Atopic dermatitis (AD) is a chronic, inflammatory skin disease affecting all age groups, particularly children. This systematic review provides an overview of the humanistic and economic disease burden in the pediatric population with AD in Spain. The evidence, collected from 11 observational studies published over the past 10 years, exhibits the most common characteristics of the patients, disease burden, patient-reported outcomes, use of resources, and treatment patterns. The burden of AD extends beyond physical symptoms, with associated comorbidities such as asthma and impaired health-related quality of life and mental health disorders, particularly in severe cases. Traditional therapies, primarily topical corticosteroids, face adherence and efficacy challenges. Despite promising innovative treatments and available biological therapies, their use is still limited in the pediatric population. The findings of the present review highlight the scarce scientific evidence on the economic burden of pediatric AD, as well as the most updated humanistic evidence on this disease. At the same time, the need for individualized care and innovative therapeutic interventions to address the multifaceted challenges of pediatric AD in Spain is evident.
La dermatitis atópica (DA) es un trastorno cutáneo crónico e inflamatorio que afecta a todos los grupos de edad, pero especialmente a los niños. Esta revisión sistemática proporciona una visión general de la carga de la enfermedad en la población pediátrica con DA en España. La evidencia recopilada de 11 estudios observacionales publicados en los últimos 10 años presenta las características más comunes de los pacientes, la carga de la enfermedad, los resultados reportados por los pacientes, el uso de recursos y los patrones de tratamiento más frecuentes. La carga de la DA se extiende más allá de los síntomas físicos, con comorbilidades asociadas como el asma, el deterioro de la calidad de vida relacionada con la salud y trastornos de salud mental, particularmente en los casos graves. Los tratamientos tradicionales, principalmente los corticosteroides tópicos, enfrentan desafíos de adherencia y eficacia. A pesar de las prometedoras innovaciones terapéuticas y la disponibilidad de terapias biológicas, su uso permanece limitado en población pediátrica. Los resultados de la presente revisión resaltan la escasa evidencia científica sobre la carga económica de la DA pediátrica, así como la evidencia humanística más actualizada de la enfermedad. Asimismo, se hace patente la necesidad de una atención personalizada e intervenciones terapéuticas innovadoras para abordar los desafíos multifacéticos de la DA pediátrica en España.
Atopic dermatitis (AD) is a prevalent, chronic, relapsing, and inflammatory skin disorder of a multifactorial etiology characterized by intense itching and skin lesions.1 AD is a global disease that affects individuals across all age groups.2 The prevalence of AD among the pediatric population is notably high, with estimates indicating that 15% up to 30% of children seem to be affected by this disease3–5 vs a slightly lower prevalence among the adult population ranging from 7% up to 14%.2 The prevalence of AD among Spanish children remains unclear due to the lack of comprehensive, age-specific studies and scarce available data.1
AD in pediatric patients is often associated with a range of comorbidities, including other diseases such as asthma or allergic rhinitis.1 Additionally, psychiatric and psychological disorders are likely to be more prevalent in this population. In fact, there is evidence of an increased incidence of mental issues, such as attention deficit hyperactivity disorder (ADHD) and depression, which can also manifest as suicide ideation among these patients.1,6,7
The presence of these comorbidities, along with symptoms of AD such as intense pruritus, significantly impairs health-related quality of life (HRQoL), especially in severe cases.6,8
Due to disease heterogeneity, comorbidities, complexity in treatment care and differences between national or regional health care systems, managing AD in the pediatric population remains challenging.9 Short regimens of topical corticosteroids are the most widely prescribed treatment.10 However, adherence to these conventional therapies is often low,11 and their efficacy in severe cases is also limited.12,13 Fortunately, in recent years, innovative, and highly effective treatments have emerged such as biologic drugs and Janus kinase (JAK) inhibitors. However, their use in children is still restricted in some cases.10 As a matter of fact, the substantial humanistic burden of disease in patients with moderate-to-severe AD suggests potential undertreatment in this group, with few evidence available on new therapies, patient-reported outcomes (PROs), AD severity and impairment.14 On the other hand, AD represents a substantial economic burden on the health system, as well as on the patients and caregivers.14 The total direct cost was estimated to be nearly €2700 per patient per year.14 As expected, the severity of the condition had a direct impact on cost, with severe cases resulting in higher costs.14 Specifically, compared with patients with mild and moderate AD, severe AD presented significantly higher total direct costs (€1512 [SD, 854] and €1984 [SD, 2093] vs €5377 [SD, 3.518]; p<0.001, respectively).
