JAK inhibitors target specific inflammatory cytokines involved in various inflammatory diseases. Four molecules have been approved for dermatological use: upadacitinib, baricitinib, abrocitinib and topical ruxolitinib. Off-label prescriptions for other dermatological conditions have been reported. We conducted a narrative review of the literature to assess the long-term safety profile of currently approved JAK inhibitors in dermatology, and their off-label use in skin disorders. We performed literature searches with Pubmed and Google Scholar from January 2000 to January 2023, using the keywords “Janus kinase inhibitors”, “JAK inhibitors”, “off-label”, “dermatology”, “safety”, “adverse events”, “ruxolitinib”, “upadacitinib”, “abrocitinib” and “baricitinib”. Our search yielded a total of 37 dermatological disorders with studies supporting the use of these JAK inhibitors. Preliminary studies indicate that JAK inhibitors generally have a favorable safety profile and can be considered as an option in many dermatological disorders.
Los inhibidores de JAK actúan bloqueando la acción de ciertas citoquinas inflamatorias involucradas en varias enfermedades inflamatorias. Cuatro moléculas han sido aprobadas para uso en dermatología: upadacitinib, baricitinib, abrocitinib y ruxolitinib tópico. Se han reportado usos fuera de indicación para diferentes enfermedades dermatológicas. Se realizó una revisión narrativa de la literatura sobre la seguridad a largo plazo de los inhibidores de JAK aprobados en dermatología y su uso fuera de indicación en enfermedades dermatológicas, mediante búsquedas bibliográficas en Pubmed y Google Scholar desde enero de 2000 hasta enero de 2023, incluyendo las palabras clave: «Janus kinase inhibitors», «JAK inhibitors», «off-label», «dermatology», «safety», «adverse events», «ruxolitinib», «upadacitinib», «abrocitinib» y «baricitinib». Se encontraron un total de 37 trastornos dermatológicos con estudios que respaldan el uso de estos fármacos. Los estudios preliminares indican que los inhibidores de JAK tienen un perfil de seguridad generalmente favorable y pueden considerarse una opción en muchas enfermedades dermatológicas.
In recent years, the field of dermatology has witnessed significant advancements with the development of multiple biological drugs and small molecules that selectively target specific molecules within the immune system. One particularly noteworthy signaling pathway, implicated in both innate and adaptative immunity, is the JAK–STAT pathway. The JAK–STAT pathway involves intracellular tyrosine kinases called Janus kinases (JAKs), which are comprised of four isoforms: JAK1, JAK2, JAK3, and TYK2. JAK inhibitors act by reversibly inhibiting JAK phosphorylation through occupation of the catalytic ATP-binding site.1 While more selective JAK inhibitors may avoid adverse events associated with non-desired JAK isoforms, the long-term safety implications of this selectivity remain unclear.2
Oral upadacitinib and abrocitinib (selective JAK 1 inhibitors), and oral baricitinib and topical ruxolitinib (JAK1/2 inhibitors) have been approved by the Food and Drug Administration (FDA) and/or the European Medicines Agency (EMA) for several dermatologic indications (Table 1). In this study, we aim to review the long-term safety profile of these JAK inhibitors in dermatology and describe their off-label use in skin disorders.
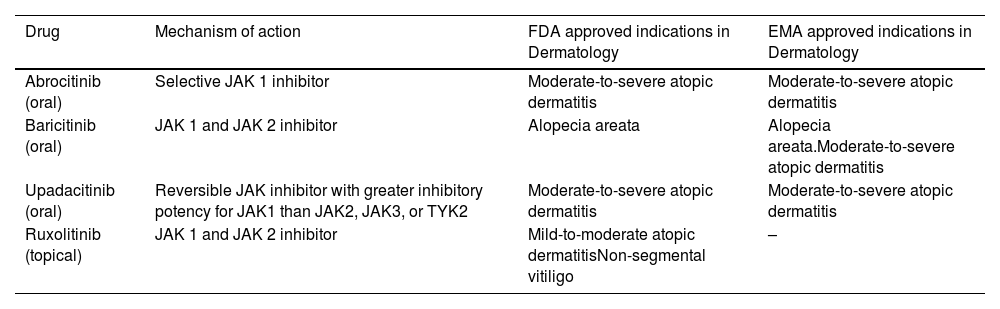
Mechanism of action and approved indications for JAK inhibitors in Dermatology.
| Drug | Mechanism of action | FDA approved indications in Dermatology | EMA approved indications in Dermatology |
|---|---|---|---|
| Abrocitinib (oral) | Selective JAK 1 inhibitor | Moderate-to-severe atopic dermatitis | Moderate-to-severe atopic dermatitis |
| Baricitinib (oral) | JAK 1 and JAK 2 inhibitor | Alopecia areata | Alopecia areata.Moderate-to-severe atopic dermatitis |
| Upadacitinib (oral) | Reversible JAK inhibitor with greater inhibitory potency for JAK1 than JAK2, JAK3, or TYK2 | Moderate-to-severe atopic dermatitis | Moderate-to-severe atopic dermatitis |
| Ruxolitinib (topical) | JAK 1 and JAK 2 inhibitor | Mild-to-moderate atopic dermatitisNon-segmental vitiligo | – |
Abbreviations: FDA, Food and Drugs Administration; EMA, European Medicines Agency; JAK, Janus kinase; TYK2, tyrosine kinase 2.
A narrative review of the literature was carried out. We performed literature searches with Pubmed and Google Scholar from January 2000 to January 2023 using the keywords “Janus kinase inhibitors”, “JAK inhibitors”, “off-label”, “dermatology”, “safety”, “adverse events”, “ruxolitinib”, “upadacitinib”, “abrocitinib”, “baricitinib”. We also included cutaneous inflammatory diseases in the search strategy: “granuloma annulare”, “histiocytosis”, “sarcoidosis”, “morphea”, “livedoid vasculopathy”, “Sweet syndrome”, “VEXAS syndrome”, “hypereosinophilic syndrome”, “Kimura disease”, “acrodermatitis continua of Hallopeau”, “erythema multiforme”, “DRESS syndrome”, “Steven Johnson syndrome”, “toxic epidermal necrolysis”, “autoinflammatory diseases”, “panniculitis”, “cutaneous vasculitis”, “cutaneous lupus”, “lichen planus”, “graft versus host disease”, “Still disease”, “necrobiosis lipoidica”, “chronic nodular prurigo”. The search strategy included clinical trials, meta-analyses, observational studies, case series and case reports, and was restricted to English and Spanish language articles.
