Vitamin D deficiency associates with the risk of developing many diseases, including cancer. At the molecular level, vitamin D appears to have an antineoplastic effect. However, the role of vitamin D deficiency in cancer pathogenesis remains unelucidated and numerous studies have resulted in discordant results. This study aimed to determine whether vitamin D deficiency during melanoma diagnosis increases the risk of developing non-cutaneous second primary cancers (SPC).
Materials and methodsA retrospective study on 663 patients diagnosed with melanoma between 1 January 2011 and 31 October 2022. The effect of each variable on the development of a subsequent non-cutaneous cancer was performed using Kaplan–Meier curves and differences were assessed by log-rank tests. Cox proportional hazard univariate and multivariate models were used to quantify the effect of each variable in the time to develop a non-cutaneous neoplasia.
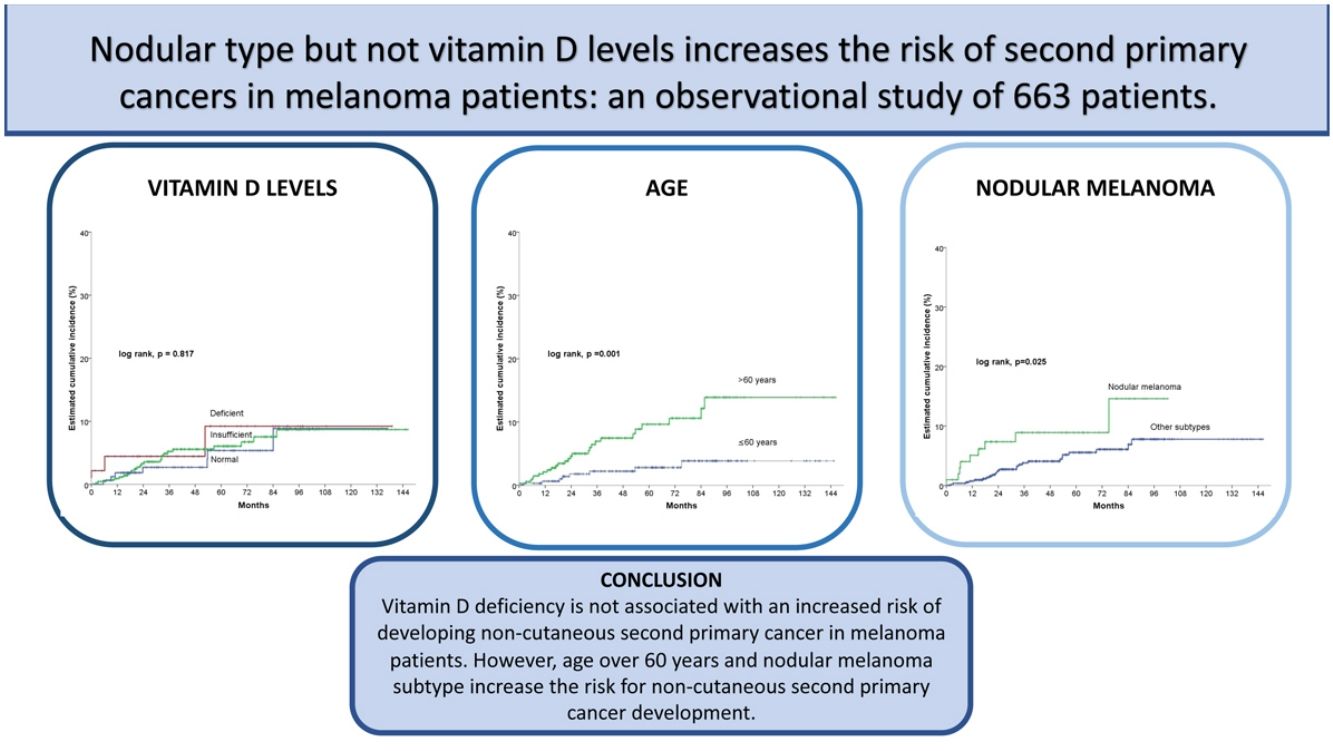
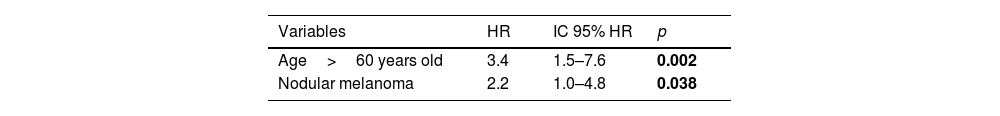
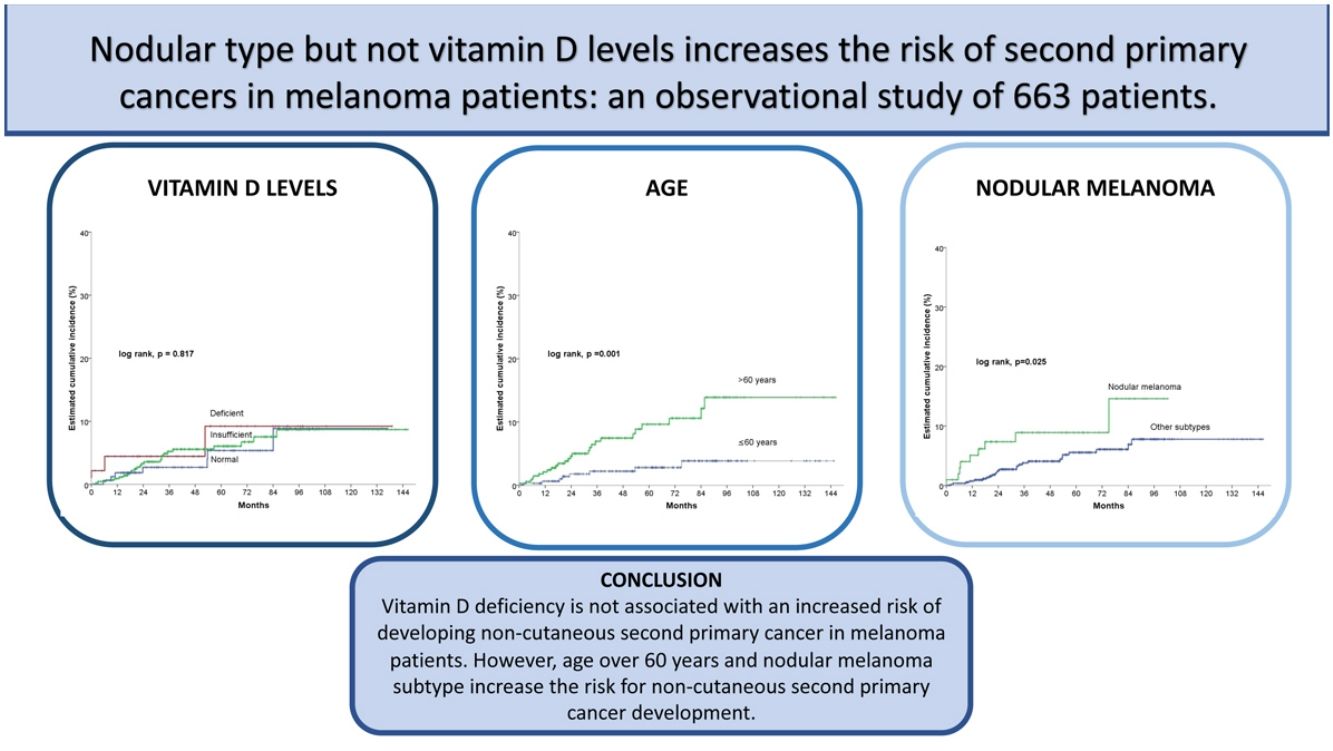
ResultsOut of 663 patients, 34 developed a non-cutaneous SPC. There was no statistically significant association between vitamin D levels and non-cutaneous SPC development (log-rank, p=0.761). Age>60 years, stage III/IV, and nodular melanoma subtype were significantly associated with the development of a SPC. After multivariate analysis, only age>60 years (HR 3.4; HR CI 95%: 1.5–7.6) and nodular melanoma subtype (HR 2.2; HR CI 95%: 1.0–4.8) were included in the final model.
ConclusionsOur results suggest that vitamin D deficiency is not associated with an increased risk of developing non-cutaneous SPC in melanoma patients. However, age over 60 years and nodular melanoma subtype increase the risk for non-cutaneous SPC development.
