Patients with chronic spontaneous urticaria frequently relapse after discontinuing omalizumab and require its reintroduction. Although prior optimization might reduce recurrences, there is scarce evidence on this issue. Moreover, predictors of relapse have been identified in non-optimized patients before suspension.
MethodsWe conducted a multicenter retrospective study with patients who discontinued omalizumab after optimization with a 12-month follow-up. Univariate and multivariate (tree classification method and Cox regression) analyses were performed.
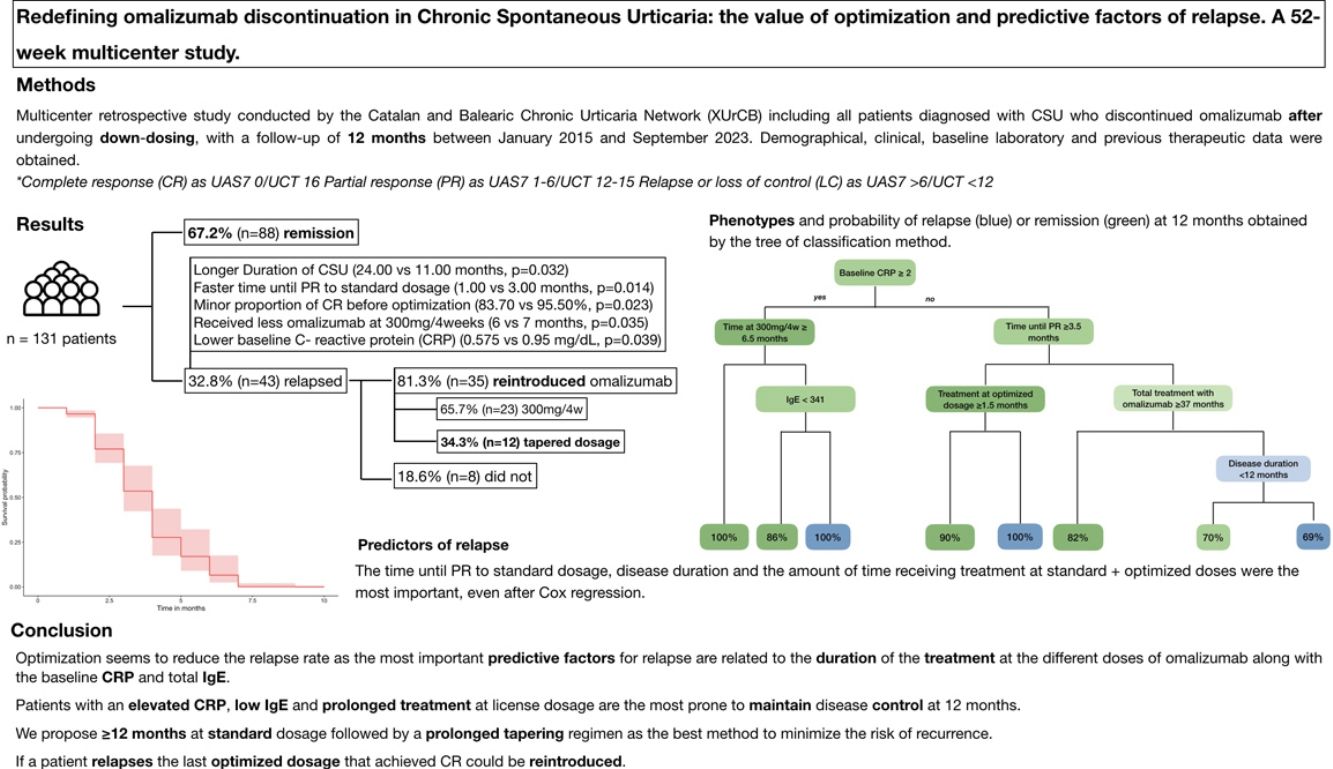
ResultsA total of 131 patients were included, 32.8% of whom relapsed after 12 months. Relapsed patients had longer disease duration (24.00 vs 11.00 months; p=0.032), quicker response to standard dosage (1.00 vs. 3.00 months; p=0.014), fewer complete responses pre-optimization (83.70% vs 95.50%; p=0.023), and shorter treatment duration at 300mg/4 weeks (6 vs 7 months; p=0.035). Multivariate analysis revealed that patients with low baseline C-reactive protein (CRP) and total immunoglobulin E (IgE) who underwent prolonged treatment were more likely to maintain a sustained remission at 12 months.
ConclusionOptimization seems to reduce the relapse rate after discontinuation. The most relevant factors for recurrence are associated with the duration of treatment at different doses of omalizumab, along with the baseline CRP and total IgE levels. To minimize relapse after suspension, a 12-month treatment regimen at 300mg/4 weeks followed by an 18-month dose tapering is proposed.
Los pacientes con urticaria crónica espontánea suelen rebrotar tras suspender omalizumab, requiriendo su reintroducción. La optimización previa a la retirada puede reducir las recurrencias, aunque todavía no existe suficiente evidencia. Por otro lado, los factores predictores de recaída reportados se han estudiado mayormente en pacientes que no optimizaron antes de la suspensión.
MétodosEstudio retrospectivo multicéntrico que incluyó los pacientes que suspendieron omalizumab tras ser optimizados con un seguimiento de 12 meses. Se realizaron análisis univariantes y multivariantes (un árbol de clasificación y una regresión de Cox).