There is no available comprehensive, updated information on the humanistic, and economic burden associated with children with AD in Spain. This systematic review aims to provide an overview based on the most recent Spanish real-world evidence.
MethodsWe conducted a systematic review of observational studies on the humanistic and economic burden associated with pediatric patients with AD over the past 10 years (from March 2013 through March 2023) following the recommendations established by the “Preferred Reporting Items of Systematic Reviews and Meta-analysis” (PRISMA).15
Data sources and search strategyThe international PubMed/MEDLINE, Cochrane Library, the Spanish national Medicina en Español (MEDES), and Índice Bibliográfico Español en Ciencias de la Salud (IBECS) databases were searched to identify relevant publications for review. Additionally, manual searches in the grey literature (Google and Google Scholar) were conducted to identify documents such as non-indexed articles and conference abstracts published over the past 3 years at key national and European congresses organized by the following medical societies: Spanish Academy of Dermatology and Venerology (AEDV), Spanish Society of Allergology and Clinical Immunology (SEAIC), European Academy of Dermatology and Venereology (EADV), American Academy of Dermatology (AAD) and Society for Pediatric Dermatology (SDP).
The different databases were searched using both free-text and MeSH (medical subject headings) terms, combined with the Boolean connectors OR and AND. The list of terms and search strategy are detailed in Table S1 of the supplementary data.
Study selectionTwo reviewers independently screened all identified articles at two levels (levels 1 and 2). Level 1 consisted of a broad screening based on article titles and/or abstracts, as available. Level 2 involved two reviewers who independently reviewed the full-text articles and applied the inclusion/exclusion criteria. At both levels, discrepancies were resolved by consensus or with the involvement of a third team member.
Eligibility criteriaObservational studies conducted in Spain including pediatric population (<18 years) with a confirmed diagnosis of AD by dermatologists/physicians published in English or Spanish from March 2013 through March 2023 were eligible. Studies conducted out of Spain were included when specific data from the Spanish population were provided. Table S2 of the supplementary data lists the inclusion and exclusion criteria.
Data mining and quality assessmentData mining included data on the epidemiology of the disease (prevalence and incidence), patients’ characteristics (demographic, comorbidities), patterns on use of treatment, PROs, impact of the disease on the HRQoL, adherence, use of health care resources, and associated costs. A standardized data mining form was used to draw the data from the selected articles.
The quality of included studies was assessed by two independent reviewers using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement,16 resolving discrepancies by consensus.
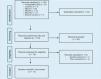
ResultsA total of 133 reports were initially identified (Fig. 1). After duplicate removal and applying the established inclusion criteria, 11 publications (10 full-text articles and 1 conference abstract) were included in the synthesis.
The main characteristics of selected articles are summarised in Table 1. Regarding the study design, 54.5% (n=6) of the studies were cross-sectional, 27.3% (n=3) retrospective, and 18.2% (n=2) prospective. Inclusion criteria were heterogeneous regarding age range, time from diagnosis, diagnostic criteria, severity, and treatment patterns. The information source included the review of health records or databases in 54.5% (n=6) reports, whereas in 36.4% (n=4) of the publications, information was retrieved through interviews conducted with the patients or their parents/caregivers. A total of 27.3% (n=3) of studies were eventually conducted in primary care health care settings, 45.5% (n=5) in hospital health care settings, and 18.2% (n=2) in both.