El déficit de vitamina D se asocia con un mayor riesgo de padecer varias enfermedades, incluido el cáncer. Molecularmente, esta parece tener un efecto antineoplásico. Sin embargo, el papel que juega en la patogénesis del cáncer no está bien esclarecido y hay resultados dispares en los estudios publicados. El objetivo del presente fue determinar si unos niveles de vitamina D deficientes en el momento del diagnóstico del melanoma aumentaba el riesgo de desarrollar un cáncer no cutáneo (CNC).
Material y métodoSe diseñó un estudio retrospectivo de 663 pacientes diagnosticados de melanoma entre el 1 de enero de 2011 y el 31 de octubre de 2022. El efecto de cada una de las variables seleccionadas en el desarrollo de un CNC durante el seguimiento tras el diagnóstico del melanoma se realizó mediante el estudio de supervivencia con el método de Kaplan-Meier y las diferencias se evaluaron con la prueba de los rangos logarítmicos. Se elaboraron modelos uni y multivariados de riesgos proporcionales de Cox para cuantificar el efecto de cada valor de las variables de estudio en el tiempo para desarrollar un CNC.
ResultadosDe los 663 pacientes, 34 desarrollaron un CNC tras el melanoma. No hubo diferencias estadísticamente significativas entre los grupos definidos por los niveles de vitamina D (log-rank, p = 0,761). Sin embargo, una edad > 60, el estadio III/IV, y el tipo nodular se asociaron significativamente al desarrollo de un CNC. Tras el análisis multivariado, solo la edad > 60 (hazard ratio [HR] 3,4; intervalo de confianza [IC] 95% HR:1,5-7,6) y el subtipo nodular de melanoma (HR 2,2; IC 95% HR:1,0-4,8) se mantuvieron en el modelo predictivo final.