ResultadosSe incluyeron un total de 131 pacientes, de los cuales el 32,8% recayó a los 12 meses. Los pacientes que rebrotaron tenían mayor duración de la enfermedad (24,00 vs. 11,00 meses; p=0,032), una respuesta más rápida a la dosis estándar (1,00 vs. 3,00 meses; p=0,014), menores respuestas completas antes de optimizar (83,70% vs. 95,50%; p=0,023) y menor tiempo de tratamiento a 300mg/4 semanas (6 vs. 7 meses; p=0,035). El análisis multivariante reveló que los pacientes con proteína C reactiva (PCR) e inmunoglobulina E (IgE) basales bajas que habían sido tratados durante un tiempo más prolongado tenían más probabilidades de mantener una remisión sostenida a los 12 meses.
ConclusiónLa optimización parece reducir la tasa de recaída tras suspender omalizumab. Los factores más relevantes de recurrencia son la duración del tratamiento con omalizumab junto con los niveles basales de PCR e IgE total. Para minimizar la recaída, proponemos realizar tratamiento a 300mg/4 semanas durante 12 meses seguido de una optimización progresiva durante 18 meses.
Chronic spontaneous urticaria (CSU) is a mast cell-driven skin disease characterized by the spontaneous appearance of transient wheals, angioedema, or both; persisting>6 weeks. Although antihistamines are recommended as the first-line of therapy, 50% of patients do not achieve disease control even with quadrupled dosage.1 In such cases, omalizumab at a licensed dosage of 300mg every 4 weeks is a safe and effective treatment for CSU, successfully controlling the disease in more than 70% of patients.2
Once the CSU is well-controlled; patients may discontinue omalizumab due to the self-resolving nature of the disease. However, upon discontinuation patients frequently relapse and require its reintroduction, regaining disease control.3,4 To reduce the relapse rate; patients could benefit from dose tapering prior to cessation, although its efficacy has not been firmly established. Two main initial methods of optimization have been proposed: reducing dosage down to 150mg every 4 weeks and maintaining the license dosage but prolonging the interval of administration.5–7 No comparative studies have been conducted to determine which approach is better in terms of toleration or sustained remission after suspension.
To date, various predictors of relapse after omalizumab discontinuation have been identified; predominantly in patients who did not optimize prior to interruption. Most reported predictors are clinical variables, including an elevated baseline Urticaria Activity Score 7 (UAS7), longer disease duration, older age, or an initial fast response.3,8,9 In contrast, limited evidence has been published regarding biomarkers, with some studies suggesting that elevated baseline total Immunoglobulin E (IgE) levels are associated with a higher risk of relapse.10–12
EndpointsThe primary endpoints of this study are (i) to identify the predictive factors of relapse after omalizumab suspension and (ii) to determine if optimization reduces the rate of relapse. Secondary endpoints are to analyze the management of these relapses and the effectiveness of retreatment.
Materials and methodsStudy populationThe Catalan and Balearic Chronic Urticaria Network (XUrCB) conducted a multicenter retrospective study including all patients diagnosed with CSU who discontinued omalizumab after undergoing dose tapering, with a minimum follow-up 12 months from January 2015 through September 2023. The demographic, clinical and baseline laboratory data of each patient was obtained from the health records. We excluded patients who stopped omalizumab without prior optimization; patients with <12-month follow-up and chronic inducible urticarias (CIndU) who do not associate CSU. This study was approved by the Ethics Committee (2024/26-DER-HUSC).
We defined complete response (CR) as UAS7 0 and/or Urticaria Control test (UCT) 16; partial response (PR) as UAS7 1–6 and/or UCT 12–15 and relapse or loss of control (LC) as UAS7>6 and/or UCT<12.
Statistical analysisStatistical analysis was performed using R Core Team 2022 v.4.2.2. Categorical variables were expressed as percentages. Continuous variables were expressed as mean±standard deviation (SD) or median (interquartile range) based on the distribution of each variable determined with the Shapiro–Wilk or Kolmogorov–Smirnov test.
For the univariate analysis, comparison between the qualitative data of 2 groups was performed using the Pearson chi-squared test. Similarly, when comparing quantitative data the Mann–Whitney U or T-Student's tests were used, when appropriate. We performed a multivariate analysis using the tree classification method including demographic, analytical and clinical variables. We limited the number of decision nodes to 4 and determined the most relevant variables of the obtained model to predict relapse. These variables were combined with the statistically significant ones obtained in the univariate analysis and the main ones previously reported in the literature as predictive factors in a Cox regression analysis. The level of statistical significance was set at p<0.05.
We estimated cut-off points for the duration of treatment at standard and optimized doses that better discriminate between patients who relapse and those who do not.