ResultsLong-term safety profileJAK inhibitors are commonly associated with various adverse events. These include cytopenias, urinary and upper respiratory tract infections, herpes virus reactivation, nausea, diarrhea, headache, alteration of liver function tests, hypercholesterolemia and increase in creatine phosphokinase (CPK).3 More serious and rare adverse events comprise thromboembolic events, reactivation of hepatitis B virus (HBV), disseminated tuberculosis, gastrointestinal perforation (particularly tofacitinib4), and solid cancers.3 Rare dermatologic adverse events include non-melanoma skin cancer, disseminated molluscum contagiosum, and drug-induced reactions.5
On September 1st, 2021, the Food and Drug Administration (FDA) reviewed the results of the post-marketing safety trial that compared tofacitinib with tumor necrosis factor alpha (TNF-α) inhibitors in rheumatoid arthritis. The study involved patients aged ≥50 years who were concurrently treated with methotrexate and had preexisting cardiovascular risk factors. It was concluded that tofacitinib posed an increased risk of major cardiovascular events (MACE), blood clots, malignancies, and death. Based on these findings, the FDA issued a Boxed Warning, which was also extended to other JAK inhibitors that had not been evaluated in similar clinical trials.6 However, a study analyzing an extensive dataset of 126,815 adverse events reports associated with the use of JAK inhibitors failed to identify any statistically significant increase in major cardiovascular events.4 Furthermore, two meta-analyses investigating JAK inhibitors in inflammatory diseases and atopic dermatitis reported a similar incidence of venous thromboembolism compared to controls.7,8
Data from randomized controlled trials (RCTs) suggest that certain adverse effects may act in a dose-dependent manner, due to off-target effects.9
AbrocitinibIn a study evaluating the long-term incidence rates of serious adverse events from a cohort of the integrated safety analysis study for abrocitinib with 2856 patients and 1614 person years (PY), abrocitinib at doses of 100mg and 200mg showed 0.6 and 0.4 non-melanoma skin cancers/100PY; 0.6 and 0.2 MACE events/100PY, and 0.0 and 0.4 venous thromboembolic events (VTE)/100PY, respectively. Other malignancies (excluding non-melanoma skin cancer) occurred at a rate of 0.2/100PY.6,10 Dose-related adverse events included mainly nausea, headache, acne, and thrombocytopenia. Incidence rates were 2.65/100PY and 2.33/100PY for serious infection, 2.04/100PY and 4.34/100PY for herpes zoster, and 8.73/100PY and 11.83/100PY for herpes simplex in the 100mg and 200mg groups, respectively. Three deaths were reported, attributed to gastric carcinoma, sudden death, and COVID-19.10
In adolescents with atopic dermatitis, the safety of oral abrocitinib has been evaluated in a phase 3 placebo-controlled RCT, demonstrating a lower incidence of serious adverse events compared to the placebo group.11 A network meta-analysis in atopic dermatitis showed that abrocitinib 100mg was related to more serious adverse events than dupilumab (OR 2.6).12 An analysis of platelet counts from data obtained from five clinical trials of abrocitinib reported a higher risk of thrombocytopenia in the first 4 weeks of treatment in patients with low baseline platelet counts.13
BaricitinibThe incidence of severe adverse events associated with baricitinib aligns with the inherent risk posed by the specific disease population being treated. Rheumatologic diseases are commonly associated with a higher prevalence of MACE, VTE, malignancies, serious infections, and herpes zoster. Conversely, cases of herpes simplex are more frequently reported among patients with atopic dermatitis.14
In a pooled safety analysis of 8 RCTs of baricitinib in 2531 patients with atopic dermatitis, the overall rate of treatment-emergent adverse events was higher in patients under baricitinib compared to those on placebo. The adjusted incidence rate for serious infections was 3.0/100PY and 1.5/100PY for baricitinib 4mg and 2mg daily, respectively. Two cases of MACE were reported in patients receiving baricitinib 2mg and two cases of VTE were observed in those receiving the 4mg dose. There was one death in the baricitinib 4mg group, due to gastrointestinal bleeding. Common laboratory-related adverse events were increased CPK, hyperlipidemia, and mild hematologic, hepatic, and renal alterations.15 The extended safety analysis of baricitinib 2mg showed similar results.16
Among 1303 patients with alopecia areata included in an integrated safety analysis, the most frequent treatment-emergent adverse events were upper respiratory tract infection, nasopharyngitis, headache, acne and elevated CPK. The analysis identified 34 cases of herpes zoster, three malignancies (excluding non-melanoma skin cancer), one opportunistic infection, one myocardial infarction, one pulmonary embolism, and one gastrointestinal perforation.17
Regarding psoriasis, baricitinib underwent a phase 2b clinical trial (n=271), with comparable safety reports.18
UpadacitinibA meta-analysis from 2 RCT was conducted to assess the long-term incidence rates of adverse events in patients with atopic dermatitis. The results indicated that upadacitinib at doses of 15mg and 30mg demonstrated lower and similar rates of malignancies, respectively, compared to the overall incidence rate of all malignancies in the United States population. Upadacitinib also exhibited low rates of non-melanoma skin cancer (0.4events/100PY), MACE (0.0–0.1events/100PY) and VTE (0.1events/100PY).6 In both RCT, the incidence of serious adverse events was similar among groups. The most frequently observed treatment-emergent adverse effects (TEAE) included acne, upper respiratory tract infection, elevation in CPK levels, and atopic dermatitis.19 Additional RCTs conducted in patients with atopic dermatitis reported a similarly favorable safety profile.20–22 One study showed slightly higher rates of serious infection (1.1% vs 0.6%), eczema herpeticum (0.3% vs 0%), herpes zoster (2.0% vs 0.9%), and laboratory-related adverse events in patients who received upadacitinib compared to those who received dupilumab.20 Placebo-controlled trials yielded similar results, although the increased risk of herpes zoster and serious infections was not consistent in every study.21,22
A meta-analysis conducted in patients with psoriatic arthritis showed that a daily dose of upadacitinib at 30mg was associated with a relative risk of adverse events of 1.20 compared to placebo, while a daily dose of 15mg did not reach statistical significance.23 Although another meta-analysis on the safety profile of upadacitinib demonstrated similar rates of TEAE in patients with atopic dermatitis and those with rheumatologic conditions, serious TEAE, herpes zoster and elevations in creatin phosphokinase were less frequent in patients with atopic dermatitis. However, higher rates of acne were observed in patients with atopic dermatitis. The same study concluded that upadacitinib was associated with a higher risk of herpes zoster, non-melanoma skin cancer, and elevation of CPK when compared with methotrexate and adalimumab.24
Topical ruxolitinibTopical ruxolitinib is generally well-tolerated with adverse effects mainly restricted to local skin reactions (application-site pain, erythema, exfoliation, folliculitis, pruritus).25,26 No systemic toxicity has been reported. Interestingly, in a double-blind study of ruxolitinib 0.5% or 1.0% cream daily or 1.5% cream twice daily in psoriasis, no inhibition of phosphorylated STAT3 was observed in blood cells, and low steady-state plasma concentrations of ruxolitinib were detected.27 A study in atopic dermatitis estimated that systemic exposure corresponded to approximately 4–5% of the dose applied.28 In two phase 3 RCTs in atopic dermatitis (n=1251), a lower rate of application site reactions compared to vehicle was reported.29
Off-label use of JAK inhibitors in skin disordersAbrocitinib (Table 2)Alopecia areataA teenage female with atopic dermatitis and alopecia areata universalis received abrocitinib 200mg/day, following hair regrowth after 12 weeks. Terminal hairs were noted on several areas at week 52.30
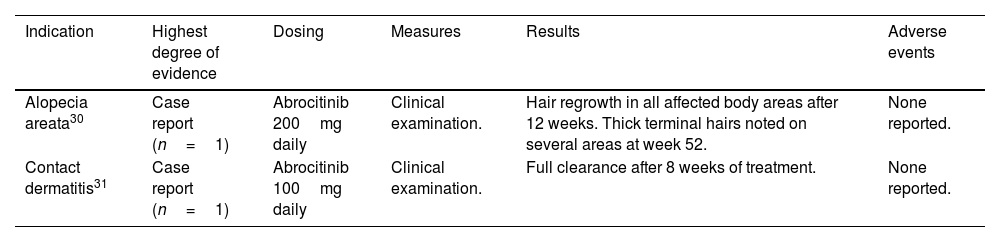
Off-label use of abrocitinib in dermatology.
| Indication | Highest degree of evidence | Dosing | Measures | Results | Adverse events |
|---|---|---|---|---|---|
| Alopecia areata30 | Case report (n=1) | Abrocitinib 200mg daily | Clinical examination. | Hair regrowth in all affected body areas after 12 weeks. Thick terminal hairs noted on several areas at week 52. | None reported. |
| Contact dermatitis31 | Case report (n=1) | Abrocitinib 100mg daily | Clinical examination. | Full clearance after 8 weeks of treatment. | None reported. |
An adult patient treated with abrocitinib 100mg/day for an occupational airborne allergic contact dermatitis reached full clearance after 8 weeks.31
Baricitinib (Table 3)Autoinflammatory disorders with cutaneous manifestationsOral baricitinib has been tested in certain autoinflammatory disorders. Clinical improvement was observed in series and/or reports of patients with CANDLE syndrome,32 CANDLE-like syndrome,32 Aicardi-Goutières,32 SAVI,32 GOF mutations of STAT1′,32 refractory Blau syndrome33 and systemic juvenile idiopathic arthritis.34 In VEXAS syndrome, a retrospective multicenter study showed that baricitinib and upadacitinib led to poorer outcomes than oral ruxolitinib.35 Two case series (n=3 and n=2) of adult Still's disease showed complete (40%) or partial resolution (20%)34 with baricitinib 4mg/day, and a case of clinical remission after associating baricitinib to anakinra and corticoids was reported.36
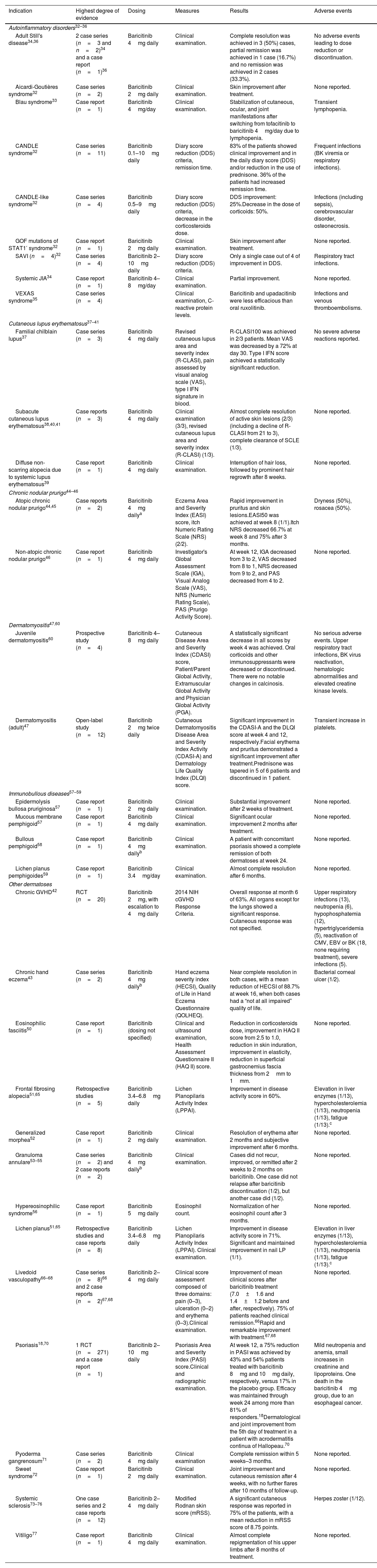
Off-label use of baricitinib in dermatology.