ConclusionesNuestros resultados sugieren que unos niveles de vitamina D deficientes en el diagnóstico de melanoma no se asocian a un mayor riesgo de desarrollar un CNC. Sin embargo, en una edad > 60 y el subtipo nodular sí que aumentan el riesgo de desarrollar un CNC.
The role of vitamin D in calcium metabolism and bone homeostasis is well known, and its deficiency is a cause of rickets and osteoporosis. Vitamin D deficiency is associated with the risk of developing multiple pathologies, as it can cause cardiovascular diseases, multiple sclerosis, rheumatoid arthritis, type I diabetes mellitus, and cancer.1
Most of the nucleated cells express the vitamin D receptor. Numerous tissues besides the kidney express 1-alpha-hydroxylase, the enzyme responsible for the conversion of vitamin D into its active form, 1,25-(OH)2D3, which acts at the genomic level by binding to the VDR/RXR receptor and participating in multiple molecular pathways involved in immunomodulation, proliferation, differentiation and cellular apoptosis.2
In recent decades, epigenomic and transcriptomic analyses, as well as numerous experimental studies in different types of cancer, have provided reliable data that, taken together, indicate a protective action of vitamin D against several types of cancer.3,4
Vitamin D appears to exert antineoplastic effects both directly, by controlling the differentiation, proliferation, and apoptosis of neoplastic cells, and indirectly, by regulating the stromal and immune cells of the tumor microenvironment5 and thus decreasing the risk of metastasis.4,6
Vitamin D deficiency in tumor pathogenesis, however, remains contentious. Numerous studies have reported the relationship between vitamin D deficiency and the development of different neoplasms, with contradictory results. Observational epidemiological studies showed that a low vitamin D status is a risk factor for different types of cancer and suggest that vitamin D3 supplementation could prevent cancer development. However, randomized controlled clinical trials have not been able to confirm these findings for the general population.5
A 2021 review showed that, although observational studies indicate in many cases that low vitamin D levels are associated with an increased risk of cancer, randomized studies show that vitamin supplementation slightly reduces total cancer mortality but not cancer incidence.7
In our experience, vitamin D deficiency was associated with more aggressive melanomas.8,9 Moreover, in a recent systematic review, vitamin D deficiency, established as levels<20ng/mL, has been associated with a greater Breslow thickness and a worse prognosis in patients diagnosed with melanoma and, on the contrary, did not associate with an increased incidence of melanoma.10
Patients who survive a melanoma are known to have an increased risk of developing a second primary malignant non-cutaneous cancer.11
In 2022, an analysis of 120,299 patients from the SEER database showed an increased incidence of secondary primary cancers (SPCs) in patients with non-acral cutaneous melanoma, acral lentiginous melanoma, mucosal melanoma, and uveal melanoma compared with the general population.12
The evidence for an association between vitamin D deficiency and non-cutaneous SPC in patients diagnosed with melanoma is uncertain. Therefore, we believe this issue needs further investigation, as there are not enough studies to provide scientific evidence that vitamin D deficiency at the time of melanoma diagnosis is a risk factor for developing second non-cutaneous malignant neoplasms. This hypothesis is thus worth an analysis.
Materials and methodsStudy designWe conducted an analytical, observational, longitudinal, retrospective study based on a cohort of 663 patients diagnosed with melanoma from 1 January 2011 to 31 October 2022 at our center. The selection criteria were adult patients diagnosed with melanoma with available vitamin D levels and information on the development or not of non-cutaneous SPC. All the information came from the database of the center.
Study variablesThe outcome variable was the development of a non-cutaneous SPC after the diagnosis of a first cutaneous melanoma. Non-cutaneous SPC was defined as non-cutaneous cancer occurring two or more months after a melanoma diagnosis. The variable was also considered as the event for estimating cumulative incidence and for this purpose date of melanoma and date of SPC development were retrieved from the database. Patients that did not develop a SPC were censored at the date of last follow-up or death.
The main variable was the vitamin D level at the time of melanoma diagnosis, defining three categories according to their value: deficient (<10ng/mL), insufficient (10–29.99ng/mL), and normal (30–100ng/mL). We included the following characteristics in the study as covariates: gender, age (≤60 or >60 years), family history of melanoma, family history of other cancer, age at diagnosis of primary melanoma and age at diagnosis of non-cutaneous SPC, melanoma stage (I–II, III–IV), number of nevi (<20, ≥20), histological type (lentigo maligna melanoma LMM, superficial spreading melanoma SMM, nodular melanoma NM, acral melanoma AM), personal history of other non-cutaneous cancer, presence of senile angiomas, personal history of non-melanoma skin cancer (basal cell or squamous cell carcinoma), sun exposure (≤20 years, >20 years).
Statistical methodologyFor continuous variables, measures of central tendency were used, such as mean and standard deviation or median and interquartile range, depending on their distribution. On the other hand, for categorical variables, the absolute number of observations in each category and their percentage of the total number of observations were used to determine the distribution and proportion of the population. The differences between the distributions of each covariate according to patients’ groups were evaluated using contingency tables and, as a contrast test, Pearson's χ2 test or Fisher's exact test, where appropriate.