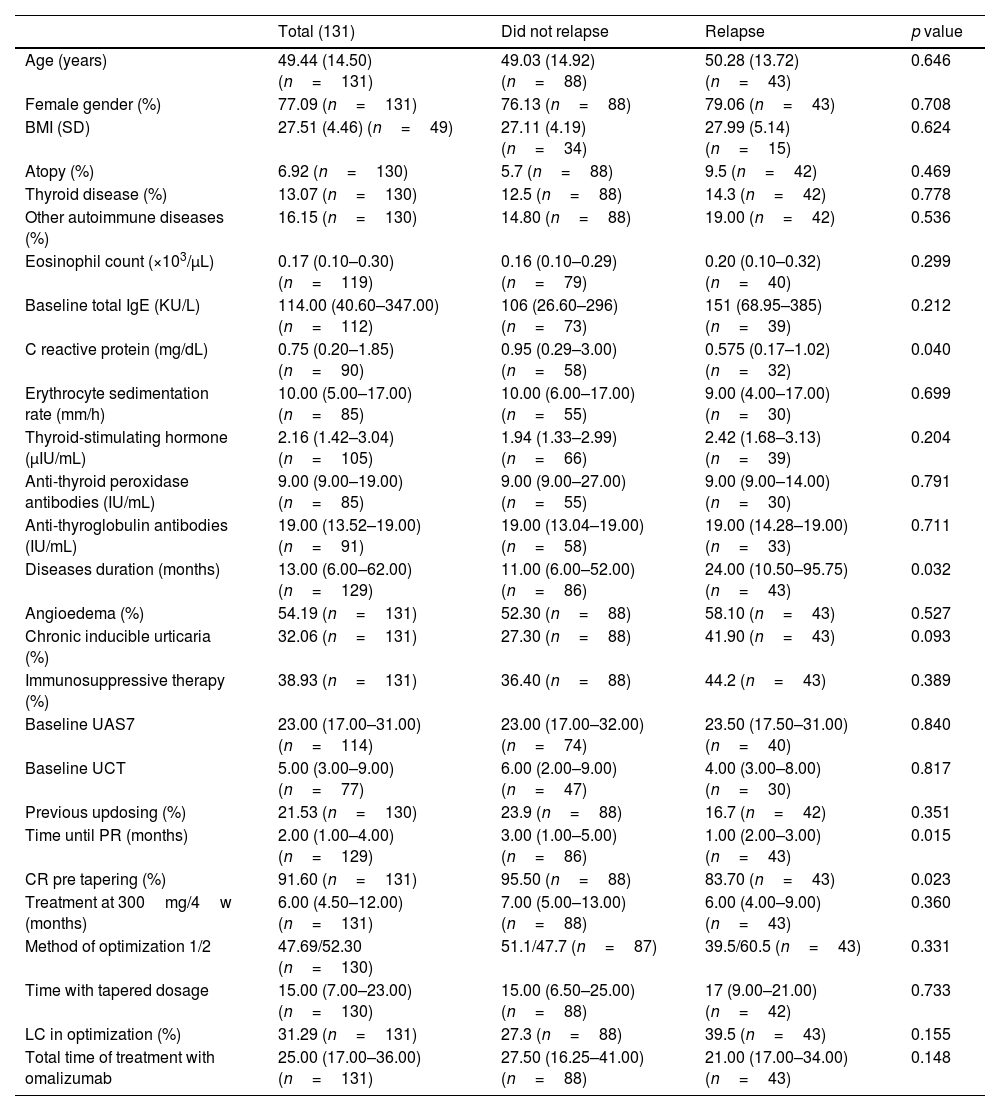
ResultsWe identified a total of 257 patients who received a tapered regimen of omalizumab. A total of 131 patients (50.97%) discontinued omalizumab and had a minimum follow-up of 12 months (Table 1). Patients were predominantly women (n=101; 77%) with a mean age of 49.44±14.50 years (n=131) and body mass index (BMI) of 27.51±4.46 (n=49). A past medical history of thyroid disease and other autoimmune diseases were present in 13.07% (n=17) and 16.15% (n=21) of patients, respectively.
Demographical, clinical, baseline lab test results and previous therapeutic data.
| Total (131) | Did not relapse | Relapse | p value | |
|---|---|---|---|---|
| Age (years) | 49.44 (14.50) (n=131) | 49.03 (14.92) (n=88) | 50.28 (13.72) (n=43) | 0.646 |
| Female gender (%) | 77.09 (n=131) | 76.13 (n=88) | 79.06 (n=43) | 0.708 |
| BMI (SD) | 27.51 (4.46) (n=49) | 27.11 (4.19) (n=34) | 27.99 (5.14) (n=15) | 0.624 |
| Atopy (%) | 6.92 (n=130) | 5.7 (n=88) | 9.5 (n=42) | 0.469 |
| Thyroid disease (%) | 13.07 (n=130) | 12.5 (n=88) | 14.3 (n=42) | 0.778 |
| Other autoimmune diseases (%) | 16.15 (n=130) | 14.80 (n=88) | 19.00 (n=42) | 0.536 |
| Eosinophil count (×103/μL) | 0.17 (0.10–0.30) (n=119) | 0.16 (0.10–0.29) (n=79) | 0.20 (0.10–0.32) (n=40) | 0.299 |
| Baseline total IgE (KU/L) | 114.00 (40.60–347.00) (n=112) | 106 (26.60–296) (n=73) | 151 (68.95–385) (n=39) | 0.212 |
| C reactive protein (mg/dL) | 0.75 (0.20–1.85) (n=90) | 0.95 (0.29–3.00) (n=58) | 0.575 (0.17–1.02) (n=32) | 0.040 |
| Erythrocyte sedimentation rate (mm/h) | 10.00 (5.00–17.00) (n=85) | 10.00 (6.00–17.00) (n=55) | 9.00 (4.00–17.00) (n=30) | 0.699 |
| Thyroid-stimulating hormone (μIU/mL) | 2.16 (1.42–3.04) (n=105) | 1.94 (1.33–2.99) (n=66) | 2.42 (1.68–3.13) (n=39) | 0.204 |
| Anti-thyroid peroxidase antibodies (IU/mL) | 9.00 (9.00–19.00) (n=85) | 9.00 (9.00–27.00) (n=55) | 9.00 (9.00–14.00) (n=30) | 0.791 |
| Anti-thyroglobulin antibodies (IU/mL) | 19.00 (13.52–19.00) (n=91) | 19.00 (13.04–19.00) (n=58) | 19.00 (14.28–19.00) (n=33) | 0.711 |
| Diseases duration (months) | 13.00 (6.00–62.00) (n=129) | 11.00 (6.00–52.00) (n=86) | 24.00 (10.50–95.75) (n=43) | 0.032 |
| Angioedema (%) | 54.19 (n=131) | 52.30 (n=88) | 58.10 (n=43) | 0.527 |
| Chronic inducible urticaria (%) | 32.06 (n=131) | 27.30 (n=88) | 41.90 (n=43) | 0.093 |
| Immunosuppressive therapy (%) | 38.93 (n=131) | 36.40 (n=88) | 44.2 (n=43) | 0.389 |
| Baseline UAS7 | 23.00 (17.00–31.00) (n=114) | 23.00 (17.00–32.00) (n=74) | 23.50 (17.50–31.00) (n=40) | 0.840 |
| Baseline UCT | 5.00 (3.00–9.00) (n=77) | 6.00 (2.00–9.00) (n=47) | 4.00 (3.00–8.00) (n=30) | 0.817 |
| Previous updosing (%) | 21.53 (n=130) | 23.9 (n=88) | 16.7 (n=42) | 0.351 |
| Time until PR (months) | 2.00 (1.00–4.00) (n=129) | 3.00 (1.00–5.00) (n=86) | 1.00 (2.00–3.00) (n=43) | 0.015 |
