Psoriatic patients have shown higher levels of neutrophil extracellular traps (NETs) vs healthy controls. Psoriasis is often associated with other comorbidities such as cardiovascular disease. An increase in indirect markers of NETs has been found in patients with increased cardiovascular events in patients without psoriasis. We conducted a prospective observational study with psoriatic patients. A total of 39 of patients were included. Myeloperoxidase, neutrophil elastase and double-stranded DNA were significantly higher in patients with severe-to-moderate vs mild psoriasis. Myeloperoxidase was also significantly higher in patients of moderate high vs low cardiovascular risk (p=.01) in patients with mild psoriasis. Patients with psoriatic arthritis show higher myeloperoxidase levels higher vs those without arthritis (p=.048). Myeloperoxidase was also significantly correlated (p=.0375) with the patients’ BMI. The detection of NETs through indirect markers (myeloperoxidase, neutrophil elastase and double-stranded DNA) is associated with the severity of psoriasis. In addition, myeloperoxidase can be useful in psoriatic patients as biomarkers of comorbidities.
Los pacientes con psoriasis presentan niveles más elevados de trampas extracelulares de neutrófilos que los controles sanos. La psoriasis a menudo se asocia con otras comorbilidades como las enfermedades cardiovasculares. Se realizó un estudio prospectivo en pacientes con psoriasis. Se incluyeron 39 pacientes. La mieloperoxidasa, elastasa de neutrófilos y el ADN de doble cadena fueron significativamente más altas en los pacientes con psoriasis grave-moderada frente a leve. La mieloperoxidasa también fue significativamente mayor en pacientes con riesgo cardiovascular moderado- alto frente a bajo (p=0,01). Los pacientes con artritis psoriásica tienen niveles de mieloperoxidasa más altos en comparación con los que no tienen artritis (p=0,048). La mieloperoxidasa también se correlacionó significativamente (p=0,0375) con el Índice de Masa Corporal de los pacientes. La detección de las trampas extracelulares de neutrófilos a través de marcadores indirectos está relacionada con la gravedad de la psoriasis. Además, la mieloperoxidasa, podría ser útil como biomarcador de comorbilidades.
Psoriasis is a chronic inflammatory disease that affects almost 2% of the population.1 In addition to skin involvement, patients with psoriasis often associate other diseases such as psoriatic arthritis (PsA), metabolic syndrome, or cardiovascular disease.
Although it was initially linked to an increased prevalence of other cardiovascular risk factors, it has been hypothesized that psoriasis may behave as an independent cardiovascular risk factor, especially in young patients with severe psoriasis.2
Identifying patients with psoriasis and high cardiovascular risk would allow prevention strategies to be implemented early. To this end, in recent years multiple biomarkers3 or imaging modalities have been studied,4 the latter techniques being difficult to generalize to all patients.
Several observations have convinced researchers that T cells (and specially Th17 cells) are crucial in the pathogenesis of psoriasis. Although neutrophils are present in psoriatic lesions, their possible involvement in the pathogenesis of the disease is unclear.5 The role of innate immunity in the pathogenesis of psoriasis is becoming increasingly important, and an integrated system of innate and adaptive immunity has been suggested.6
Neutrophils are important components of innate immunity and a primary defense vs microbial infections. Neutrophil extracellular traps (NETs) were described for the first time in 2024. NETosis is a form of programmed cell death like apoptosis or necrosis, in which neutrophils, after decondensing their chromatin, expel it into the extracellular medium forming 3D chromatin networks. The presence of NETosis can be detected either directly by immunohistochemical staining in circulating tissue/cells or indirectly by detection of biomarkers in blood by ELISA.7
Although the main function of NETs was initially thought to trap and kill pathogens, NET formation has been observed during chronic inflammatory disease, autoimmune diseases, or thrombosis.8 Higher levels of NETs have been shown in other skin diseases such as hidradenitis suppurativa,9 or pyoderma gangrenosum.10
On the other hand, diseases that present an intense inflammatory cascade and immunothrombosis phenomena, such as COVID-19, may present skin rashes that have shown the presence of neutrophils, suggesting the presence of NETosis.11 In fact, it has been hypothesized that NETosis plays a role in the shared pathophysiological links between psoriasis and COVID-19 comorbidities.12
Prospective studies have found higher dsDNA levels in patients with increased cardiovascular events at the 2-year follow-up.13
Elevated levels of NETs markers such as double-stranded DNA (dsDNA) have been independently associated with severe coronary atherosclerosis.14
In atherosclerosis, both the process of atherosclerotic lesion development and atherothrombosis has been related to NETs. In murine models of atherosclerosis, blocking the formation of NETs reduced the size of plaques and the occurrence of thrombosis, suggesting a significant role of NETs in pathogenesis.15
Indirect markers of NETs, both myeloperoxidase (MPO) and neutrophil elastase (NE), are elevated in patients with plaque psoriasis vs healthy controls.16,17 In addition, Hu et al.18 found that the number of NETotic cells in peripheral blood was higher in patients with psoriasis vs healthy controls. Furthermore, the cells increased according to the severity measured with psoriasis area and severity index (PASI). However, indirect markers of NETs were not studied in this case.