The cumulative incidence of SPC development was estimated by the Kaplan–Meier method, and univariate analysis for each variable tested by the log-rank test.
Univariate and multivariate Cox proportional hazard (PH) regression models were used to quantify the association between covariates and the development of non-cutaneous SPC. All the covariates with a p-value<0.1 in the univariate models were introduced in the backward stepwise multivariate analysis. We considered a statistically significant result for a p-value<0.05 in all tests. Statistical analysis was performed using IBM SPSS version 20.0.
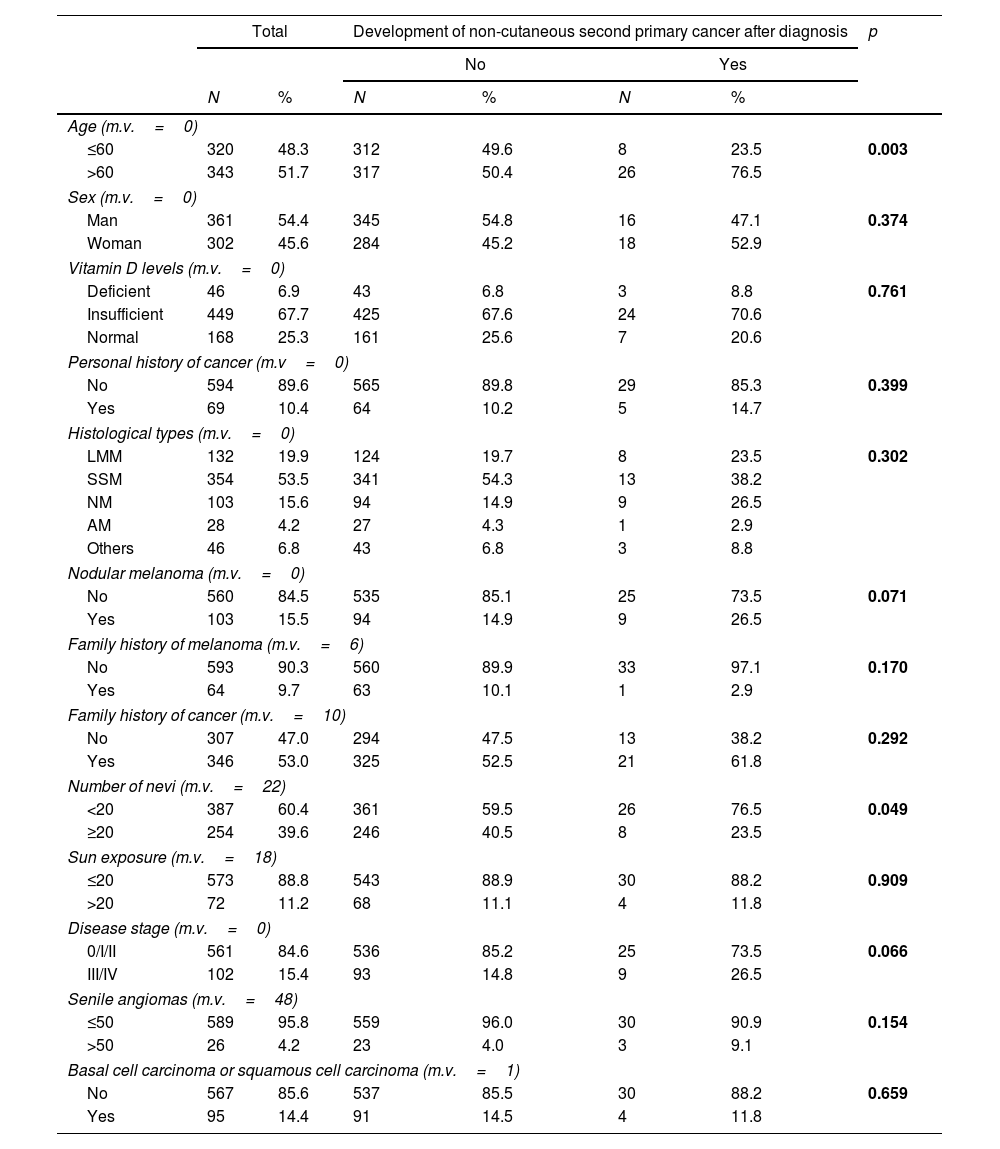
ResultsThe study included 663 patients, 34 of whom developed a non-cutaneous SPC after the diagnosis of melanoma. The patients included 361 (54.4%) men and 302 (45.6%) women. The median age at diagnosis was 61 years (interquartile range=48–72 years). Forty-six (6.9%) patients showed deficient levels of Vitamin D, and 449 (67.7%) showed insufficient levels.
Five hundred sixty-one patients (84.6%) had localized melanoma (stages 0, I, and II), and 102 (15.4%) had metastasis at diagnosis (stages III or IV). More than 50 senile angiomas were present in 26 patients (4.2%), and a personal history of non-melanoma skin cancer in 95 patients (4.4%).
The characteristics of the population and contingency tables of the study groups are detailed in Table 1.