Main characteristics of the selected publications.
| Author (year) | Study characteristics | Main variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Design | Source | Health care setting | Inclusion criteria | Sample size (N) | Demographic and clinical characteristics | Epidemiology | Treatment patterns | PROs | HRU/costs | |
| Sánchez-Pérez (2013)17 | Prospective | Medical chart review | Primary care | Age ≥2 to <18 years, ≥1 year since AD diagnosis (Hanifin and Rajka criteria) | 151 | X | X- HRQoL | |||
| Torrelo (2013)18 | Cross-sectional | Patient interviews | Hospital | Age ≥2 to <15 years. Moderate-to-severe AD (Hanifin and Rajka criteria), ≥16-month history of AD, topical treatment ≥4 months | 141 | X | X- HRQoL- Satisfaction- Adherence | |||
| Ortiz de Frutos (2014)19 | Cross-sectional | Medical chart review | Hospital | Age ≥2 to <15 years. Clinically diagnosed moderate-to-severe AD (specialist's judgment, excluding mild AD), ≥12-month history of AD, IGA >2 | 116 | X | X | X- HRQoL- Adherence | ||
| Draaisma (2015)20 | Cross-sectional | Parent or caregiver interviews | Primary care | Age >12 to <15 month-old infants | 6937 | X | X- Prevalence | |||
| Barroso (2019)21 | Cross-sectional | Electronic health records | Hospital | Age <18 years with moderate-to-severe AD (IGA >3), treatmentsa | 8 | X | X- Prevalence | X | ||
| Sicras-Mainar (2019)22 | Retrospective | Review of medical records (computerized databases) | Primary care and hospital | Age ≥6 years, AD diagnosis ≥1 year prior to the index date, prescribed medication, ≥2 prescriptions at the follow-up, regular monitoring with ≥2 health records, including a visit to the dermatology unit | 24,374 with AD844 with severe AD | X | X- Prevalence | X | ||
| Arnedo-Pena (2020)23 | Prospective | Patient interviews | Hospital | NA | 3607 (1st survey, 1994)1805 (2nd survey, 2002) | X | X- Prevalence- Incidence | |||
| Darbà (2021)24 | Retrospective | Database | Primary care and hospital | NA | 1266 | X | X- Incidence | X | ||
| Silverberg (2021)25 | Cross-sectional | Patient interviews | Hospital | Age >6 months to 18 years | 3465 | X | X- Prevalence | |||
| Almenara-Blasco (2022)26 | Retrospective | Medical chart review | Primary care | NA | 31,757 | X | ||||
| Lázaro (2022)27[ABSTRACT SEAIC 2022] | Cross-sectional | NA | NA | Age >6 months to 18 years, diagnosed with AD (criteria-based)b | 564 | X | ||||
AD, atopic dermatitis; PROs, patient-reported outcomes; HRQoL, health-related quality of life; NA, not available; X marks the reported variables in each study.
≥ one of the following therapies: immunosuppressants (e.g., cyclosporine, off-label methotrexate, off-label azathioprine, off-label mycophenolate mofetil), biologic drugs (including off-label omalizumab and off-label ustekinumab, dupilumab), systemic corticosteroids, and/or other drugs (e.g., off-label immunoglobulins, off-label apremilast, UVB phototherapy).
Details on the population's age and gender are detailed in Table 2. Four studies21,25–27 included a broad age spectrum from 6-month infants to younger than 18-year-old patients. In contrast, two studies20,23 focused on a narrower age range, 12–15-month-olds, and 6–7/14–15-year-olds, respectively. Overall, the most represented age group was from 2- to 15-year-olds. Regarding gender, the male/female proportion was similar, with the female representation varying from 42% up to 54.5% according to different studies and age ranges.