| CR pre tapering (%) | 91.60 (n=131) | 95.50 (n=88) | 83.70 (n=43) | 0.023 |
| Treatment at 300mg/4w (months) | 6.00 (4.50–12.00) (n=131) | 7.00 (5.00–13.00) (n=88) | 6.00 (4.00–9.00) (n=43) | 0.360 |
| Method of optimization 1/2 | 47.69/52.30 (n=130) | 51.1/47.7 (n=87) | 39.5/60.5 (n=43) | 0.331 |
| Time with tapered dosage | 15.00 (7.00–23.00) (n=130) | 15.00 (6.50–25.00) (n=88) | 17 (9.00–21.00) (n=42) | 0.733 |
| LC in optimization (%) | 31.29 (n=131) | 27.3 (n=88) | 39.5 (n=43) | 0.155 |
| Total time of treatment with omalizumab | 25.00 (17.00–36.00) (n=131) | 27.50 (16.25–41.00) (n=88) | 21.00 (17.00–34.00) (n=43) | 0.148 |
Regarding CSU characteristics, the median duration, assessed at the time of initiating omalizumab, was 13.00 months (IQR, 6.00–62.00 months; n=129). The CSU associated angioedema in 54.19% (n=71) and CIndU in 32.06% (n=42) of patients. At baseline, the median UAS7 was 23.00 (IQR, 17.00–31.00; n=114) and UCT was 5.00 (IQR, 3.00–9.00; n=77). Lab test results: median total baseline IgE levels of 114.00UI/ml (IQR, 40.60–347.00), and C reactive protein (CRP) levels of 0.75mg/dL (IQR, 0.20–1.85; n=90).
Patients were treated for a median of 6.00 months (IQR, 4.50–12.00; n=131) at 300mg every 4 weeks, requiring up dosing to 450 or 600mg every 2 or 4 weeks in 21.53% (n=28). PR was achieved at a median of 2 months (IQR, 1.00– 4.00; n=129) after initiation, with 91.6% obtained a CR prior to optimization. All patients underwent down dosing for a median of 15.00 months (IQR, 7.00–23.00; n=131). Initially, 47.69% of patients reduced the dosage to 150mg every 4 weeks while 52.30% maintained the dose and prolonged interval administration to 300mg every 6 weeks. LC during tapering occurred in 31.29% of patients without differences across the 2 tapering methods.
Overall; patients stopped omalizumab after a total of 25.00 months (IQR, 17.00–36.00) of treatment. During the 12 months following discontinuation, 32.8% (n=43) of patients relapse at a median time of 3.00 (IQR, 2.00–4.50) months. Omalizumab was reintroduced in 81.3% (n=35) of patients. The license dosage of 300mg every 4 weeks was initiated in 65.7% (n=23) patients whereas 34.28% restarted an optimized dosage. All patients regained disease control.
The univariate analysis revealed that patients who relapsed had statistically significant longer disease courses (24.00 vs 11.00 months; p=0.032), faster time until PR to standard dosage (1.00 vs 3.00 months; p=0.014), fewer CR before optimization (83.70 vs 95.50%; p=0.023) and were treated for a shorter period with omalizumab at 300mg every 4 weeks (6 vs 7 months; p=0.035). Moreover, the median baseline CRP was lower in patients who relapsed (0.575 vs 0.95mg/dL; p=0.039) and the total baseline IgE was higher but did not reach statistical significance (151 vs 106UI/ml; p=0.204).