Characteristics of the study population.
| Total | Development of non-cutaneous second primary cancer after diagnosis | p | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| N | % | N | % | N | % | ||
| Age (m.v.=0) | |||||||
| ≤60 | 320 | 48.3 | 312 | 49.6 | 8 | 23.5 | 0.003 |
| >60 | 343 | 51.7 | 317 | 50.4 | 26 | 76.5 | |
| Sex (m.v.=0) | |||||||
| Man | 361 | 54.4 | 345 | 54.8 | 16 | 47.1 | 0.374 |
| Woman | 302 | 45.6 | 284 | 45.2 | 18 | 52.9 | |
| Vitamin D levels (m.v.=0) | |||||||
| Deficient | 46 | 6.9 | 43 | 6.8 | 3 | 8.8 | 0.761 |
| Insufficient | 449 | 67.7 | 425 | 67.6 | 24 | 70.6 | |
| Normal | 168 | 25.3 | 161 | 25.6 | 7 | 20.6 | |
| Personal history of cancer (m.v=0) | |||||||
| No | 594 | 89.6 | 565 | 89.8 | 29 | 85.3 | 0.399 |
| Yes | 69 | 10.4 | 64 | 10.2 | 5 | 14.7 | |
| Histological types (m.v.=0) | |||||||
| LMM | 132 | 19.9 | 124 | 19.7 | 8 | 23.5 | 0.302 |
| SSM | 354 | 53.5 | 341 | 54.3 | 13 | 38.2 | |
| NM | 103 | 15.6 | 94 | 14.9 | 9 | 26.5 | |
| AM | 28 | 4.2 | 27 | 4.3 | 1 | 2.9 | |
| Others | 46 | 6.8 | 43 | 6.8 | 3 | 8.8 | |
| Nodular melanoma (m.v.=0) | |||||||
| No | 560 | 84.5 | 535 | 85.1 | 25 | 73.5 | 0.071 |
| Yes | 103 | 15.5 | 94 | 14.9 | 9 | 26.5 | |
| Family history of melanoma (m.v.=6) | |||||||
| No | 593 | 90.3 | 560 | 89.9 | 33 | 97.1 | 0.170 |
| Yes | 64 | 9.7 | 63 | 10.1 | 1 | 2.9 | |
| Family history of cancer (m.v.=10) | |||||||
| No | 307 | 47.0 | 294 | 47.5 | 13 | 38.2 | 0.292 |
| Yes | 346 | 53.0 | 325 | 52.5 | 21 | 61.8 | |
| Number of nevi (m.v.=22) | |||||||
| <20 | 387 | 60.4 | 361 | 59.5 | 26 | 76.5 | 0.049 |
| ≥20 | 254 | 39.6 | 246 | 40.5 | 8 | 23.5 | |
| Sun exposure (m.v.=18) | |||||||
| ≤20 | 573 | 88.8 | 543 | 88.9 | 30 | 88.2 | 0.909 |
| >20 | 72 | 11.2 | 68 | 11.1 | 4 | 11.8 | |
| Disease stage (m.v.=0) | |||||||
| 0/I/II | 561 | 84.6 | 536 | 85.2 | 25 | 73.5 | 0.066 |
| III/IV | 102 | 15.4 | 93 | 14.8 | 9 | 26.5 | |
| Senile angiomas (m.v.=48) | |||||||
| ≤50 | 589 | 95.8 | 559 | 96.0 | 30 | 90.9 | 0.154 |
| >50 | 26 | 4.2 | 23 | 4.0 | 3 | 9.1 | |
| Basal cell carcinoma or squamous cell carcinoma (m.v.=1) | |||||||
| No | 567 | 85.6 | 537 | 85.5 | 30 | 88.2 | 0.659 |
| Yes | 95 | 14.4 | 91 | 14.5 | 4 | 11.8 | |
m.v.: missing values.
LMM: lentigo maligna melanoma; SSM: superficial spreading melanoma; MN: nodular melanoma; AM: acral melanoma.
p-value <0.05
When analyzing the distribution of the independent variables studied in the groups defined by the presence or absence of a non-cutaneous SPC, we found that the group that had developed a non-cutaneous SPC had a significantly higher proportion of patients older than 60 years (76.5% vs. 50.4%; p=0.003) and patients with 20 or fewer nevi (76.5% vs. 59.5%; p=0.049), and a trend for presenting with a nodular melanoma (26.5% vs. 14.9%; p=0.071) and an advanced stage (III/IV) (26.5% vs. 14.8%; p=0.066).
There were no statistically significant differences found for the variables like sex, vitamin D levels, personal history of cancer, histological type, family history of melanoma, family history of cancer, sun exposure, senile angiomas and history of basal cell or epidermoid carcinoma.
