The objective of this article is to collect and describe the most relevant BIOBADADERM findings since its beginning in 2008. BIOBADADERM is a prospective cohort registry whose main aim is to analyze the safety profile of the new systemic therapy for the management of psoriasis. Data included in the registry allowed the publication of numerous articles over the past 15 years (2008–2023). Data from the registry join those obtained from clinical trials regarding safety and efficacy profile. Additionally, it has expanded our knowledge of population groups excluded from clinical trials and in less frequent settings. Collaboration with other registries has given us a few fact-checking answers in smaller groups.
El objetivo de este artículo es recopilar y describir los hallazgos más relevantes aportados por BIOBADADERM desde su inicio en 2008. BIOBADADERM es un registro prospectivo de cohortes cuyo objetivo principal es analizar la seguridad de los nuevos tratamientos sistémicos para la psoriasis. El análisis de los datos incluidos en el registro ha permitido la publicación de numerosos artículos en los últimos 15 años (2008-2023), que complementan los ensayos clínicos en cuanto a seguridad y eficacia. Asimismo, ha ampliado los conocimientos en grupos poblacionales excluidos de los ensayos clínicos y en escenarios poco frecuentes. La colaboración con otros registros nos ha permitido dar respuesta a preguntas sobre grupos menos frecuentes.
In 2008, the Psoriasis Working Group of the Spanish Academy of Dermatology and Venereology, in collaboration with the Spanish Agency for Medicines and Health Products and the Spanish Foundation of Rheumatology, launched the BIOBADADERM registry.1 As of November 2023, it included a total of 21 participant hospitals, 6000 psoriatic patients on systemic therapy, and almost 30,000 patient-years of follow-up in a prospective cohort. Data quality is checked through periodic monitoring. Its main objective is to analyze the safety profile of new systemic therapies for psoriasis.
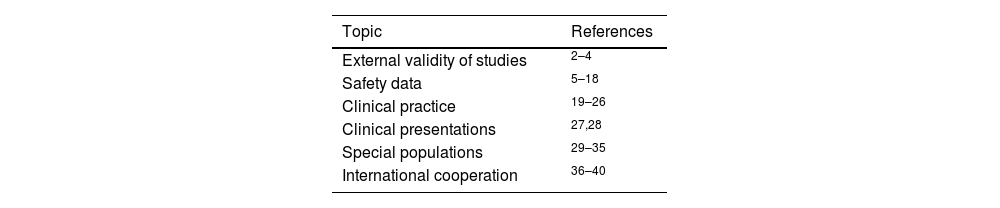
With 15 years of evolution, BIOBADADERM is probably the longest-running scientific project of the AEDV. The objective of this article is to compile its most relevant scientific contributions for daily clinical practice. Table 1 groups the articles by topic.
Data on new drugs come primarily from clinical trials and experience in other diseases, but, as BIOBADADERM has demonstrated, these data are not always generalizable to the psoriatic population.
In the field of safety, data extrapolation from other diseases has clear limitations. BIOBADADERM has analyzed the mid- and long-term adverse events (AEs) of recently marketed drugs, with results supporting their safety profile in the routine clinical practice. In addition, comparative studies of different drugs facilitate therapeutic selection. Of note, the occurrence of AEs in the registry is not the same at all times during therapy. They are more frequent within year 1, especially with systemic treatments, while the risk of serious AEs remains constant over time. Notifications of unknown adverse effects are essential for the continuous evaluation of drug safety. BIOBADADERM has detected an alert regarding symptomatic urinary tract infection with cyclosporine, infliximab, and anti-IL17 drugs, and data have supported the hypothesis that anti-TNF drugs and ustekinumab can infrequently induce paradoxical arthritis after several months into therapy.
During the COVID-19 pandemic, suspicion abounded on the safety of drugs used daily in the treatment of psoriasis. Having an active registry made it possible to conclude that neither classic nor biological drugs increase the susceptibility or severity of COVID-19 infection.
The increasing availability of safe and effective drugs has changed the therapeutic approach to psoriasis, with biologics being used more and more frequently. In addition, the biological drug prescribed first has changed over time, and in participating hospitals, the use of biologics at different doses than recommended is common. In clinical practice, variable dosage has numerous advantages, such as individual adaptation, reduction of side effects, economic optimization, and greater flexibility. BIOBADADERM has confirmed the safety of these drugs in various day-to-day situations, such as surgery or their use as first-line therapy.
Regarding associated comorbidities, we have found that an increase in body mass index is associated with a higher risk of treatment discontinuation due to lack of efficacy and with an increased risk of AEs. Similarly, patients with psoriatic arthritis had a higher number of comorbidities, especially hypertension and liver disease, used a greater number of treatments, and exhibited more AEs and infections/infestations regardless of associated comorbidities and current or past treatments.