| Indication | Highest degree of evidence | Dosing | Measures | Results | Adverse events |
|---|---|---|---|---|---|
| Autoinflammatory disorders32–36 | |||||
| Adult Still's disease34,36 | 2 case series (n=3 and n=2)34 and a case report (n=1)36 | Baricitinib 4mg daily | Clinical examination. | Complete resolution was achieved in 3 (50%) cases, partial remission was achieved in 1 case (16.7%) and no remission was achieved in 2 cases (33.3%). | No adverse events leading to dose reduction or discontinuation. |
| Aicardi-Goutières syndrome32 | Case series (n=2) | Baricitinib 2mg daily | Clinical examination. | Skin improvement after treatment. | None reported. |
| Blau syndrome33 | Case report (n=1) | Baricitinib 4mg/day | Clinical examination. | Stabilization of cutaneous, ocular, and joint manifestations after switching from tofacitinib to baricitinib 4mg/day due to lymphopenia. | Transient lymphopenia. |
| CANDLE syndrome32 | Case series (n=11) | Baricitinib 0.1–10mg daily | Diary score reduction (DDS) criteria, remission time. | 83% of the patients showed clinical improvement and in the daily diary score (DDS) and/or reduction in the use of prednisone. 36% of the patients had increased remission time. | Frequent infections (BK viremia or respiratory infections). |
| CANDLE-like syndrome32 | Case series (n=4) | Baricitinib 0.5–9mg daily | Diary score reduction (DDS) criteria, decrease in the corticosteroids dose. | DDS improvement: 25%.Decrease in the dose of corticoids: 50%. | Infections (including sepsis), cerebrovascular disorder, osteonecrosis. |
| GOF mutations of STAT1′ syndrome32 | Case report (n=1) | Baricitinib 2mg daily | Clinical examination. | Skin improvement after treatment. | None reported. |
| SAVI (n=4)32 | Case series (n=4) | Baricitinib 2–10mg daily | Diary score reduction (DDS) criteria. | Only a single case out of 4 of improvement in DDS. | Respiratory tract infections. |
| Systemic JIA34 | Case report (n=1) | Baricitinib 4–8mg/day | Clinical examination. | Partial improvement. | None reported. |
| VEXAS syndrome35 | Case series (n=4) | Clinical examination, C-reactive protein levels. | Baricitinib and upadacitinib were less efficacious than oral ruxolitinib. | Infections and venous thromboembolisms. | |
| Cutaneous lupus erythematosus37–41 | |||||
| Familial chilblain lupus37 | Case series (n=3) | Baricitinib 4mg daily | Revised cutaneous lupus area and severity index (R-CLASI), pain assessed by visual analog scale (VAS), type I IFN signature in blood. | R-CLASI100 was achieved in 2/3 patients. Mean VAS was decreased by a 72% at day 30. Type I IFN score achieved a statistically significant reduction. | No severe adverse reactions reported. |
| Subacute cutaneous lupus erythematosus38,40,41 | Case reports (n=3) | Baricitinib 4mg daily | Clinical examination (3/3), revised cutaneous lupus area and severity index (R-CLASI) (1/3). | Almost complete resolution of active skin lesions (2/3) (including a decline of R-CLASI from 21 to 3), complete clearance of SCLE (1/3). | None reported. |
| Diffuse non-scarring alopecia due to systemic lupus erythematosus39 | Case report (n=1) | Baricitinib 4mg daily | Clinical examination. | Interruption of hair loss, followed by prominent hair regrowth after 8 weeks. | None reported. |
| Chronic nodular prurigo44–46 | |||||
| Atopic chronic nodular prurigo44,45 | Case reports (n=2) | Baricitinib 4mg dailya | Eczema Area and Severity Index (EASI) score, itch Numeric Rating Scale (NRS) (2/2). | Rapid improvement in pruritus and skin lesions.EASI50 was achieved at week 8 (1/1).Itch NRS decreased 66.7% at week 8 and 75% after 3 months. | Dryness (50%), rosacea (50%). |
| Non-atopic chronic nodular prurigo46 | Case report (n=1) | Baricitinib 4mg daily | Investigator's Global Assessment Scale (IGA), Visual Analog Scale (VAS), NRS (Numeric Rating Scale), PAS (Prurigo Activity Score). | At week 12, IGA decreased from 3 to 2, VAS decreased from 8 to 1, NRS decreased from 9 to 2, and PAS decreased from 4 to 2. | None reported. |
| Dermatomyositis47,60 | |||||
| Juvenile dermatomyositis60 | Prospective study (n=4) | Baricitinib 4–8mg daily | Cutaneous Disease Area and Severity Index (CDASI) score, Patient/Parent Global Activity, Extramuscular Global Activity and Physician Global Activity (PGA). | A statistically significant decrease in all scores by week 4 was achieved. Oral corticoids and other immunosuppressants were decreased or discontinued. There were no notable changes in calcinosis. | No serious adverse events. Upper respiratory tract infections, BK virus reactivation, hematologic abnormalities and elevated creatine kinase levels. |
| Dermatomyositis (adult)47 | Open-label study (n=12) | Baricitinib 2mg twice daily | Cutaneous Dermatomyositis Disease Area and Severity Index Activity (CDASI-A) and Dermatology Life Quality Index (DLQI) score. | Significant improvement in the CDASI-A and the DLQI score at week 4 and 12, respectively.Facial erythema and pruritus demonstrated a significant improvement after treatment.Prednisone was tapered in 5 of 6 patients and discontinued in 1 patient. | Transient increase in platelets. |
| Immunobullous diseases57–59 | |||||
| Epidermolysis bullosa pruriginosa57 | Case report (n=1) | Baricitinib 2mg daily | Clinical examination. | Substantial improvement after 2 weeks of treatment. | None reported. |
| Mucous membrane pemphigoid57 | Case report (n=1) | Baricitinib 4mg daily | Clinical examination. | Significant ocular improvement 2 months after treatment. | None reported. |
| Bullous pemphigoid58 | Case report (n=1) | Baricitinib 4mg dailyb | Clinical examination. | A patient with concomitant psoriasis showed a complete remission of both dermatoses at week 24. | None reported. |
| Lichen planus pemphigoides59 | Case report (n=1) | Baricitinib 3.4mg/day | Clinical examination. | Almost complete resolution after 6 months. | None reported. |
| Other dermatoses | |||||
| Chronic GVHD42 | RCT (n=20) | Baricitinib 2mg, with escalation to 4mg daily | 2014 NIH cGVHD Response Criteria. | Overall response at month 6 of 63%. All organs except for the lungs showed a significant response. Cutaneous response was not specified. | Upper respiratory infections (13), neutropenia (6), hypophosphatemia (12), hypertriglyceridemia (5), reactivation of CMV, EBV or BK (18, none requiring treatment), severe infections (5). |
| Chronic hand eczema43 | Case series (n=2) | Baricitinib 4mg dailyb | Hand eczema severity index (HECSI), Quality of Life in Hand Eczema Questionnaire (QOLHEQ). | Near complete resolution in both cases, with a mean reduction of HECSI of 88.7% at week 16, when both cases had a “not at all impaired” quality of life. | Bacterial corneal ulcer (1/2). |