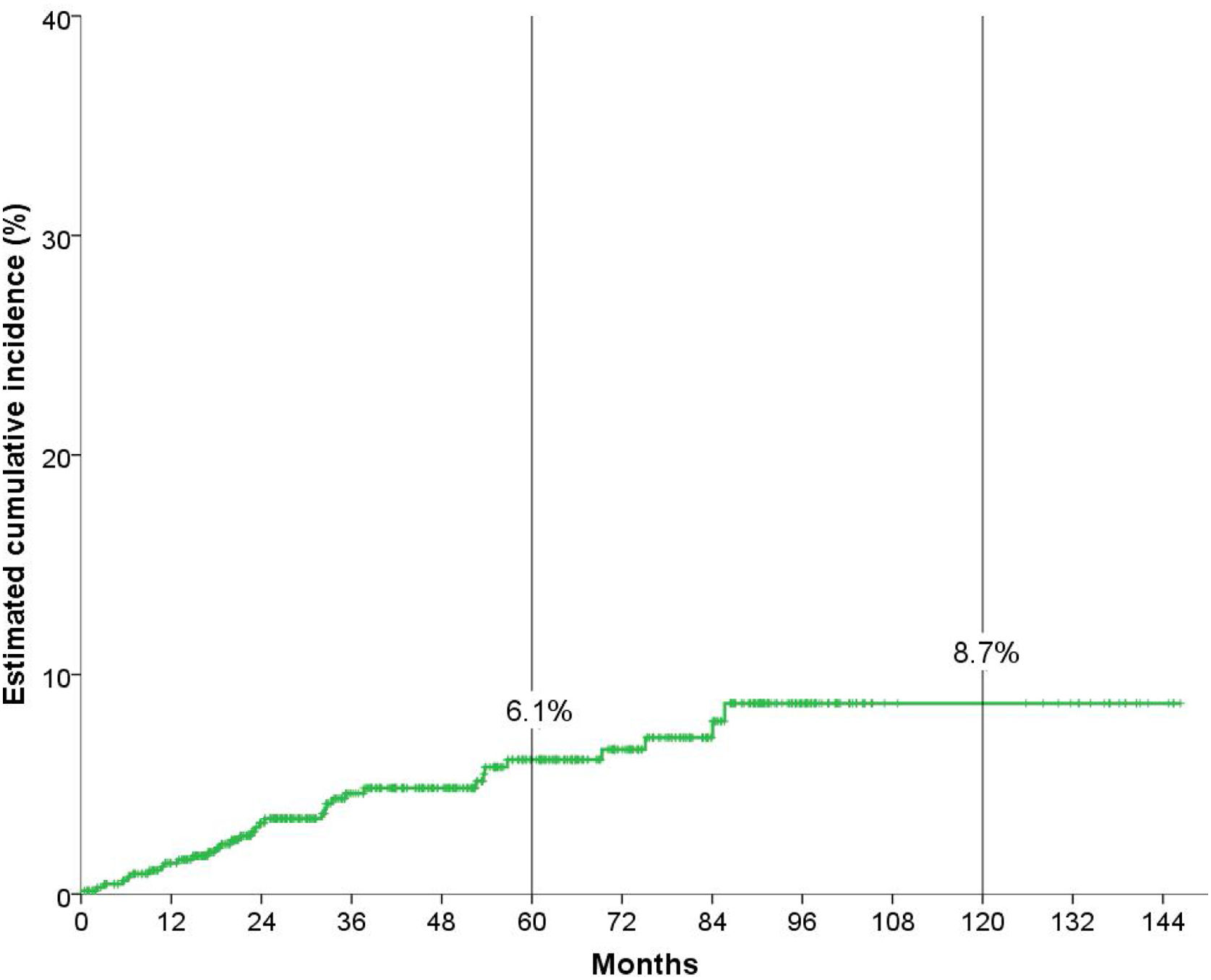
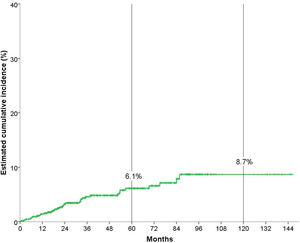
Cumulative incidence analysisAfter a median follow-up of 51.3 months, 34 (5.1%) patients developed a non-cutaneous SPC. Fig. 1 shows that the overall cumulative incidence of non-cutaneous SPC at five years was 6.1%, and at ten years, 8.7%.