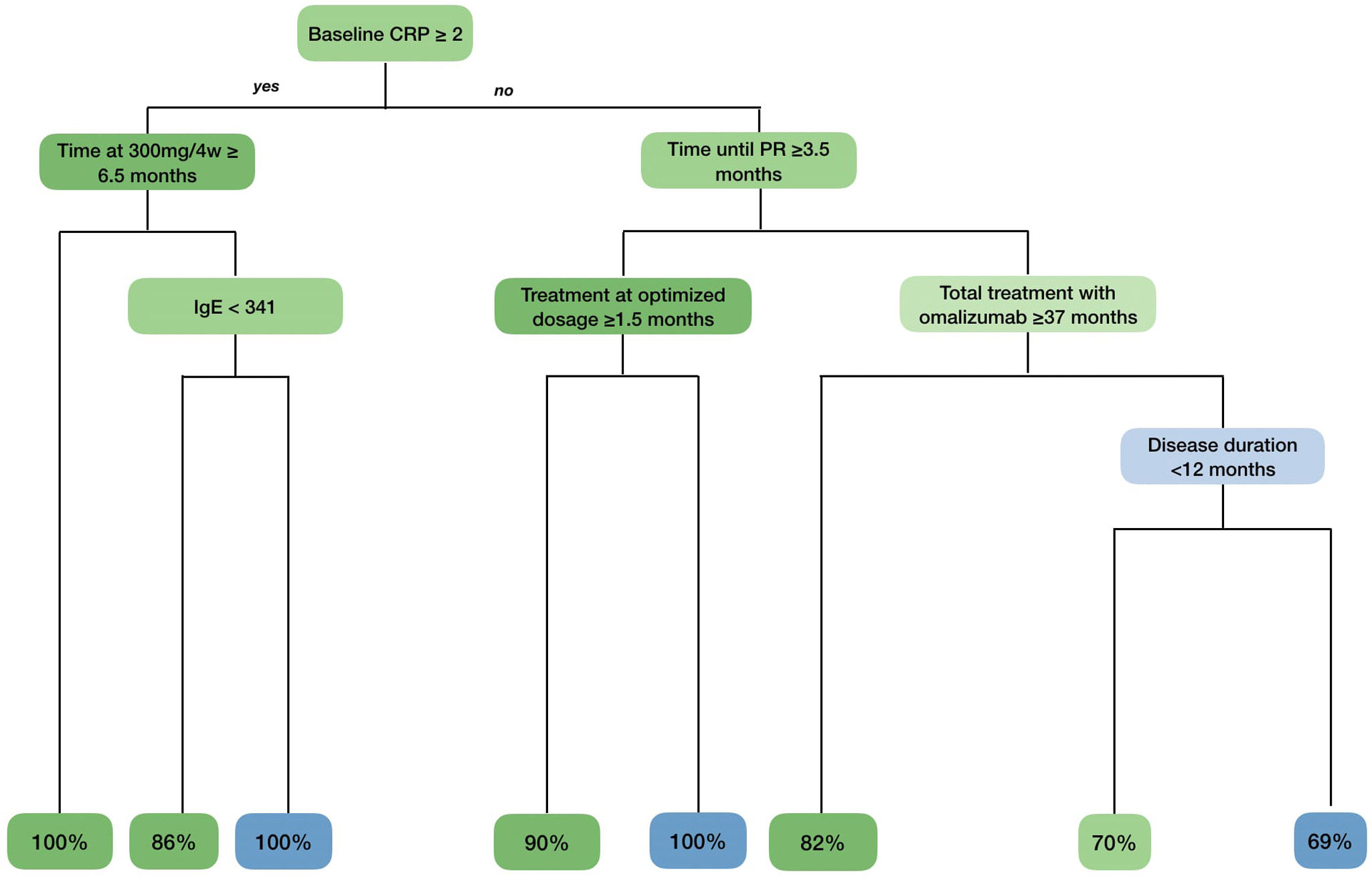
A tree classification method was constructed including all variables in Table 1, with an overall accuracy rate of 81% (Fig. 1). As shown in the algorithm, we identified one phenotype that sustained a CR after 12 months in all cases: patients with a baseline CRP≥2mg/dL who received, at least, 6.5 months of omalizumab at license dosage. We also found 2 phenotypes that were strongly associated with relapse: patients with baseline CRP≥2mg/dL and total IgE≥341 KU who received <6.5 months of omalizumab at license dosage and patients with baseline CRP≤2mg/dL, who achieved a PR<3.5 months and received tapering regimens for <1.5 months.
Phenotypes and probability of relapse obtained by the tree of classification method. Variables related to the absence of relapse are shown in green, while those associated with relapse are shown in blue. The intensity of the green and blue colors correlates with their importance in the algorithm (the darker the more important). In the last row, the proportion of patients who do not relapse (in green) and those who do actually relapse (in blue) is shown based on their phenotype.