One of the measurements used to indirectly assess the safety and efficacy profile of a drug is survival, defined as the duration of a specific therapy. However, after analyzing the survival of different biologics and systemic drugs in the registry, we have concluded that survival is not adequate as a measure of safety (and probably effectiveness), especially when comparing different drugs. This is due to the number of factors that can influence survival independent of safety or efficacy.
The possibility of identifying prognostic factors that help us predict treatment response is an interesting question. Patients with fewer previous treatments, who are thinner, older at the start of treatment, and with a previous history of good response have had a higher probability of obtaining good results.
Data obtained from the registries on epidemiology, comorbidities, and management provide valuable information on rare clinical forms. The number of cases of erythrodermic psoriasis included in BIOBADADERM has gradually decreased at the follow-up, which may reflect better disease control and supports the idea that EP in some patients is a severe stage of other types of psoriasis. Patients with generalized pustular psoriasis had a higher prevalence of kidney and liver disease, while patients with palmoplantar pustulosis showed a higher risk of hypercholesterolemia. These previously undescribed findings should be taken considered in the routine clinical practice.
BIOBADADERM includes a more diverse and representative population as it does not have inclusion and exclusion criteria as strict as those in clinical trials.
After analyzing the differences by sex, women were more likely to receive a biological drug. No sex differences were detected in terms of efficacy, but women had a higher risk of developing AEs. Women of childbearing age showed a reduction in fertility rate. The only significant variables related were age and disease duration. The review of pregnancies and experience in women with inflammatory bowel disease indicated a probable low risk of complications. A striking decrease in the percentage of breastfeeding has been observed, probably due to psoriasis outbreaks.
The different age groups have also been studied. Those younger than 21 years represented a very small group. These data could reflect a lower disease burden or undertreatment, delaying the initiation of systemic treatment until adulthood. Patients were more frequently managed with classic treatments, and the average age at the start of classic or biological systemic treatment in those younger than 18 years was 14.5 years. In the elderly, the use of biologics was associated with a lower risk of AEs in young and old patients. Serious AEs were more common in elderly patients, but may be related to other particularities of the group and not be directly due to the drug used.
The management of patients with human immunodeficiency virus (HIV) is controversial and limited. BIOBADADERM analyzed 23 HIV-positive patients followed for an average of 3.2 years. Anti-TNF drugs and ustekinumab were effective and safe in combination with adequate antiviral therapy.
BIOBADADERM is part of Psonet (network of independent European registries of patients with psoriasis and PsA on systemic therapy), which has not detected an increased risk of cancer in the mid-term, although data heterogeneity and methodological limitations did not allow ruling out a small increased risk of some tumor subtypes. Similarly, in the routine clinical practice, anti-TNFs for psoriasis have not been associated with a significantly increased risk of serious infections compared to classic treatments.
The analysis of prescription trends in different countries has observed large differences in the use of drugs, especially in terms of availability and management, with variability between countries in the distribution of covariates that potentially influence the choice of treatment (e.g., age, PASI, proportion of patients with psoriasis vulgaris vs other forms of psoriasis). Some variables were directly related to the chances of receiving a biological or a systemic drug in most registries (BMI, PsA, psoriatic onychopathy, or proportion of current smokers).
Psonet data from Spain, France, and the United Kingdom are being analyzed in a global study comparing the survival and safety of biosimilar adalimumab versus the original. The results are still pending publication.
In conclusion, BIOBADADERM, as a prospective cohort focused on describing drug safety, has provided data that complement the knowledge generated by clinical trials, especially in special populations or those not included in them, in infrequent exposures and outcomes such as drug combinations or long-term effects, and in risks of late onset. Collaboration with other registries has allowed us to answer questions about less frequent groups.
FundingThe BIOBADADERM project was promoted by Fundación Piel Sana of the Spanish Academy of Dermatology and Venereology and is funded by the Spanish Agency for Medicines and Health Products (AEMPS) and pharmaceutical companies, such as AbbVie, Almirall, Amgen, Boehringer Ingelheim, BMS, Janssen, and UCB. The following companies have also collaborated in the past (Biogen, Leo Pharma, Lilly, MSD, Novartis, and Pfizer).
The collaborating pharmaceutical companies did not participate in the design or the study; nor in the collection, management, analysis, and interpretation of data, or in the preparation, review, or approval of the manuscript and the decision to submit the manuscript for publication.
Conflict of interestsNone declared.
BIOBADADERM Group, Andrea Montes Torres, Beatriz Gonzalez Sixto.