Summary of data regarding the population's age and gender.
| Author (year) | Sample size (N) | Population age, yr* | Mean age, years (SD) | Gender, female (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Range | 0.5–1 | 2–5 | 5–15 | 15–18 | ||||
| Sánchez-Pérez (2013)17 | 151 | 2–17 | X | X | X | 9.4 (4.5) | 48.3 | |
| Torrelo (2013)18 | 141 | 2–15 | X | X | 8.7 (3.6) | 41.8 | ||
| Ortiz de Frutos (2014)19 | 161 | 2–15 | X | X | 7.7 (3.9) | 50.0 | ||
| Draaisma (2015)20 | 6937 | 12–15 months | X | NA | 46.9–50.1a | |||
| Barroso (2019)21 | 8 | <18 | X | X | X | X | 12.5 | NA |
| Sicras-Mainar (2019)22 | 24,374 with AD844 with severe AD | 6–18 | X | X | 6–12 yr: 9.1 (2.0)13–18 yr: 14.8 (1.6) | 6–12 yr: 4213–18 yr: 51.4 | ||
| Arnedo-Pena (2020)23 | 3607(year 1994) | 6–7 | X | 6.3 (0.5) | 49.5 | |||
| 1805(year 2002) | 14–15 | X | 14.4 (0.5) | 50.1 | ||||
| Darbà (2021)24 | 1266 | 0–5 | X | X | 1.5 | 40.5 | ||
| Silverberg (2021)25 | 3465 | 0.5–<18 | X | X | X | X | 6 mo–<6 yr: 3.26–<12 yr: 9.212–<18 yr: 14.9 | 6 mo–<6yr: 54.56–<12 yr: 45.112–<18 yr: 46.7 |
| Almenara-Blasco (2022)26 | 31,757 | 0–17 | X | X | X | X | NA | 50.0 |
| Lázaro (2022)27 | 564 | 0.5–<18 | X | X | X | X | NA | NA |
NA, not available; X marks the reported age range.
Six studies17,21,25,27 assessed the severity of AD using different tools, including the Investigator's Global Assessment (IGA) (n=3),17,19,21 the SCORing Atopic Dermatitis (SCORAD) (n=1),19 the Clinical Global Impressions scale – Severity of Illness (CGI-SI) (n=1),19 the Patient Global Assessment (PtGA) (n=2),25,27 the Patient-Oriented Eczema Measure (POEM) (n=1),25 and the physician's perspective18 (Fig. 2).
AD severity. (A) Studies including mild, moderate and severe patients measured with IGA, PtGA and POEM. (B) Studies restricted to moderate-to-severe patients measured with SCORAD, IGA, CGI-SI, IGA and medical history. NA, not available; aIGA has six categories of response and its score ranges from 0 (no illness, with no inflammatory signs of AD) up to 5 (very serious illness, with intense erythema and intense papule/infiltration with crusts/exudation). Barroso et al. ranged severity from 0 up to 4, considering scores of 3 as moderate and scores of 4 as severe AD; bPatient Global Assessment (PtGA), which asks, “Please check one answer that best describes the severity of your or your child's eczema over the past week,” with responses of clear, mild, moderate, or severe; cPatient-Oriented Eczema Measure (POEM), with a total score ranging from 0 (lower severity) up to 28 (higher severity); severity groupings have been defined as bands of 0–7 indicating mild AD, 8–16 moderate AD, and >16 severe AD; dSCORAD takes into account the extent and intensity of the lesions, as well as the symptoms (pruritus and loss of sleep) it causes; extent (A): the body surface is divided into four segments (head and neck, trunk, upper and lower extremities) to which a percentage is assigned based on the extent represented; intensity (B); eThe Clinical Global Impression – Severity scale (CGI-S) is a valuable tool used by clinicians to assess the severity of a patient's illness vs their past experience with similar diagnoses. It involves rating the patient's current condition on a 7-point scale, reflecting different levels of illness severity: (1) normal, not at all ill, (2) borderline mentally ill, (3) mildly ill, (4) moderately ill, (5) markedly ill, (6) severely ill, and (7) extremely ill.