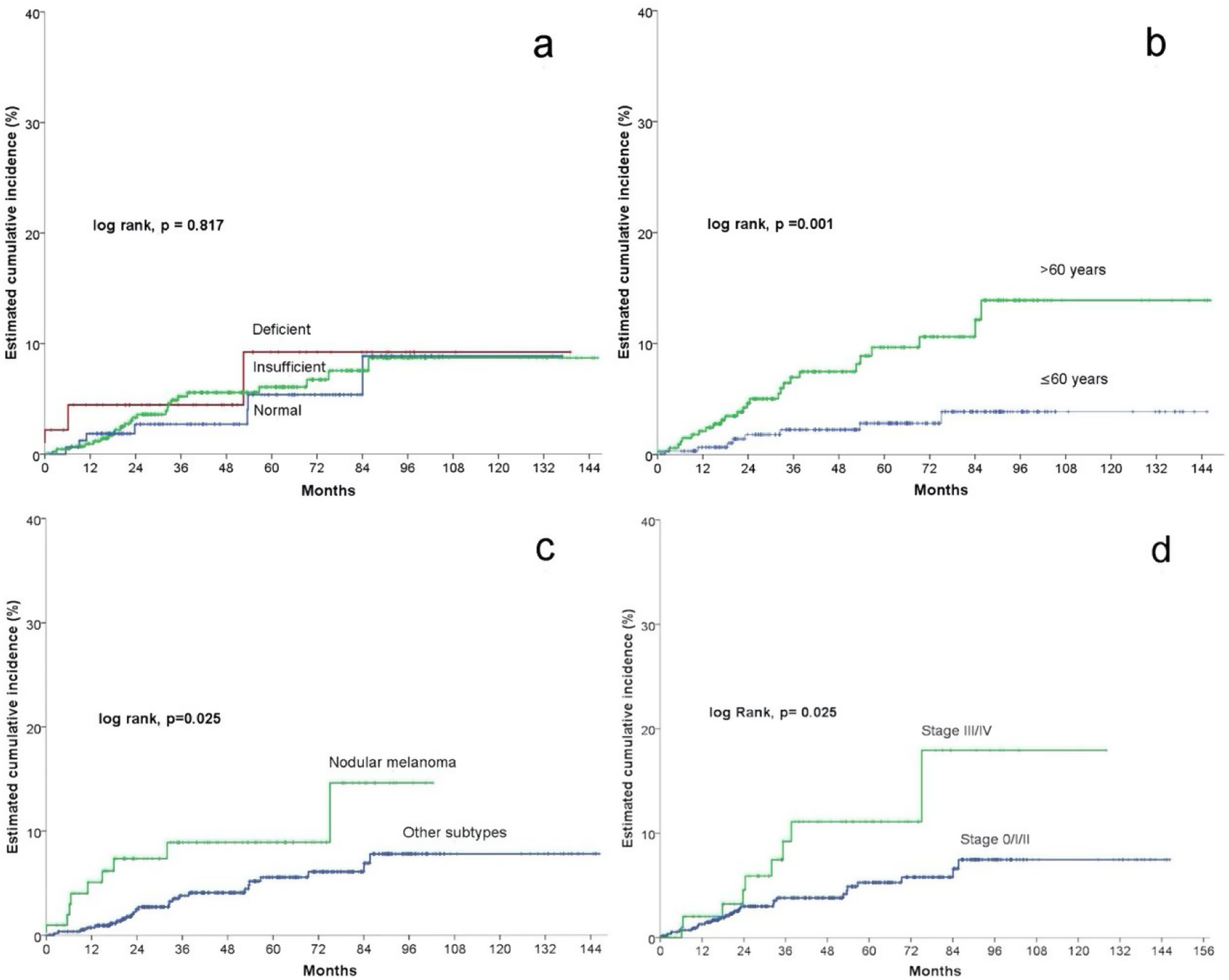
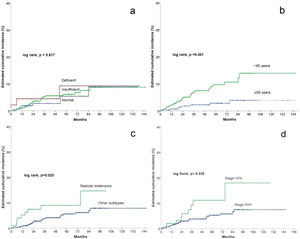
In the univariate Cox statistical analysis, 5- and 10-years estimated cumulative incidence of non-cutaneous SPC was of 9.2% for patients with deficient vitamin D levels, 6.1% and 8.7% for patients with insufficient levels, respectively, and 5.4 and 8.9% in patients with sufficient levels, respectively, showing that Vitamin D levels did not significantly differ in survival time for the development of non-cutaneous SPC (log-rank, p=0.817) (Fig. 2a). Among the remaining variables, significant differences were observed for age, number of nevi, nodular melanoma, and stage III/IV (Table 2).
Univariate survival model for cumulative incidence (Cox regression).
| Covariates | Cumulative incidence at five years | Cumulative incidence at ten years | Log rank, p |
|---|---|---|---|
| Total | 6.1% | 8.7% | – |
| Vitamin D levels | |||
| Deficient | 9.2% | 9.2% | 0.817 |
| Insufficient | 6.1% | 8.7% | |
| Normal | 5.4% | 8.9% | |
| Age | |||
| ≤60 | 2.8% | 3.9% | 0.001 |
| >60 | 9.6% | 13.9% | |
| Number of nevi | |||
| <20 | 8.5% | 10.9% | 0.038 |
| ≥20 | 3.3% | 6.1% | |
| Nodular melanoma | |||
| No | 5.6% | 7.8% | 0.025 |
| Sí | 8.9% | 14.6% | |
| Disease stage | |||
| 0/I/II | 5.3% | 7.5% | 0.025 |
| III/IV | 11.1% | 17.9% | |
Patients older than 60 had a significantly higher incidence of non-cutaneous SPC (log rank, p=0.001). For patients>60 years, the cumulative incidence at 5 and 10 years was 9.6% and 13.9%, respectively. And 2.8% and 3.9% for patients≤60 years (Fig. 2b).
Regarding the presence of nodular melanoma (log-rank, p=0.025), the incidence in patients with nodular melanoma was 8.9% at five years and 14.6% at ten years, while patients without nodular melanoma had a cumulative incidence at 5 and 10 years of 5.6% and 7.8%, respectively (Fig. 2c).