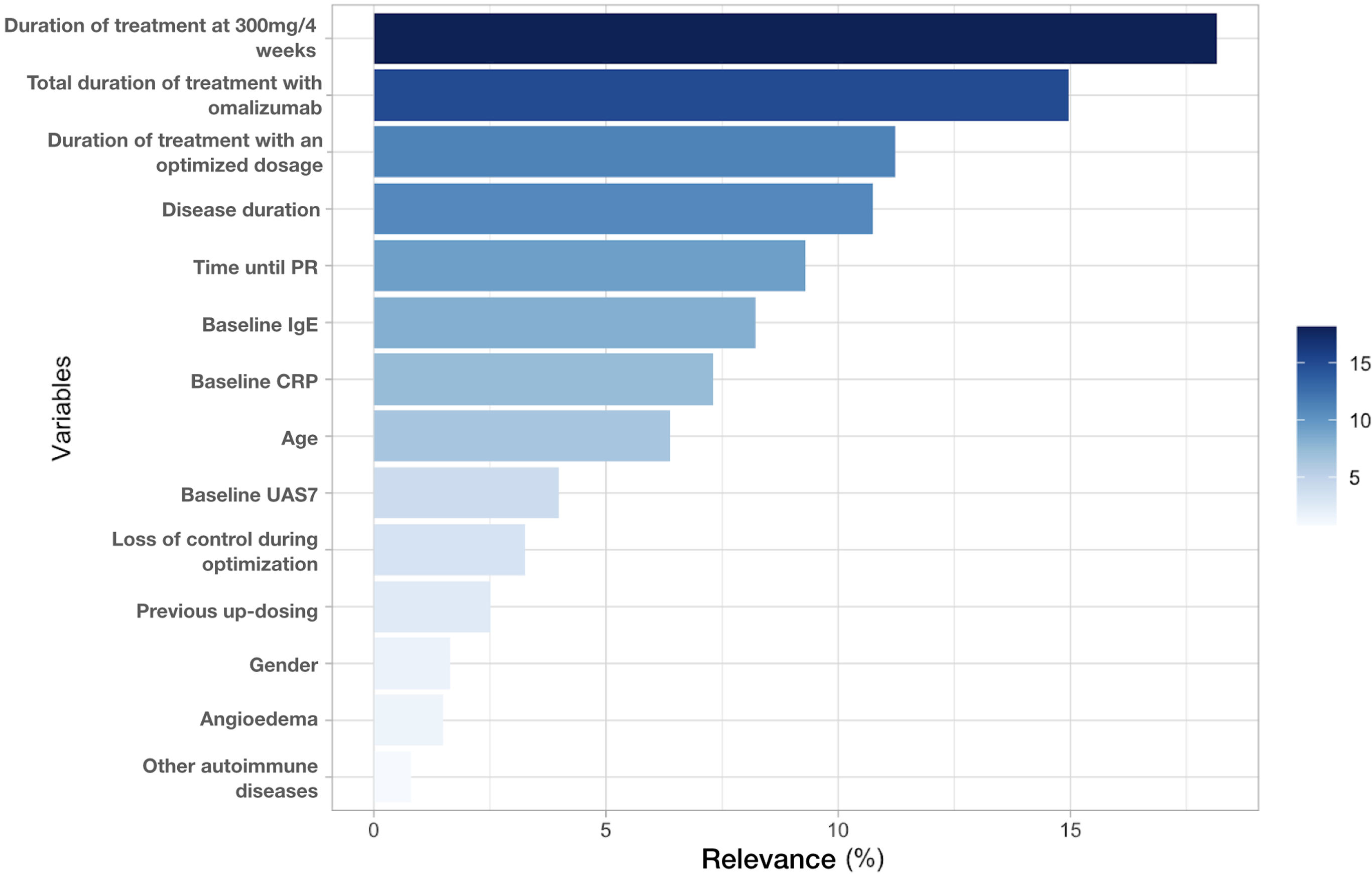
Afterwards, we organized the variables based on their relevance in the classification algorithm (Fig. 2). The time until PR and disease duration along with the amount of time during which the patient received treatment at standard doses, optimized doses, and the sum of these last 2 were the most important predictive variables, accounting for 65% of the capacity of the model.