| Eosinophilic fasciitis50 | Case report (n=1) | Baricitinib (dosing not specified) | Clinical and ultrasound examination, Health Assessment Questionnaire II (HAQ II) score. | Reduction in corticosteroids dose, improvement in HAQ II score from 2.5 to 1.0, reduction in skin induration, improvement in elasticity, reduction in superficial gastrocnemius fascia thickness from 2mm to 1mm. | None reported. |
| Frontal fibrosing alopecia51,65 | Retrospective studies (n=5) | Baricitinib 3.4–6.8mg daily | Lichen Planopilaris Activity Index (LPPAI). | Improvement in disease activity score in 60%. | Elevation in liver enzymes (1/13), hypercholesterolemia (1/13), neutropenia (1/13), fatigue (1/13).c |
| Generalized morphea52 | Case report (n=1) | Baricitinib 2mg daily | Clinical examination. | Resolution of erythema after 2 months and subjective improvement after 6 months. | None reported. |
| Granuloma annulare53–55 | Case series (n=2) and 2 case reports (n=2) | Baricitinib 4mg dailyb | Clinical examination. | Cases did not recur, improved, or remitted after 2 weeks to 2 months on baricitinib. One case did not relapse after baricitinib discontinuation (1/2), but another case did (1/2). | None reported. |
| Hypereosinophilic syndrome56 | Case report (n=1) | Baricitinib 5mg daily | Eosinophil count. | Normalization of her eosinophil count after 3 months. | None reported. |
| Lichen planus51,65 | Retrospective studies and case reports (n=8) | Baricitinib 3.4–6.8mg daily | Lichen Planopilaris Activity Index (LPPAI). Clinical examination. | Improvement in disease activity score in 71%. Significant and maintained improvement in nail LP (1/1). | Elevation in liver enzymes (1/13), hypercholesterolemia (1/13), neutropenia (1/13), fatigue (1/13).c |
| Livedoid vasculopathy66–68 | Case series (n=8)66 and 2 case reports (n=2)67,68 | Baricitinib 2–4mg daily | Clinical score assessment composed of three domains: pain (0–3), ulceration (0–2) and erythema (0–3).Clinical examination. | Improvement of mean clinical scores after baricitinib treatment (7.0±1.6 and 1.4±1.2 before and after, respectively). 75% of patients reached clinical remission.66Rapid and remarkable improvement with treatment.67,68 | None reported. |
| Psoriasis18,70 | 1 RCT (n=271) and a case report (n=1) | Baricitinib 2–10mg daily | Psoriasis Area and Severity Index (PASI) score.Clinical and radiographic examination. | At week 12, a 75% reduction in PASI was achieved by 43% and 54% patients treated with baricitinib 8mg and 10mg daily, respectively, versus 17% in the placebo group. Efficacy was maintained through week 24 among more than 81% of responders.18Dermatological and joint improvement from the 5th day of treatment in a patient with acrodermatitis continua of Hallopeau.70 | Mild neutropenia and anemia, small increases in creatinine and lipoproteins. One death in the baricitinib 4mg group, due to an esophageal cancer. |
| Pyoderma gangrenosum71 | Case series (n=2) | Baricitinib 4mg daily | Clinical examination | Complete remission within 5 weeks–3 months. | None reported. |
| Sweet syndrome72 | Case report (n=1) | Baricitinib 2mg daily | Clinical examination. | Joint improvement and cutaneous remission after 4 weeks, with no further flares after 10 months of follow-up. | None reported. |
| Systemic sclerosis73–76 | One case series and 2 case reports (n=12) | Baricitinib 2–4mg daily | Modified Rodnan skin score (mRSS). | A significant cutaneous response was reported in 75% of the patients, with a mean reduction in mRSS score of 8.75 points. | Herpes zoster (1/12). |
| Vitiligo77 | Case report (n=1) | Baricitinib 4mg daily | Clinical examination. | Almost complete repigmentation of his upper limbs after 8 months of treatment. | None reported. |
Abbreviations: JAK, Janus kinase; SAVI, STING-associated vasculopathy with onset in infancy; JIA, juvenile idiopathic arthritis; RCT, randomized controlled trial; LP, lichen planus; GVHD, graft versus host disease.
We found a case series and 4 case reports under baricitinib 4mg/day. Patients with familial chilblain lupus showed improvement of cutaneous lupus lesions after 3 months.37 Complete clearance with concomitant frontal fibrosing alopecia stabilization,38 systemic lupus erythematosus-associated alopecia improvement39 and near complete resolution of subacute cutaneous lupus erythematosus lesions40,41 were achieved in case reports.
Chronic graft versus host disease (cGVHD)A phase 1/2 RCT of baricitinib in cGVHD (n=20), including 19 cases with sclerotic cGVHD, demonstrated an overall response at month 6 of 63% with 88% durable responses.42
Chronic hand eczemaA case series (n=2) with baricitinib 4mg/day showed a near complete resolution after 16 weeks.43
Chronic nodular prurigoBaricitinib 4mg/day led to rapid improvement in pruritus and prurigo lesions in two case reports of patients with an atopic predisposition,44,45 and in a patient with non-atopic chronic nodular prurigo.46
Dermatomyositis (adult form)An open-label study (n=12) showed that baricitinib 2mg/12h decreased the disease activity and improved the Dermatology Life Quality Index (DLQI) score.47 A case series (n=3)48 and a case report49 of baricitinib 4mg/day also documented promising results.
Eosinophilic fasciitisIn an adult male with refractory eosinophilic fasciitis, baricitinib reduced skin induration and corticoids use, and improved cutaneous elasticity.50
Frontal fibrosing alopeciaIn a retrospective study (n=5), baricitinib improved 60% of patients with frontal fibrosing alopecia.51
Generalized morpheaA male with refractory generalized morphea was treated with baricitinib 2mg/day, with improvement after 6 months.52
Granuloma annulareAll patients from a case series (n=2) and two case reports experienced improvement or remission following 2–8 weeks on baricitinib 3–4mg/day.53–55
Hypereosinophilic syndromeA 39-year-old female with hypereosinophilic syndrome presenting with eosinophilic vasculitis on her fingers normalized eosinophil count after three months of baricitinib.56
Immunobullous diseasesCase reports have documented the use of baricitinib in the treatment of various disorders, including epidermolysis bullosa pruriginosa, ocular mucous membrane pemphigoid, bullous pemphigoid, and lichen planus pemphigoides. The reported outcomes varied from significant improvement to complete resolution.57–59
Juvenile dermatomyositis (JDM)Baricitinib (4–8mg/day) significantly reduced disease activity in refractory JDM (n=4) from week 4 in a prospective study.60 Retrospective studies (n=15 and n=3) reported cutaneous improvement in all JDM patients, including calcinosis stabilization, partial regression, and complete remission.61,62 Case reports demonstrated improved cutaneous and muscular symptoms,63 as well as reductions in calcinosis.64
Lichen planus (LP)In a retrospective study (n=7), the use of baricitinib demonstrated improvement in 71% of patients with LP pilaris.51 A woman with severe nail LP65 experienced significant and sustained improvement with baricitinib.