No studies on the association of NETs with the comorbidities frequently associated with psoriatic patients have been reported.
Materials and methodsWe conducted a prospective observational study with psoriatic patients without systemic treatment attending our hospital dermatology department. This study was approved by our hospital Medical Ethics Committee, and all patients gave their informed consent prior to being included in the study,
We collected the patients’ demographic characteristics, and quantified the severity of psoriasis using the PASI (Psoriasis Area severity Index), considering mild psoriasis PASI <7 and moderate-to-severe psoriasis PASI ≥7,19 and the Physicians’ Global Assessment (PGA) scale (0–4), considering mild psoriasis PGA 1-2 and moderate-to-severe psoriasis, PGA 3-4. The cardiovascular risk of patients was quantified using the SCORE2 (European Society of Cardiology – ESC), and patients were categorized as mild-to-moderate and high-very high cardiovascular risk according to this scale.20
After obtaining platelet-poor plasma from venous blood samples, enzyme-linked immunosorbent assays were performed to measure the levels of three different NETs biomarkers: dsDNA, MPO and NE.
Means and standard deviations were used for normally distributed continuous variables. Medians with interquartile ranges were used for non-normally distributed continuous variables. Inferences on quantitative variables were made using the Student t test or the Mann–Whitney U test, according to normality. p-Values <0.05 were considered statistically significant.
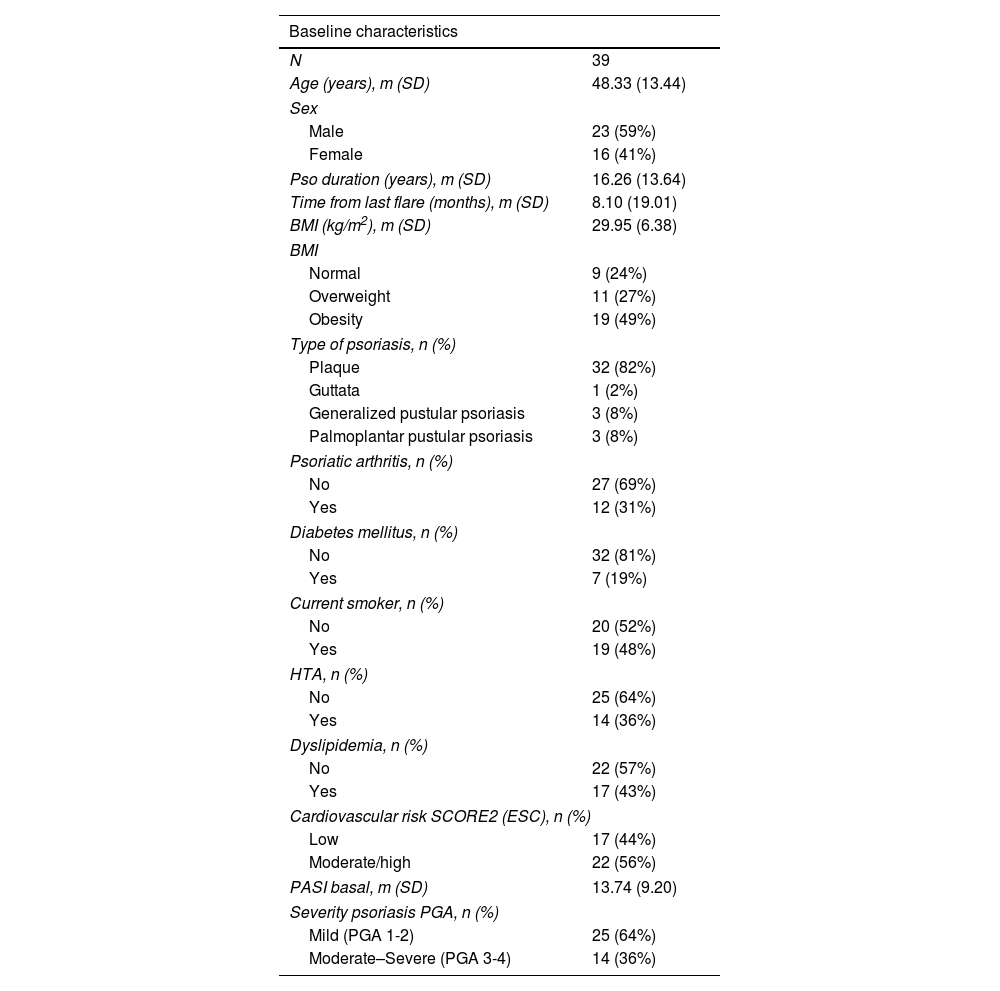
ResultsA total of 39 patients were included. Clinical characteristics are shown in Table 1.
Clinical characteristics of the patients.
| Baseline characteristics | |
|---|---|
| N | 39 |
| Age (years), m (SD) | 48.33 (13.44) |
| Sex | |
| Male | 23 (59%) |
| Female | 16 (41%) |
| Pso duration (years), m (SD) | 16.26 (13.64) |
| Time from last flare (months), m (SD) | 8.10 (19.01) |
| BMI (kg/m2), m (SD) | 29.95 (6.38) |
| BMI | |
| Normal | 9 (24%) |
| Overweight | 11 (27%) |
| Obesity | 19 (49%) |
| Type of psoriasis, n (%) | |
| Plaque | 32 (82%) |