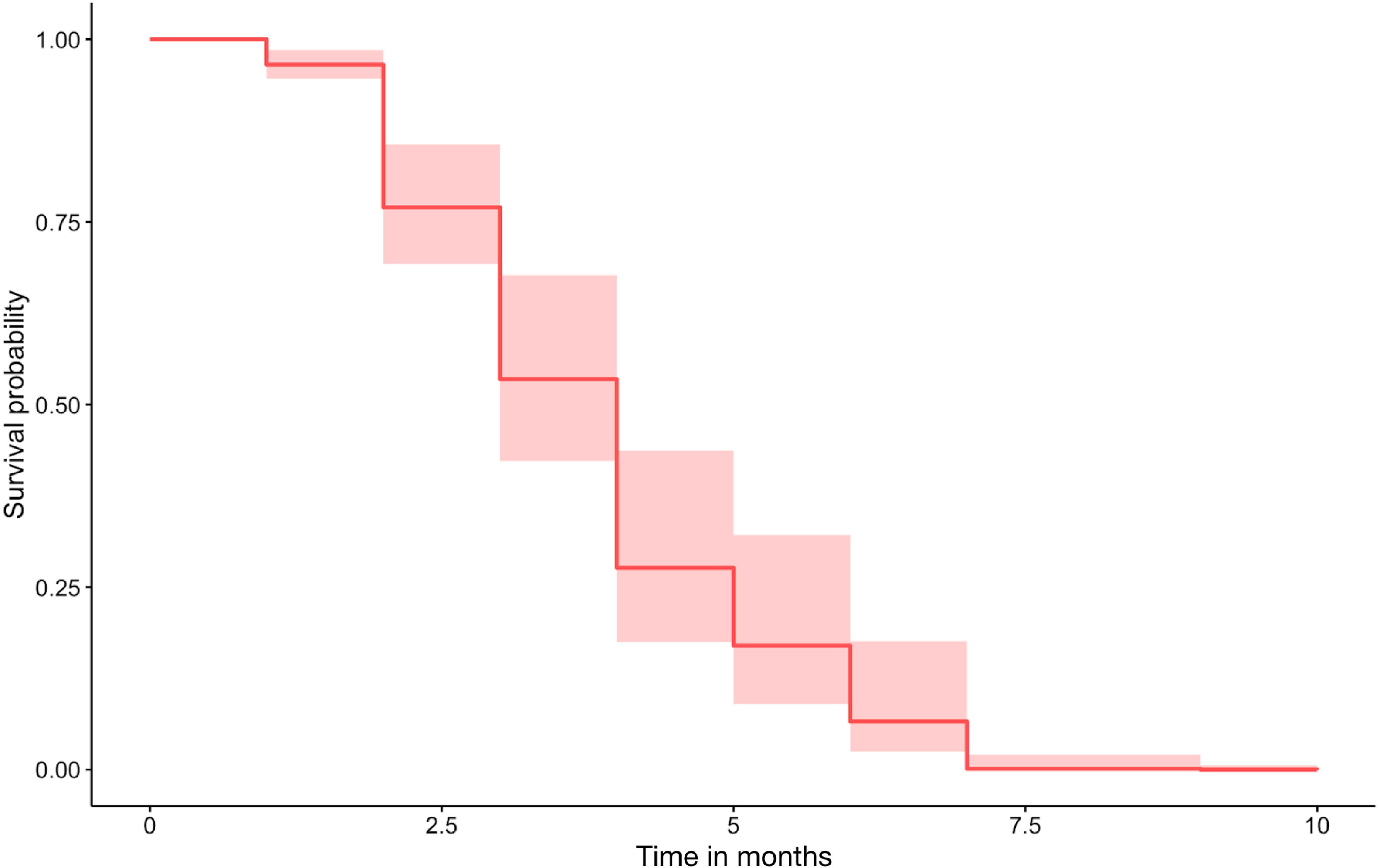
These 5 variables were included with baseline CRP, baseline total IgE, age and gender in a Cox regression model. A survival curve was obtained (Fig. 3). All variables except gender (p=0.394) and disease duration (p=0.829) remained statistically significant (p<0.001). Considering the importance of treatment duration, we calculated the cut-off points to reduce the risk of relapse: 12 months at the licensed dosage and 18 months for the optimized regimen.
DiscussionPatients diagnosed with CSU often experience relapse after stopping omalizumab within the first year, with the greatest risk occurring in the first 2 months.9,13 Without prior optimization, the relapse rate is approximately 60% within 2 months.3 To maintain the long-term remission of the disease, various optimization methods have been proposed. Different tapering strategies have noted a slight decrease in the rate of relapses, approximately (from 35% up to 56%).9,14–17 Additionally, down titration may delay the onset of relapse.15
In our study, we observed a relapse rate of 32.8% (n=43) at a median time of 3.00 (IQR, 2.00–4.50) months. This is the lowest recurrence rate reported and strongly advocates for the benefit of tapering prior to cessation. Of note, no differences were found between the proportion of patients that relapse with the different optimization methods. The OPTIMA trial confirmed that re-treatment with omalizumab successfully regained symptomatic control in patients who flared after suspension.4 Nevertheless, the therapeutic management has not been defined. Omalizumab was restarted in 81.3% (n=35) of our patients. Interestingly, 34.28% (n=15) reinitiated with an optimized dosage and achieved a CR. Thus; patients who optimized and relapsed could reintroduce the last tapered dosage that they were in CR rather than 300mg every 4 weeks.
Several biomarkers have been identified which allows to predict the response to omalizumab at a license dosage.18 However, less evidence is available on the predictive factors of relapse, especially in patients who previously underwent optimization. Biomarkers can be categorized into 3 groups: demographic, CSU characteristics, and molecular parameters.
Demographic data does not seem to influence the risk of relapse based on the current available evidence. Age is associated with a higher severity of CSU, but its potential as a biomarker for relapse is controversial with disparate results having been published. Merteens et al. reported that elderly patients have higher risk of relapse, while a different study demonstrated that patients younger than 40 years often relapsed the most.9,17 Similarly, opposite results have been published regarding gender, with Grieco et al. reporting a higher risk in women vs men according to Sirufo et al.,12,19 BMI is associated with response to standard dosage and tapering, yet it has not been described as a biomarker for relapse. When elevated, it associates a poor response to omalizumab and need for up dosing, meanwhile when low it correlates with better tolerance to down titration.15,20 We did not find significant differences in age, gender, or BMI in our study neither in the univariate nor multivariate analysis.