Livedoid vasculopathyA case series (n=8) of baricitinib 2mg/day for refractory livedoid vasculopathy showed statistically significant improvement in disease activity. Clinical remission was achieved in 6 cases.66 Two case reports showed rapid67 and remarkable improvement with baricitinib 4mg/day.68
PsoriasisIn a 12-week dose-ranging phase 2b RCT (n=271), a 75% reduction in Psoriasis Area and Severity Index (PASI) was achieved by 43–54% patients treated with baricitinib.18 A network meta-analysis showed lower efficacy of baricitinib compared to tofacitinib.69 A 28-year-old female with Acrodermatits Continua of Hallopeau showed a rapid and maintained skin and joint symptoms remission with baricitinib 2mg/day.70
Pyoderma gangrenosumBaricitinib 4mg/day led to a complete response in a case series (n=2) of refractory pyoderma gangrenosum on the lower leg and scalp.71
Sweet syndromeA 59-year-old female with refractory rheumatoid arthritis-associated Sweet syndrome improved her joint and cutaneous symptoms after 4 weeks with baricitinib.72
Systemic sclerosisA case series (n=10)73 and 2 case reports investigated the use of baricitinib in systemic sclerosis.74,75 Significant cutaneous response was observed in nine patients.76
VitiligoA 67-year-old man with vitiligo affecting both hands and forearms received baricitinib 4mg/day for rheumatoid arthritis, showing repigmentation after 8 months.77
Upadacitinib (Table 4)Alopecia areataFour case reports (n=4) demonstrated hair regrowth with upadacitinib 15–30mg/day. In three cases, this regimen also improved a concurrent severe atopic dermatitis.78–81
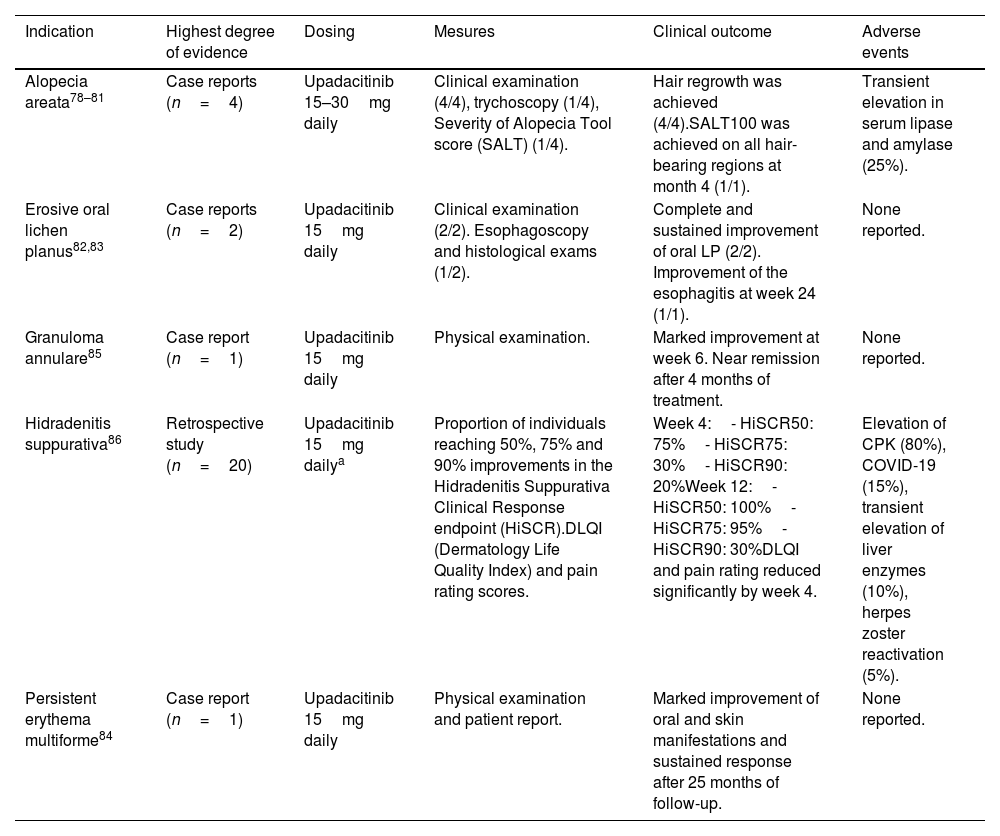
Off-label use of upadacitinib in dermatology.
| Indication | Highest degree of evidence | Dosing | Mesures | Clinical outcome | Adverse events |
|---|---|---|---|---|---|
| Alopecia areata78–81 | Case reports (n=4) | Upadacitinib 15–30mg daily | Clinical examination (4/4), trychoscopy (1/4), Severity of Alopecia Tool score (SALT) (1/4). | Hair regrowth was achieved (4/4).SALT100 was achieved on all hair-bearing regions at month 4 (1/1). | Transient elevation in serum lipase and amylase (25%). |
| Erosive oral lichen planus82,83 | Case reports (n=2) | Upadacitinib 15mg daily | Clinical examination (2/2). Esophagoscopy and histological exams (1/2). | Complete and sustained improvement of oral LP (2/2). Improvement of the esophagitis at week 24 (1/1). | None reported. |
| Granuloma annulare85 | Case report (n=1) | Upadacitinib 15mg daily | Physical examination. | Marked improvement at week 6. Near remission after 4 months of treatment. | None reported. |
| Hidradenitis suppurativa86 | Retrospective study (n=20) | Upadacitinib 15mg dailya | Proportion of individuals reaching 50%, 75% and 90% improvements in the Hidradenitis Suppurativa Clinical Response endpoint (HiSCR).DLQI (Dermatology Life Quality Index) and pain rating scores. | Week 4:- HiSCR50: 75%- HiSCR75: 30%- HiSCR90: 20%Week 12:- HiSCR50: 100%- HiSCR75: 95%- HiSCR90: 30%DLQI and pain rating reduced significantly by week 4. | Elevation of CPK (80%), COVID-19 (15%), transient elevation of liver enzymes (10%), herpes zoster reactivation (5%). |
| Persistent erythema multiforme84 | Case report (n=1) | Upadacitinib 15mg daily | Physical examination and patient report. | Marked improvement of oral and skin manifestations and sustained response after 25 months of follow-up. | None reported. |
Abbreviations: CPK, creatin phosphokinase; LP, lichen planus.
A 45-year-old woman with erosive oral LP and psoriatic arthritis,82 and a 59-year-old woman with refractory erosive oral and esophageal LP83 received upadacitinib 15mg daily. Drastic and sustained improvement of the oral lesions was observed in both cases.
Erythema multiformeA female in her 30s with persistent erythema multiforme showed significant improvement with upadacitinib 15mg/day.84
Granuloma annulareA woman in her 60s with refractory patch-type granuloma annulare showed a near-complete remission with upadacitinib 15mg/day.85
Hidradenitis suppurativaA retrospective study (n=20) of moderate-to-severe hidradenitis suppurativa treated with upadacitinib showed significant improvement in hidradenitis suppurativa clinical response (HiSCR), DLQI and pain rating scores from week 4 of therapy.86
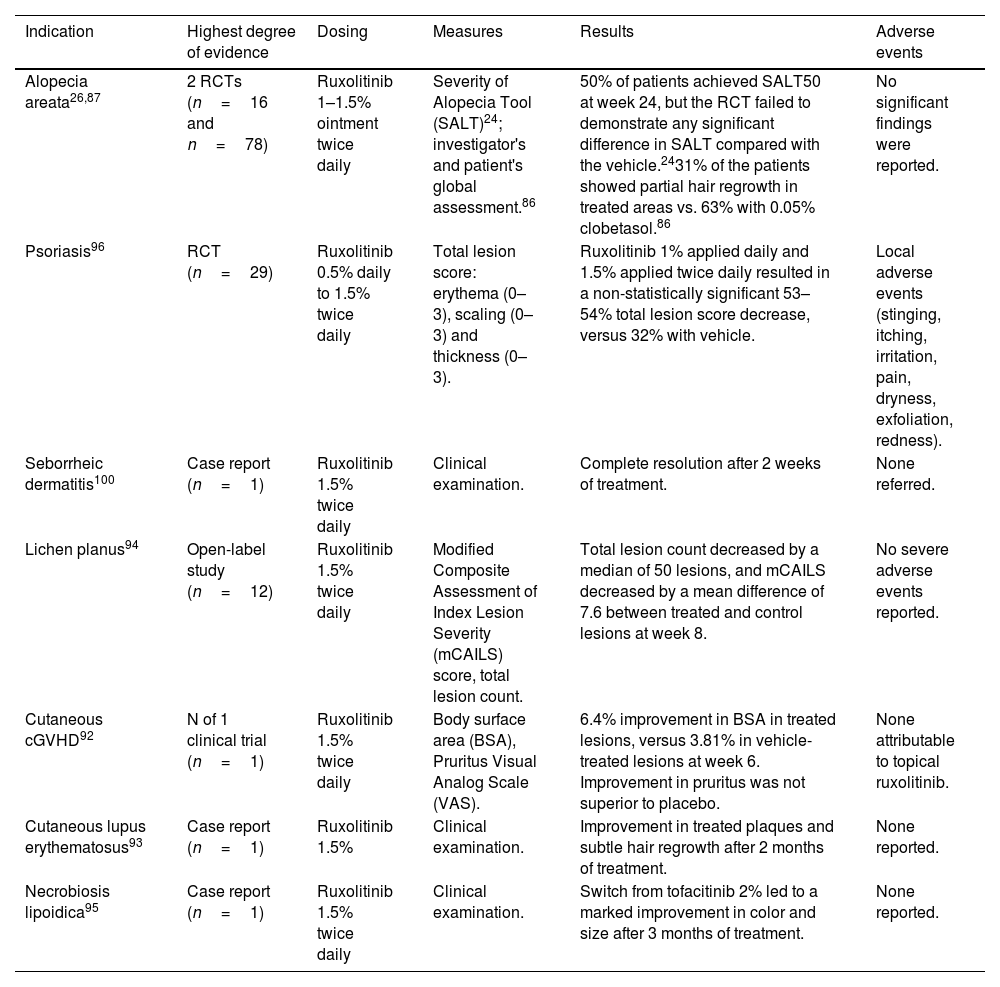
Topical ruxolitinib (Table 5)Alopecia areata (AA)In a phase 1 RCT (n=16) comparing ruxolitinib 1% ointment to clobetasol 0.05% in individuals with AA universalis, 31% exhibited partial hair regrowth in ruxolitinib-treated areas.87 An open-label pilot study followed by an RCT, including patients with 25–99% hair loss at baseline, reported that 50% achieved >50% reduction in Severity of Alopecia Tool (SALT50) at week 24 with ruxolitinib 1.5% cream. However, the RCT failed to demonstrate superior efficacy compared to the vehicle.26
Off-label use of topical ruxolitinib in dermatology.