Three studies focused on moderate-to-severe AD patients,18,19,21 while the other three studies included patients from all AD severities.17,25,27 In the latter studies, moderate AD was reported in 28.8% up to 50.3% of patients, and severe AD in 4.1% up to 12.6% of patients.17,25,27 Mild AD was the most prevalent disease in two of those studies according to PtGA/POEM25,27; while moderate AD was the most prevalent in study #3 according to IGA.17
Ortiz de Frutos et al. used several tools to assess disease severity. A total of 70.7% of pediatric patients had been initially diagnosed with moderate AD, based on the specialist's best clinical judgment, and, therefore, had been included in the study. However, at the time of medical consultation, only 53.4% and 50.9% exhibited moderate-to-severe AD according to IGA and CGI-SI respectively.19 Six percent of the patients had been initially included in the study as having moderate-to-severe AD, but obtained a SCORAD score <15, which ended up categorizing their AD as mild. In contrast, Silverberg et al. evaluated disease severity using PtGA and POEM, observing similar rates.25
ComorbiditiesFive studies17,21,22,24,27 provided specific data on comorbidities associated with AD in Spanish pediatric patients (Fig. 3). An additional study26 listed comorbidities without providing prevalence percentages.
One study presented aggregate data, confirming that 84.4% of pediatric patients with AD had, at least, one comorbidity, for a mean of 2.5 comorbidities per patient.27 In general, respiratory conditions such as asthma and allergies such as allergic rhinitis were the most widely reported conditions in the observational studies reviewed ranging from 2.6% up to 47% and from 7.9% up to 50%, respectively.
Interestingly, in the study conducted by Almenara-Blasco et al.,26 different gender-related comorbidity patterns were identified. Mental health disorders, dyslipidemia, and respiratory conditions were more common among men, whereas respiratory-allergic, sensitive-digestive, menstrual-dysphoric-metabolic, and cardiometabolic conditions were more common among women (percentages not provided).26
Disease burdenEpidemiologySix articles20–25 provided epidemiological data, including prevalence (n=5) and incidence (n=2) of AD in the pediatric population of Spain. The reported prevalence rates varied significantly across the studies ranging from 0.01% up to 30% (Fig. 4).
Sicras-Mainar et al.22 assessed the prevalence of AD by different age groups, with both overall and severe AD prevalence being higher in 6–12-year-olds (11.5% vs 6.4%; p<0.001) vs 13–18-year-olds (0.39% vs 0.23; p<0.001), respectively.22 Similarly, Arnedo-Pena et al.23 reported a higher prevalence in adolescents older than 12 years vs younger children, as well as in women compared to men in both 2002 and 2012 (29.80% vs 21.50% and 41.40% vs 28.30%, respectively). This study also reported an increase in the prevalence ratio from 1994 (1.02 [95% CI, 0.86–1.21]) through 2012 (1.41 [95% CI, 1.21–1.73]).23
For the incidence of AD, data were drawn from two studies.23,24 Arnedo-Pena et al.23 identified a total of 182 new cases of AD from 1994 through 2002, with an incidence rate of 15.9 per 1000 persons years. Darbà and Marsà24 confirmed an incidence rate of AD from 2000 through 2017 of 5.8 per 100,000 persons, with a higher incidence being reported in children aged 0–5 years (30.0 per 100,000 persons), and the overall incidence being stable throughout the study period.24
Treatment patternsThree studies19,21,22 assessed treatment patterns in pediatric patients with moderate-to-severe AD in Spain (Fig. 5).