| Guttata | 1 (2%) |
| Generalized pustular psoriasis | 3 (8%) |
| Palmoplantar pustular psoriasis | 3 (8%) |
| Psoriatic arthritis, n (%) | |
| No | 27 (69%) |
| Yes | 12 (31%) |
| Diabetes mellitus, n (%) | |
| No | 32 (81%) |
| Yes | 7 (19%) |
| Current smoker, n (%) | |
| No | 20 (52%) |
| Yes | 19 (48%) |
| HTA, n (%) | |
| No | 25 (64%) |
| Yes | 14 (36%) |
| Dyslipidemia, n (%) | |
| No | 22 (57%) |
| Yes | 17 (43%) |
| Cardiovascular risk SCORE2 (ESC), n (%) | |
| Low | 17 (44%) |
| Moderate/high | 22 (56%) |
| PASI basal, m (SD) | 13.74 (9.20) |
| Severity psoriasis PGA, n (%) | |
| Mild (PGA 1-2) | 25 (64%) |
| Moderate–Severe (PGA 3-4) | 14 (36%) |
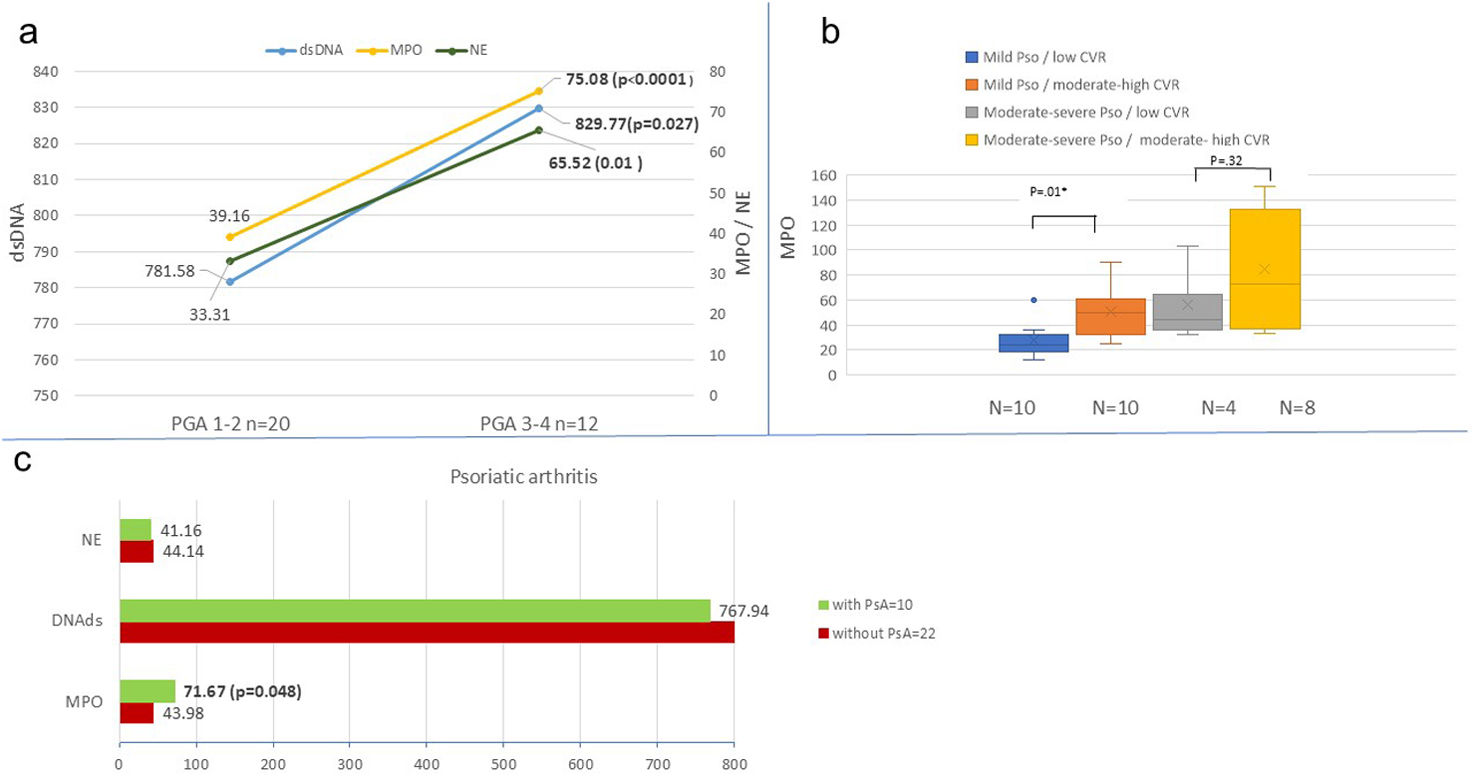
Indirect markers of NETs were not related to the severity of psoriasis according to PASI scale (p=.61) However, if the psoriasis severity was quantified with the PGA scale, MPO, NE and dsDNA were statistically significantly higher in patients with severe-to-moderate (PGA 3-4) vs mild psoriasis (PGA 1-2) (Fig. 1). Indirect markers were not related to body surface area (BSA) either.