| Indication | Highest degree of evidence | Dosing | Measures | Results | Adverse events |
|---|---|---|---|---|---|
| Alopecia areata26,87 | 2 RCTs (n=16 and n=78) | Ruxolitinib 1–1.5% ointment twice daily | Severity of Alopecia Tool (SALT)24; investigator's and patient's global assessment.86 | 50% of patients achieved SALT50 at week 24, but the RCT failed to demonstrate any significant difference in SALT compared with the vehicle.2431% of the patients showed partial hair regrowth in treated areas vs. 63% with 0.05% clobetasol.86 | No significant findings were reported. |
| Psoriasis96 | RCT (n=29) | Ruxolitinib 0.5% daily to 1.5% twice daily | Total lesion score: erythema (0–3), scaling (0–3) and thickness (0–3). | Ruxolitinib 1% applied daily and 1.5% applied twice daily resulted in a non-statistically significant 53–54% total lesion score decrease, versus 32% with vehicle. | Local adverse events (stinging, itching, irritation, pain, dryness, exfoliation, redness). |
| Seborrheic dermatitis100 | Case report (n=1) | Ruxolitinib 1.5% twice daily | Clinical examination. | Complete resolution after 2 weeks of treatment. | None referred. |
| Lichen planus94 | Open-label study (n=12) | Ruxolitinib 1.5% twice daily | Modified Composite Assessment of Index Lesion Severity (mCAILS) score, total lesion count. | Total lesion count decreased by a median of 50 lesions, and mCAILS decreased by a mean difference of 7.6 between treated and control lesions at week 8. | No severe adverse events reported. |
| Cutaneous cGVHD92 | N of 1 clinical trial (n=1) | Ruxolitinib 1.5% twice daily | Body surface area (BSA), Pruritus Visual Analog Scale (VAS). | 6.4% improvement in BSA in treated lesions, versus 3.81% in vehicle-treated lesions at week 6. Improvement in pruritus was not superior to placebo. | None attributable to topical ruxolitinib. |
| Cutaneous lupus erythematosus93 | Case report (n=1) | Ruxolitinib 1.5% | Clinical examination. | Improvement in treated plaques and subtle hair regrowth after 2 months of treatment. | None reported. |
| Necrobiosis lipoidica95 | Case report (n=1) | Ruxolitinib 1.5% twice daily | Clinical examination. | Switch from tofacitinib 2% led to a marked improvement in color and size after 3 months of treatment. | None reported. |
Abbreviations: RCT, randomized controlled trial; cGVHD, chronic graft versus host disease.
In a pediatric case series (n=2), topical ruxolitinib 1–2% twice daily led to >75% regrowth of upper eyelash hair in one patient, and no regrowth of eyebrows in the other.88 Two case reports showed partial hair regrowth with topical ruxolitinib.89,90 Another case reported no efficacy with ruxolitinib 0.6% twice daily.91
Cutaneous chronic graft versus host disease (cGVHD)A 51-year-old male showed a 6.4% improvement in total body surface area in lesions treated with topical ruxolitinib 1.5% at week 6.92
Cutaneous lupus erythematosusA woman with refractory discoid lupus erythematosus showed improvement and hair regrowth after two months of ruxolitinib 1.5% cream.93
Lichen planusA prospective phase 2 open-label study with ruxolitinib 1.5% twice daily in cutaneous LP (n=12) exhibited a statistically significant reduction in the number of lesions and their severity after 8 weeks.94
Necrobiosis lipoidicaA woman with a refractory necrobiosis lipoidica exhibited a marked improvement after switching from tofacitinib 2% cream to ruxolitinib 1.5% twice daily.95
PsoriasisA phase 2 RCT (n=29) found that ruxolitinib 1% and 1.5% resulted in 53% and 54% plaque reduction.96 A phase IIb open-label trial showed a 40% mean PASI improvement after 3 months of ruxolitinib 1% cream.97 Another open-label trial comparing ruxolitinib 1% and 1.5% cream applied once or twice daily for 4 weeks showed a mean reduction in erythema score (42–55%), scaling (46–78%) and thickness (50–65%) across all groups.98 A phase 2 open-label study (n=25) of ruxolitinib 1.5% twice daily, showed a statistically significant improvement at day 28.99
Seborrheic dermatitisA 74-year-old man with concomitant rosacea exhibited a complete resolution of seborrheic dermatitis and a partial improvement of rosacea after 2 weeks with ruxolitinib 1.5% twice daily.100
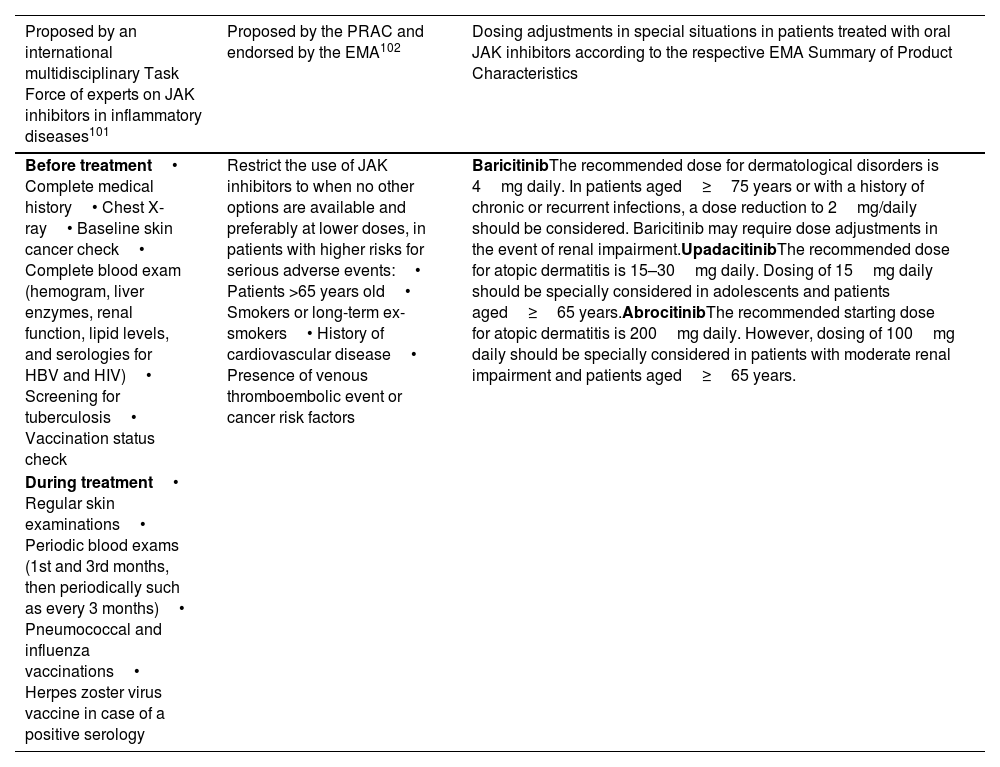
DiscussionThe inclusion of a Black Box warning for JAK inhibitors has raised concern among dermatologists regarding the safety of these medications. However, the magnitude of these concerns should not be overestimated. The long term side effects prompting the Black Box warning were observed in patients with ≥50 years of age with rheumatoid arthritis, concomitantly on methotrexate, and with pre-existing cardiovascular risk factors.9 In dermatological indications, patient populations usually differ greatly from the clinical setting in which this study was conducted, potentially impacting the safety profile. Moreover, a study comparing the incidence of adverse effects between traditional systemic therapies (methotrexate, cyclosporine, and systemic corticosteroids) and JAK inhibitors (upadacitinib and abrocitinib) found similar or higher rates of malignancy, MACEs and VTE with traditional therapies.6 These suggests that JAK inhibitors could offer a safer alternative in terms of long-term side effects. To mitigate the risk of serious side effects, a multidisciplinary Task Force released consensus recommendations for the management of patients on JAK inhibitors in 2021.101 In 2022, the European Medicines Agency endorsed the measures recommended by the Pharmacovigilance Risk Assessment Committee elaborated with the same purpose102 (Table 6). Prior to initiating JAK inhibitor treatment, a thorough anamnesis, focusing on factors such as MACE and VTE history, familial thrombosis, and previous malignancies should be performed. A complete physical examination and blood test should be conducted, including a hemogram, liver and renal function tests, lipid panel, CPK, and serological screening for HIV, HBV, HCV, and VZV. A tuberculosis screening should also be conducted. Given the elevated herpes zoster risk, all patients should be offered a vaccination according to their serological status. Shingrix, a zoster recombinant adjuvanted vaccine, has shown promising results in initial data from rheumatoid arthritis patients, with as low as 0.7% developing herpes zoster. However, further studies are required to confirm its preventive efficacy.103 Close follow-up and monitoring are vital throughout JAK inhibitor treatment. Patients should be followed by professionals with expertise in these medications, and regular blood examinations should be performed to monitor potential adverse effects. Dermatological check-ups are particularly important for high-risk patients to detect early signs of skin cancer.