The first-line therapy in children with moderate-to-severe AD was the use of emollients (82.8%),19 followed by topical pharmacological treatment (77.6%) including corticosteroids.21 Systemic corticosteroids were also used in children starting at the age of 6 years (37.5% up to 95.4%) being cyclosporine (40.5% up to 50%) and azathioprine (23.8%) the most widely used drugs.21,22 Only a small percentage of patients received biologic drugs (1.5%), in children older than 6 years.22
Despite pediatric patients suffering from moderate-to-severe AD, it was notable that 24.1% of patients reported not using any topical pharmacological treatment or delaying its use.19
Patient-reported outcomesThree articles17–19 provided data on PROs covering aspects such as HRQoL (n=3),17–19 treatment satisfaction (n=1),18 and treatment adherence (n=2).18,19
HRQoLThe impact of AD on the patients’ HRQoL was evaluated using the children's version of the Dermatology Life Quality Index (cDLQI), the Infant's Dermatitis Quality of Life Index (IDQoL), and the children's version of the EADA (Escala de afectación de la dermatitis atópica) scale. Lower scores represented less impairment and better HRQoL in all questionaries used, with final scores ranging from 0 up to 30 in the cDLQI and IDQoL indexes, and from 0 up to 10 in the EADA scale. A summary of the results has been presented in Table 3.
Summary of HRQoL results.
| Author (year) | Disease severity | Tool (score range) | Overall score | Symptoms and feelings | Activities of daily living | Leisure | Study | Interpersonal relationships | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Sánchez-Pérez (2013)17 | Mild | cDLQIa (0–30) | 3.8 | 1.7 | 0.4 | 0.3 | 0.3* | 0.7* | 0.4* |
| Moderate | 8.8 | 2.7 | 1.3 | 1.6 | 0.7* | 1.6* | 1.0* | ||
| Severe or very severe | 14.5 | 3.4 | 2.8 | 2.9 | 1.1* | 2.8* | 1.6* | ||
| Torrelo (2013)18 | Moderate-to-severe | cDLQIa (0–30) | 6.1 | 2.0 | 1.8 | 0.5 | 0.5 | 0.7 | 0.7 |
| EADAb (0–10) | 2.5 | NA | NA | NA | NA | NA | NA | ||
| Ortiz de Frutos (2014)19 | Moderate-to-severe | EADAb (0–10) | 2.9 | NA | NA | NA | NA | NA | NA |
NA, not available.
The cDLQI consists of 10 items, each including four response categories ranging from 3 (very much) to 0 (not at all). The questionnaire dimensions include symptoms and feelings, activities of daily living, leisure, work/study, interpersonal relationships, sexuality, and treatment. The final score ranges from 0 (minimum impact on HRQOL) up to 30 points (maximum impact on HRQOL).
The EADA scale is a brief self-report instrument consisting of 9 items for adults and 8 for pediatric patients. It includes four response options. The raw overall scores obtained in each version are linearly transformed into a scale ranging from 0 (minimal impact on patient HRQoL) up to 10 (maximum impact on patient HRQoL). For pediatric patients, the test was completed by parents or caregivers to assess the impact of AD on the HRQoL of minors
Two studies used the cDLQI: one reported an overall score of 6.1, being symptoms and feelings (2.0) and daily activities (1.8) the most affected dimensions18; while the other reported overall mean scores of 3.8, 8.8, and 14.5 based on disease severity (mild, moderate and severe or very severe, respectively).17 Similarly to the cDLQI, global IDQoL and EADA scores showed a moderate impact with mean scores of 7.6 and 2.5, respectively in these moderate-to-severe patients.18
Torrelo et al. observed higher levels of emotional, physical, and social impairment in patients with severe AD vs patients with mild AD.18 Consistent with this, in severe compared to mild ones, individual cDLQI domain scores revealed a significantly higher impact on symptoms (mean score, 3.4 [SD, 1.8] vs 1.7 [SD, 1.1], p<0.05), activities of daily living (mean score, 2.8 [SD, 1.6] vs 0.4 [SD, 1.7], p<0.05), and leisure (mean score, 2.9 [SD, 2.2] vs 0.3 [SD, 0.7], p<0.05).17 Furthermore, AD led to changes in mood (79.9%), affecting feelings of restlessness (91.6%), concentration difficulties (35.3%), and depression (7.6%), particularly among those with severe AD.17 AD significantly impaired patients’ daily life in all severity groups, with 24.5% of the overall sample experiencing strong or very strong pruritus.17 Sleep was deprived by pruritus, with most patients with moderate-to-severe AD reporting difficulty falling asleep (90.1% with moderate AD and 87.5% with severe AD), and a proportion of them awakening at night time (76.8% with moderate AD and 97.5% with severe AD).17