Indirect markers of neutrophil extracellular traps (NETs) in psoriasis. (a) Indirect NET markers by psoriasis severity (PGA): This panel shows indirect markers of NETs – myeloperoxidase (MPO, yellow), neutrophilic elastase (NE, green), and double-stranded DNA (dsDNA, blue) – in patients with plaque psoriasis. Data are stratified by disease severity using the Physician's Global Assessment (PGA): mild psoriasis (PGA 1-2) vs moderate-to-severe psoriasis (PGA 3-4). Means and p-values are displayed, with ‘n’ indicating the number of patients in each group. (B) Myeloperoxidase (MPO) levels by psoriasis severity and cardiovascular risk (SCORE2): This box plot illustrates MPO levels in plaque psoriasis patients, categorized by both psoriasis severity (PGA) and cardiovascular risk (CVR) as assessed by SCORE2. Groups are defined as: mild psoriasis with low CVR (blue), mild psoriasis with moderate-to-high CVR (orange), moderate-to-severe psoriasis with moderate-to-high CVR (yellow), and moderate-to-severe psoriasis with low CVR (grey). (C) Indirect NET markers by psoriatic arthritis (PsA) association: This panel presents means and p-values for MPO, NE, and dsDNA in plaque psoriasis patients. Data are differentiated based on the presence (green) or absence (red) of psoriatic arthritis (PsA).
Patients with moderate high cardiovascular risk (CVR) have significantly higher MPO (p=.0076) vs low, as well as higher neutrophil elastase yet not statistically significant (p=.0548).
MPO was also significantly higher in patients with moderate high vs low CVR (p=.01) in patients with mild psoriasis (PGA 1-2). However, no differences were found in patients with PGA 3-4.
Patients with psoriasis and associated psoriatic arthritis have MPO levels higher (71.67) vs those without arthritis 43.98 (p=.048). The characteristics of both groups with and without associated arthritis were compared, without finding any significant differences in any variable (severity, cardiovascular risk, …).
MPO was also significantly correlated (p=.0375) with the patients’ BMI. The remaining comorbidities and CVR factors, such as smoking, diabetes mellitus, dyslipidemia were not related to any of the indirect markers.
DiscussionBased on our results, the detection of NETs through indirect markers (MPO, NE, dsDNA) may be linked to severity on the PGA scale, not the PASI scale. Former studies had not found any associations of indirect markers of NETs with severity.16,21
In our study we found that several patients with severe localized variants of psoriasis and low PASI score had similar levels of NETs in blood as patients with very extensive forms and high PASI score.
We consider the detection of NETs as an indirect marker of the inflammatory intensity of skin lesions. However, NET levels might not specifically be related to the affected body surface area.
The difference found between both severity scales reinforces the possibility of underestimating the severity of the disease with PASI scale in localized psoriasis variants or those whose affected body surface area is minimal, which corresponds to low PASI score. However, these patients might exhibit moderate or intense inflammation. In these cases, NETs can behave as a biomarker that may play an interesting role in therapeutic decision-making.
In addition, in our patients we have also observed that the presence of psoriatic arthritis (PsA) was associated with higher levels of MPO. These data are consistent with former studies that had also found higher levels of other indirect markers of NETs in patients with PsA (such as DNA–MPO complexes).22 Since our sample is limited, we have not been able to establish MPO cut-off point indicating the presence of arthritis.
Regarding CVR, MPO could behave as a biomarker of CVR, at least, in patients with mild psoriasis (PGA 1-2). The role of NETs as a biomarker of CVR in other conditions such as gout has been studied, without finding differences.23 However, its possible association with psoriatic patients had not been studied, or at least published to this date.
Based on our study, we consider that indirect markers of NETs, especially MPO, may be useful in psoriatic patients as biomarkers of comorbidities such us PsA and CVR, at least in patients with mild psoriasis.
Furthermore, these indirect markers may help clinicians in the therapeutic decision-making especially by identifying patients with other comorbidities or patients with intense inflammation despite presenting a minimal body surface area affected.
Our study has several limitations, including its small sample size. To confirm these preliminary findings, larger studies are needed that can account for cardiovascular risk factors. Additionally, current cardiovascular risk (CVR) scales are not specifically designed for psoriasis patients, highlighting a need for future research to establish reference values and cut-off points. Prospective studies with more patients and longer follow-up periods are also essential to investigate how systemic psoriasis treatments might alter NET levels.
Conflict of interestThe authors declare that they have no conflict of interest.