2.88 (4.80) | 0.80 [0.23, 1.40] | 0.89 [0.03, 1.74] |
| Median [IR] | 5.50 [1.60, 12.5] | 1.00 [0, 3.00] | ||
| Severe psoriasis PASI ≥10 | ||||
|---|---|---|---|---|
| First quarter | ||||
| DLQI | Initial (N=42) | Quarter 1 (N=42) | Cohen's d/95% CI** | Glass's delta/95% CI |
| Mean (SD) | 12.1 (8.14) | 5.98 (7.87) | 0.68 [0.34, 1.02] | 0.78 [0.31, 1.25] |
| Median [IR] | 11.0 [6.00, 18.0] | 2.00 [0, 9.75] | ||
| Second quarter | ||||
|---|---|---|---|---|
| DLQI | Initial(N=20) | Quarter 2 (N=20) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 10.9 (8.48) | 7.05 (8.80) | 0.51 [0.04, 1.00] | 0.44 [−018, 1.06] |
| Median [IR] | 9.5 [3.00, 18.3] | 3.00 [0, 13.5] | ||
| Third quarter | ||||
|---|---|---|---|---|
| DLQI | Initial (N=17) | Quarter 3 (N=17) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 12.8 (9.21) | 4.18 (4.84) | 1.26 [0.63, 1.95] | 1.79 [0.57, 2.96] |
| Median [IR] | 15.0 [3.00, 19.0] | 2.00 [0, 4.00] | ||
| Forth quarter | ||||
|---|---|---|---|---|
| DLQI | Initial (N=10) | Quarter 4 (N=10) | Cohen's d/95% CI** | Glass’ delta/95% CI |
| Mean (SD) | 13.2 (9.34) | 7.70 (8.96) | 0.96 [0.20, 1.80] | −0.34 [0.34, 1.54] |
| Median [IR] | 14.00 [4.75, 18.8] | 3.50 [2.00, 14.8] | ||
SD: standard deviation; DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index; IR: interquartile range (25th percentile–75th percentile).
Cohen's d of 0.25 [0.10, 0.41], 0.43 [0.12, 0.74] and 0.68 [0.34, 1.02] were found in mild, moderate, and severe forms, respectively (Table 2).
Our findings suggest that the Colombian Spanish version of DLQI has a slight responsiveness in patients with psoriasis, even though a highly effective therapy such as a biologic was administered. This contrasts with other studies in which the instrument has been shown to be highly sensitive to change in the course of the disease after initiating therapy,4 although they align with recent publications calling for a review of the DLQI in patients with psoriasis.5,6 Importantly, in our study the DLQI was specifically found to be more sensitive to change in patients with severe psoriasis, a finding that is consistent with its greater specificity in severe inflammatory dermatoses.
Anti-TNFs use prevailed what contrasts with current clinical practice, but such finding can be explained by the inclusion of patients with less missing information in the earliest stage of the study, lapse in which these biologics were the only commercially available in Colombia.
Our study found an initial DLQI score with a moderate effect on HRQoL, which contrasts with what previous reports,4 although it may be due to the lack of equivalence of the DLQI translated (non-validated) Colombian Spanish version.
Differences of DLQI/PASI scores compared to other reports could be explained by therapy initiation in the majority of patients. In addition, scoring may have been influenced by the aforementioned cross-cultural non-equivalence of DLQI and/or measurement properties of the PASI instrument as it lacks sensitivity to evaluate clinical variants of psoriasis (i.e.: palmoplantar, scalp, pustular, and psoriatic arthritis).
The main strength of the study relies on its target on patients belonging to the largest cohort of patients with psoriasis on biologic therapy in Colombia and the evaluation of the responsiveness of the DLQI in patients with mild, moderate, and severe psoriasis as this psychometric property of the instrument has been poorly studied globally. Limitations of this study was its retrospective design, which is more prone to information bias due to missing data. In addition, scarcity of data in recent years in which the use of more current biologicals could have been included was influenced by the COVID-19 pandemic during years 2020 and 2021, as in-person follow-ups were limited.
In conclusion, the non-validated Colombian Spanish version of the DLQI showed a lack of responsiveness, when evaluating patients with mild and moderate psoriasis, what calls for a cross-cultural adaptation and validation of the Colombian Spanish DLQI to obtain a version more equivalent to the original instrument.
FundingThis work has been sponsored by the Group of Investigative Dermatology (GRID) of the University of Antioquia (Medellin, Colombia) and MFT and YKB have also contributed with their own resources to buy some materials and services related with the study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We are grateful to Medicarte SA and EPS Sura for granting access to their coded patient database. We also thank Catalina Orozco, Carolina Hincapié, Alejandro Berbeo and Sebastián Burgos for their collaboration. We are grateful to the Group of Investigative Dermatology (GRID) of the University of Antioquia (Medellin, Colombia) for supporting the logistics of the study.