Treatment satisfaction and adherenceTreatment satisfaction and adherence were evaluated in patients using topical pharmacological maintenance treatment for flare-ups prevention. Overall, patients expressed high satisfaction (7.2), evaluated using a Visual Analogue Scale (VAS 0-10), with no significant differences regarding disease severity.18 However, treatment adherence, reported by the Morisky–Green test, was low (18.4%), with 49.9% of the patients admitting to occasionally forgetting applying treatment, and 34.0% admitting not adhering to it during symptom-free periods.18 In contrast, another study found that most patients adhered to the pharmacological treatment.19 Discrepancies were found between physicians and patients’ perceptions of reported compliance. While dermatologists considered that many of their patients complied with maintenance treatment (88.7%), the percentage of patients who declared themselves as compliant was lower (18.4% up to 42.6%).18 Interestingly enough, patients rated excellent, good, or sufficient disease control in 62.7% of cases while dermatologists did so in 40.5% of cases.19
Use of resources and associated costsOnly one report showed data on specialized care resource use and associated costs in pediatric patients (<5 years old).24
The study conducted from 2000 through 2017 included 84.3% of ER admissions with a mean length of stay 4.3 days. The most common medical procedures upon admission included blood microscopic examination (30.6%), steroid injections (18.6%), antibiotics (12.1%), and skin biopsies (14.3%). Additionally, pharmacological interventions registered in primary care centers involved the administration of antibiotics (8.7%), corticosteroids (topical, 25.7%; systemic, 17.8%), analgesics (paracetamol, 27.1%; ibuprofen, 20.2%; other, 28.3%), and antihistamines (14.3%).
The mean annual direct medical costs per patient were estimated at €2310. These costs were mostly stable within the first half of study period (€1500–€2500/per patient approx., 2000–2009), with a major increase being observed from 2009 through 2010 up to €4000/per patient (p<0.0001), followed by a decreasing trend in the 2011–2017 period, back to 2500€/per patient approx.
DiscussionOur systematic review synthesized findings from 11 observational studies providing an overview of the population characteristics, burden of disease, epidemiology, treatment patterns, PROs, use of resources and costs associated with the management of AD in pediatric Spanish patients.
Based on the studies assessing the severity of AD, we could conclude that differences in classification may derive from the use of different instrument measures. The observed results suggested that the use of PRO measures such as PtGA and POEM would correlate well between them. However, the results obtained using clinical measures such IGA or CGI-SI might underestimate the severity vs the results obtained using SCORAD, as it combines the clinical and the patient perspective.
Among the studies included in our review that reported data on comorbidities, respiratory diseases and allergies were found to be the most common. Notably, one of the studies of pediatric patients with severe AD reported a high prevalence (>70%) of mental illnesses.17,21,22,24,27 In this context, there is evidence of a significant association between AD in children and mental health symptoms.28 A longitudinal, population-based birth control cohort study found that children with severe AD were >2-fold more likely to have symptoms of depression vs those without AD.28 Additionally, children with AD were more likely to internalize behaviors, such as anxiety or somatic complaints.28 Moreover, a higher prevalence of ADHD in pediatric AD patients has been reported too.29
As seen in the three studies included in our review, AD affects not only the emotional domain but also other domains of HRQoL, as physical and social impairment, particularly in severe AD.17–19 Pruritus is the symptom with the greatest impact on HRQoL, as it negatively interferes with sleep quality, mood swings and restlessness.8 Consistent with this, AD has been pointed out to be the most common skin disease that causes the greatest impairment in HRQoL on account of symptoms such as pruritus and insomnia.30 One study revealed that 79% of children with AD reported sleep disturbances,31 even among patients with mild and inactive disease.32
The prevalence data from the analyzed studies revealed wide heterogeneity. For instance, significant variability in AD prevalence and incidence was observed across regions and age groups. This variability could be attributed to variations in study methodologies and design, research settings and AD definitions.