Regarding stage (log rank, p=0.025), patients with stage III/IV had a cumulative incidence at 5 and 10 years of 11.1% and 17.9%, respectively. In contrast, stage 0/I/II patients had a 5-year cumulative incidence of 5.3% and 7.5% at ten years (Fig. 2d).
After multivariable Cox proportional hazards analysis, only age>60 years (HR 3.4; 95% CI HR 1.5–7.6; p=0.002) and nodular melanoma (HR 2.2; 95% CI HR 1.0–4.8; p=0.038) retained their significant association with the development of non-cutaneous SPC (Table 3).
DiscussionWe conducted an analytical, observational, longitudinal, and retrospective study based on a cohort of 663 patients diagnosed with melanoma to evaluate the relationship between vitamin D levels measured at diagnosis and the development of a second non-cutaneous malignancy. The results have shown that vitamin D deficiency at the time of melanoma diagnosis does not increase the risk of developing a second non-cutaneous malignant neoplasm. However, analyzing different variables such as sex, age, history of melanoma, and other neoplasms, we have observed that presenting the melanoma at an age over 60 years and nodular melanoma subtype associated with an increased risk for developing second non-cutaneous malignant neoplasms.
Although some studies had previously associated younger age at melanoma diagnosis with an increased risk of developing second primary cancers,13 numerous recent studies have shown that older age increases the risk of multiple primary tumors, Adam C. Krajewski et al. conducted a prospective study in 222 patients aged > or =65 years diagnosed with melanoma and found that, at a median follow-up of 5 years, about 10% developed a second melanoma, and 4% developed three or more melanomas, highlighting the importance of close follow-up in these patients.14 In our study, age over 60 years was statistically significantly associated with an increase in other non-cutaneous primary tumors.
The relationship between aging and cancer incidence is well established, and aging represents one of the most critical risk factors for tumor development. That age and cancer share some complex biological mechanisms, including genomic instability with progressive accumulation of genetic damage, epigenetic alterations (primarily due to prolonged exposure to environmental carcinogens), and a progressive weakening of oncosuppression mechanisms linked to immunosenescence.15,16
The association between nodular melanoma and the risk of second malignant cutaneous neoplasms is an incidental and unexpected finding in our study. Nodular melanoma is, by histologic definition, a melanoma subtype that does not show a radial growth phase and does not show intradermal melanocytic proliferation beyond three epidermal ridges at each margin of the tumor mass.17 Nodular melanoma has been associated with a worse prognosis due to a generally higher Breslow thickness at diagnosis. It exhibits inherently more aggressive biological processes than superficial spreading melanoma.18
From a molecular point of view, nodular melanoma shows a higher frequency of NRAS mutation than superficial spreading melanoma, although no differences in BRAF mutation rate have been demonstrated in this melanoma subtype. In addition, MN appears to show a lower prevalence of mutated somatic genes than SSM.17,18 Nodular melanoma also shows a higher frequency of TERT promoter mutations than other subtypes.19,20
As patients with nodular melanoma have a higher mortality rate, the risk of second malignancies would be expectedly reduced in this subgroup of patients due to the lower survival rate. However, in a 2022 study, Yen T. Luu et al., using the Surveillance, Epidemiology, and End Results (SEER) database, showed that NM survivors, compared with other histotypes, had a 27% higher risk of developing second primary malignancies.21 This result seems to align with ours, but further supporting data is currently lacking in the literature.
The possible causes are many and remain to be elucidated. Individual genetic susceptibility may play an important role in predisposing to these tumors, as may environmental or immunological risk factors.
In addition, patients with MN tend to have thicker tumors and, therefore a higher rate of treatment than patients with other types of melanoma. It cannot be excluded that therapy may play an important role in the development of SPC; however, patient therapy data were not collected in this study.
Some limitations and strengths of the present study deserve consideration. First, our study includes data from a single center, so the population studied may not represent the general population. In addition, the sample analyzed is numerically limited, as it considers 663 patients, of whom only 34 developed a second primary neoplasm. Secondly, since the vitamin D values were collected during a relatively short period, the follow-up time of the patients is also limited (51.3 months on average). As for vitamin D levels, we used the established ranges for bone metabolism, but these could vary depending on tumor development. A vitamin D cut-off point analysis was not performed. Another limitation of our study is the lack of characterization of the type of secondary primary neoplasms developed by the patients. Finally, regarding the correlation between non-cutaneous SPC and nodular melanoma, as this was an incidental and unexpected finding, we did not take into account other variables, such as the molecular characteristics, genetic substrate, and current therapies of these patients, which could provide important information and which represent to date an unexplored field worthy of further study.
On the other hand, the fact that data were obtained prospectively from a single center, with values analyzed by the same laboratory, made obtaining a homogeneous data collection possible.
In conclusion, according to our study, vitamin D deficiency is not associated with an increased risk of developing non-cutaneous SPC in melanoma patients. At the same time, age over 60 years and nodular melanoma appear to have a positive predictive value for developing non-cutaneous SPC.
Further studies are needed to characterize the relationship between NM and non-cutaneous SPC and to understand the underlying complex biological mechanisms. However, our data confirm the importance of close follow-up of these patients.
Conflict of interestsThe authors declare that they have no conflict of interest.