Summary of pretreatment and treatment follow-up recommendations in patients receiving JAK inhibitors.
| Proposed by an international multidisciplinary Task Force of experts on JAK inhibitors in inflammatory diseases101 | Proposed by the PRAC and endorsed by the EMA102 | Dosing adjustments in special situations in patients treated with oral JAK inhibitors according to the respective EMA Summary of Product Characteristics |
|---|---|---|
| Before treatment• Complete medical history• Chest X-ray• Baseline skin cancer check• Complete blood exam (hemogram, liver enzymes, renal function, lipid levels, and serologies for HBV and HIV)• Screening for tuberculosis• Vaccination status check | Restrict the use of JAK inhibitors to when no other options are available and preferably at lower doses, in patients with higher risks for serious adverse events:• Patients >65 years old• Smokers or long-term ex-smokers• History of cardiovascular disease• Presence of venous thromboembolic event or cancer risk factors | BaricitinibThe recommended dose for dermatological disorders is 4mg daily. In patients aged≥75 years or with a history of chronic or recurrent infections, a dose reduction to 2mg/daily should be considered. Baricitinib may require dose adjustments in the event of renal impairment.UpadacitinibThe recommended dose for atopic dermatitis is 15–30mg daily. Dosing of 15mg daily should be specially considered in adolescents and patients aged≥65 years.AbrocitinibThe recommended starting dose for atopic dermatitis is 200mg daily. However, dosing of 100mg daily should be specially considered in patients with moderate renal impairment and patients aged≥65 years. |
| During treatment• Regular skin examinations• Periodic blood exams (1st and 3rd months, then periodically such as every 3 months)• Pneumococcal and influenza vaccinations• Herpes zoster virus vaccine in case of a positive serology |
Abbreviations: PRAC, Pharmacovigilance Risk Assessment Committee; EMA, European Medicines Agency; JAK, Janus kinase.
Given that clinical studies tend to underrepresent pediatric or >65-year-old patients, individuals with comorbidities or at risk for malignancy or thromboembolic and cardiovascular events, there is a need for clinical trials to include these populations to comprehensively assess the safety of JAK inhibitors. Further research is needed to determine if risks can be mitigated by careful dose selection.
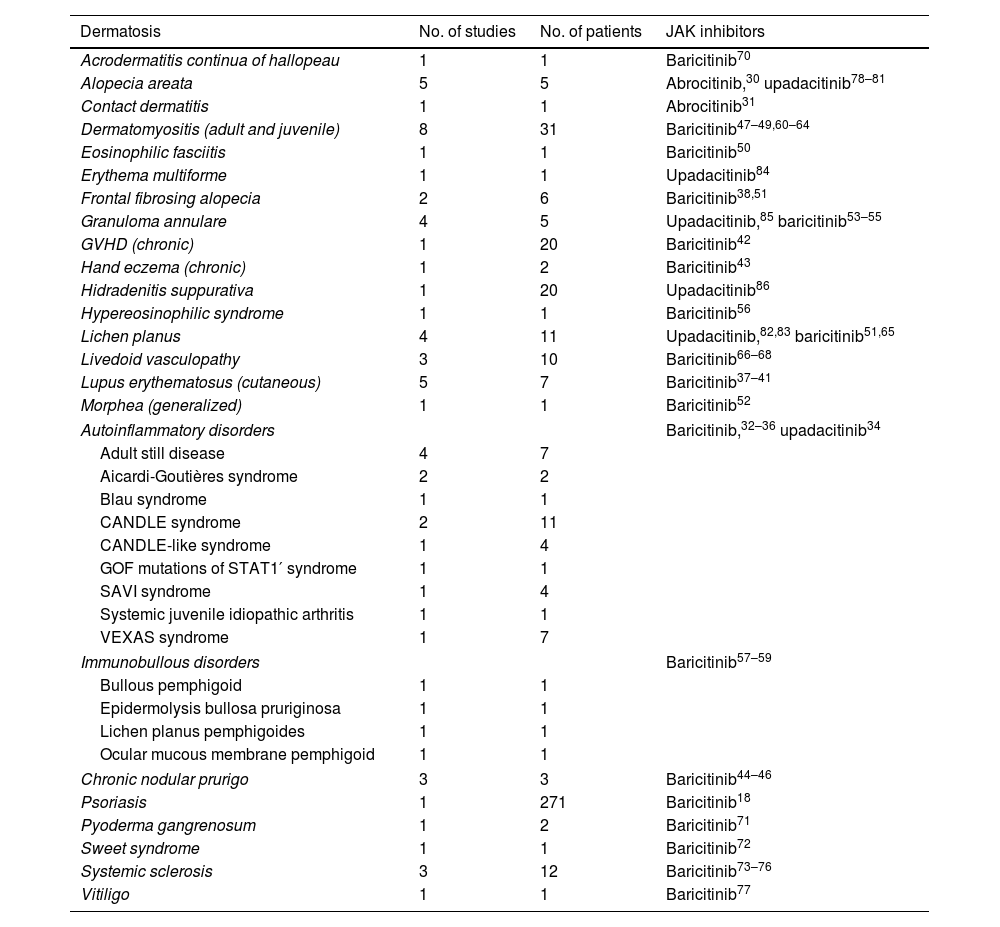
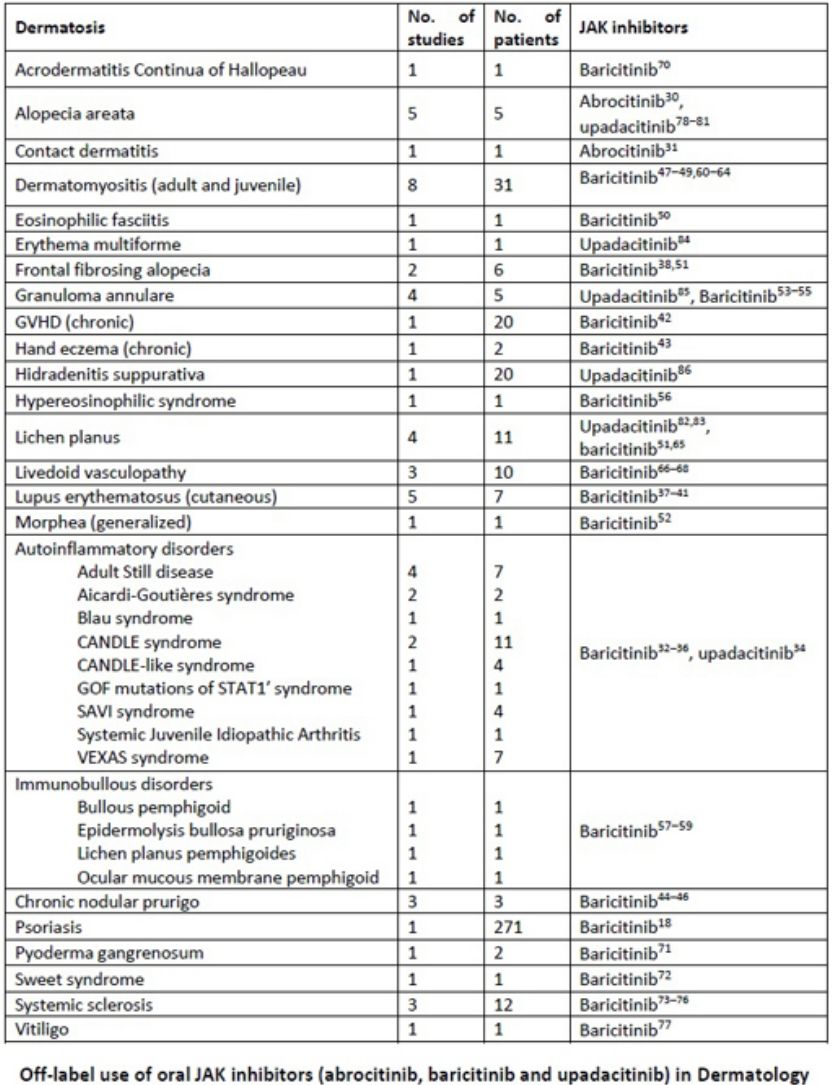
Our review includes preliminary efficacy data of JAK inhibitors in several dermatologic conditions (Table 7). Based on the promising findings examined, it is reasonable to consider JAK inhibitors as a potential treatment option for diseases such as livedoid vasculopathy, cGVHD, autoinflammatory diseases, cutaneous lupus erythematosus, dermatomyositis, and systemic sclerosis. These diseases are often refractory to conventional treatments or heavily reliant on corticosteroids, necessitating an urgent need for alternative therapies.