The most relevant CSU characteristics for relapse are its duration, the baseline UAS7 and response to omalizumab at 300mg every 4 weeks. A longer disease duration has been previously associated with a higher risk of recurrence after discontinuation.3,15 The univariate analysis revealed a longer disease duration in patients who relapse; albeit after the multivariate analysis it lost significance. Contrary to the findings of the post hoc analysis of the ASTERIA I/II clinical trials and Marzano et al. where patients did not previously optimize, we did not find any differences in baseline UAS7 between patients that experienced relapse and those who did not.3,8 No differences were found either on the presence of angioedema, association of CIndU, prior treatment with immunosuppressive drugs or previous up titration.
Response and duration of treatment with omalizumab greatly impact the relapse rate. Time until PR was significantly shorter in patients who relapse in our series, which is consistent with previous studies.8,9 Patients who respond faster will initiate tapering earlier and overall receive less treatment with omalizumab. The reduction in treatment duration may prevent the disease from self-limiting, leading to a higher risk of relaps.9 Although omalizumab is not a disease-modifying drug, the proportion of patients that remained asymptomatic after its suspension increases with repeated courses of treatment.21 Indeed, the variables associated with the duration of treatment with omalizumab were the most relevant in the tree of classification and remained statistically significant after Cox regression. Therefore, we propose achieving a sustained CR for 12 months at a standard dosage of omalizumab followed by a gradual tapering regimen over 18 months, as the best approach to minimize the recurrence rate.
Multiple molecular biomarkers have been studied in CSU, being the total baseline IgE the most notorious. An elevated baseline total IgE is associated with a fast response to omalizumab and the elevated 4-week serum IgE/baseline serum IgE ratio is, to date, the best predictor of good response.18 Small studies have associated an elevated baseline IgE with higher risk of recurrence, with a cut-off around 100–150KU/L, albeit larger studies have not reproduced this finding.3,10–12 We observed higher baseline IgE levels in patients who relapsed, which did not reach statistical significance in the univariate analysis but did in the Cox regression.
The inflammatory response is closely involved in CSU pathogenesis, with one third of patients presenting an elevated serum level of CRP. This biomarker is associated with disease severity and refractoriness to antihistamines.22 There is not enough evidence to support this molecule as a biomarker of poor response to omalizumab.18 Notably, a recent study found that elevated levels of CRP are associated with better tolerance to optimized doses.23 Based on this last study, we observed a higher CRP in patients who sustained the CR vs patients who relapsed. This result remained statistically significant after Cox regression, suggesting a low CRP as a predictor of recurrence.
Finally, other lab test results including baseline eosinophil count, erythrocyte sedimentation rate, thyroid stimulating hormone, anti-thyroid peroxidase or anti-thyroglobulin antibodies did not differ across groups nor have been previously reported as biomarkers.18
ConclusionsIn conclusion, the most important predictive factors for relapse in our study were associated with the duration of treatment at different doses of omalizumab along with the baseline CRP and total IgE. Patients with an elevated CRP, low IgE and prolonged treatment at license dosage are the most prone to maintain disease control at 12 months. In this regard, we propose ≥12 months of standard dosage followed by a prolonged tapering regimen as the best method to minimize the risk of recurrence. Furthermore, if a patient relapses the last optimized dosage that achieved CR could be reintroduced.
The main limitations of our study are its retrospective design, the inclusion of patients who received prior optimization only, a small sample size and the absence of data beyond 12 months. Further studies are required to assess our findings and the efficacy profile of the proposed algorithm.
Institutional review board statementThe study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of GRUPO HOSPITALARIO QUIRÓNSALUD-CATALUNYA (protocol code 2023/03-DER-HUSC, 29/08/2023).
Informed consent statementPatient consent was waived due to the retrospective nature of the study and the use of anonymized aggregated data without any identifying information about the patients.
FundingNone.
Conflicts of interestG. Melé-Ninot has been a Medical Advisor for Abbvie, Leo Pharma, Lilly, Sanofi, and Novartis. Educational activities for Almirall, Avène, Abbvie, Laboratorio Reig Jofre, Leo Pharma, Lilly, Meda, Novartis, Sanofi, and Uriage.
V. Expósito-Serrano has been a medical advisor and/or speaker and/or has received research funds from Abbvie, Lilly, LEO Pharma, Novartis and Sanofi Genzyme.
M. Bonfill-Ortí has been a Medical Advisor and/or a speaker for Leo Pharma, Abbvie, Lilly, Novartis, Sanofi Genzyme, Roche and Sun Pharma.
A.M. Giménez-Arnau is or recently has been a speaker and/or advisor for and/or has received research funds from Almirall, Amgen, AstraZeneca, Avene, Celldex, Escient Pharmaceutials, Genentech, GSK, Instituto Carlos III-FEDER, Leo Pharma, Menarini, Mitsubishi Tanabe Pharma, Novartis, Sanofi-Regeneron, Servier, Thermo Fisher Scientific, Uriach Pharma, Noucor.
Ribó P. has been a Medical Advisor and/or a speaker and/or received research fund from Sanofi and Novartis.
J. Spertino has been a medical advisor and/or a speaker and/or has received research funds from Abbie, Lilly, Leo Pharma, Novartis, Sanofi Genzyme and Noucor.
The remaining authors declared no conflicts of interest whatsoever.
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.