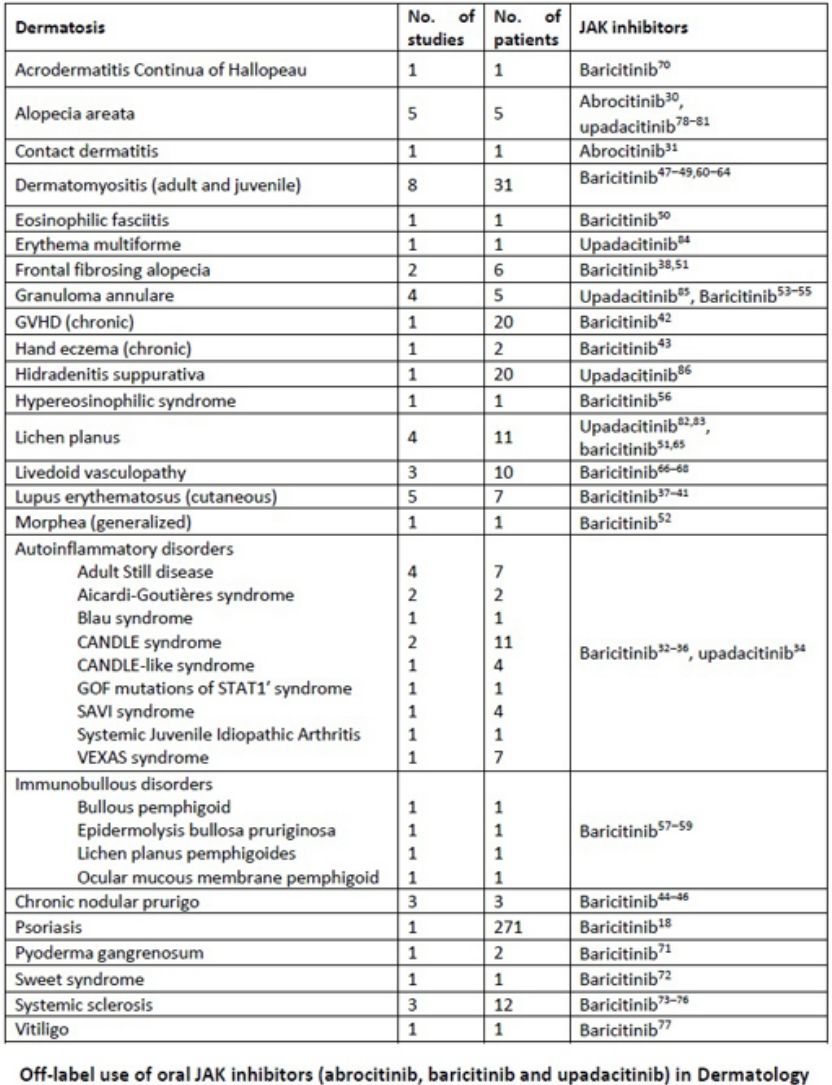
Off-label use of oral JAK inhibitors (abrocitinib, baricitinib and upadacitinib) in Dermatology.
| Dermatosis | No. of studies | No. of patients | JAK inhibitors |
|---|---|---|---|
| Acrodermatitis continua of hallopeau | 1 | 1 | Baricitinib70 |
| Alopecia areata | 5 | 5 | Abrocitinib,30 upadacitinib78–81 |
| Contact dermatitis | 1 | 1 | Abrocitinib31 |
| Dermatomyositis (adult and juvenile) | 8 | 31 | Baricitinib47–49,60–64 |
| Eosinophilic fasciitis | 1 | 1 | Baricitinib50 |
| Erythema multiforme | 1 | 1 | Upadacitinib84 |
| Frontal fibrosing alopecia | 2 | 6 | Baricitinib38,51 |
| Granuloma annulare | 4 | 5 | Upadacitinib,85 baricitinib53–55 |
| GVHD (chronic) | 1 | 20 | Baricitinib42 |
| Hand eczema (chronic) | 1 | 2 | Baricitinib43 |
| Hidradenitis suppurativa | 1 | 20 | Upadacitinib86 |
| Hypereosinophilic syndrome | 1 | 1 | Baricitinib56 |
| Lichen planus | 4 | 11 | Upadacitinib,82,83 baricitinib51,65 |
| Livedoid vasculopathy | 3 | 10 | Baricitinib66–68 |
| Lupus erythematosus (cutaneous) | 5 | 7 | Baricitinib37–41 |
| Morphea (generalized) | 1 | 1 | Baricitinib52 |
| Autoinflammatory disorders | Baricitinib,32–36 upadacitinib34 | ||
| Adult still disease | 4 | 7 | |
| Aicardi-Goutières syndrome | 2 | 2 | |
| Blau syndrome | 1 | 1 | |
| CANDLE syndrome | 2 | 11 | |
| CANDLE-like syndrome | 1 | 4 | |
| GOF mutations of STAT1′ syndrome | 1 | 1 | |
| SAVI syndrome | 1 | 4 | |
| Systemic juvenile idiopathic arthritis | 1 | 1 | |
| VEXAS syndrome | 1 | 7 | |
| Immunobullous disorders | Baricitinib57–59 | ||
| Bullous pemphigoid | 1 | 1 | |
| Epidermolysis bullosa pruriginosa | 1 | 1 | |
| Lichen planus pemphigoides | 1 | 1 | |
| Ocular mucous membrane pemphigoid | 1 | 1 | |
| Chronic nodular prurigo | 3 | 3 | Baricitinib44–46 |
| Psoriasis | 1 | 271 | Baricitinib18 |
| Pyoderma gangrenosum | 1 | 2 | Baricitinib71 |
| Sweet syndrome | 1 | 1 | Baricitinib72 |
| Systemic sclerosis | 3 | 12 | Baricitinib73–76 |
| Vitiligo | 1 | 1 | Baricitinib77 |
Abbreviations: JAK, Janus kinase; SAVI, STING-associated vasculopathy with onset in infancy.
The accessibility of JAK inhibitors as off-label medications raises concerns. A prospective cohort study in German dermatology clinics revealed lower approval rates of JAK inhibitors compared to biologics (odds ratio 0.16).104 Additionally, considering their high cost, a cost-benefit analysis is essential, especially for non-life-threatening conditions.
Our study has several limitations. It is a narrative review and not a systematic one. The sample sizes were mostly small, prospective studies were lacking in many off-label indications, and there were short follow-up periods with heterogeneous methodologies, limiting the generalizability of the findings. Many case reports and series assessing the efficacy of JAK inhibitors faced challenges in attributing positive outcomes solely to these medications, as numerous cases involved concomitant treatments that could have influenced the results.
ConclusionJAK inhibitors pose an important step forward toward precision medicine. Their safety is largely influenced by patient characteristics, disease being treated, route of administration, specific JAK inhibitor, and dosage. When compared to traditional immunosuppressant therapies, overall, JAK inhibitors demonstrate improved safety profiles. These agents hold promise as treatments for various inflammatory dermatoses that greatly impact quality of life.
Conflict of interestsThe authors declare they have no conflict of interest.