AD prevalence is heterogeneous, generally ranging from 10% up to 30% even for younger children younger than 2 years, suggesting an early onset during childhood. This early onset is usually associated with genetic predisposition and environmental factors.33 Although gender difference patterns seemed nonsignificant, the long study conducted by Arnedo-Pena et al. in a small region of Spain from 1994 though 2012 resulted in women showing a higher prevalence of AD,23 a trend not yet seen in the real-world global study conducted by Silverberg et al.25 including 18 countries. An increasing trend in AD prevalence in Spain was observed from 1994 through 2012.23 In contrast, a decrease in AD prevalence from 22.8% (1996) down to 21.3% (2006), and down to 16.3% (2017) was seen, for example, in Sweden,34 highlighting geographical variations.
Regarding AD incidence, in our review a stable trend in children aged 0–5 years from 2000 through 2017 was observed.24 Similarly, a study from Denmark and Sweden supports this stability, with consistent incidence rates throughout time.35 This stability suggests unchanged risk factors for AD development in this age group.34
In Spain, the current AD treatment guidelines for pediatric population suggest an adequate skin hydration and use of topical corticosteroids,36 which is consistent with the treatment patterns reported in the reviewed studies,19,20 yet we should mention that 24.1% of moderate-to-severe patients delayed initiation, or did not apply topical pharmacological maintenance treatment.19 The treatment adherence was low despite the patient's expressed satisfaction with treatment18 and regardless of disease severity.37 We should, also, mention the different perceptions between patients and specialists in the field, with specialists often overestimating the patient's compliance with the recommendations and prescribed treatment application.18
The use of biologic drugs as the treatment of choice among pediatric patients seemed to be limited, with only 1.5% of patients older than 6 years benefiting from this option.22 This limited usage of biologics in the Spanish pediatric population may be due to their very recent authorization for use and access barriers that might have prevented their prescription.38 However, biologic drugs may provide adequate control in moderate-to-severe cases, covering an unmet need in this pediatric population.39
Of note the significant gap in the literature on the economic burden of pediatric AD in Spain. While several studies have focused on the economic implications on adult patients,40,41 pediatric population research is lacking, despite existing research in other European countries highlighting important secondary care use of resources such as outpatient visits and pharmacy dispensations.42 The mean direct medical cost in Sweden (SD) was estimated at €1111 (3416) and €1906 (7067) for mild-to-moderate and severe AD, respectively, which is consistent with the Spanish data retrieved in our review. Nonetheless, there is a need for more targeted studies in this area.
This review presents some limitations. Variations in the definition and severity of AD across different scales had implications for the comparability and generalizability of our findings. Additionally, the heterogeneity in the available data, including differences in study design, data collection methods, and outcome measures, complicated result interpretation. Moreover, the methodologies and target populations also differed across studies, leading to variable results. There was a lack of comprehensive data on the use of resources and costs associated with AD in the pediatric population, which limits our understanding of the economic burden associated with AD.
ConclusionsThis systematic review provides a comprehensive picture of the clinical, humanistic and economic burden of AD in pediatric patients in Spain, which may be beneficial for physicians and health care decision makers who manage this condition, as well as researchers seeking to determine the gaps in knowledge that remain that need to be addressed in future research studies. The high prevalence and early onset highlight the need for comprehensive, patient-focused care tailored to this subpopulation. Despite the existence of established treatment guidelines, low adherence to treatment indicates a need for developing better and more effective strategies. On the other hand, biological therapy, including newly available treatments and small molecule JAK inhibitors, present a promising opportunity for innovation and expansion in therapeutic interventions. As modern and effective management strategies are developed, these key factors should be considered to improve patient outcomes.
Ethical approvalThis article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
FundingEli Lilly sponsored this study and the publication of this article.
Authors’ contributionsRosa Moro, Silvia Díaz-Cerezo, Luis Lizán and Mercedes Núñez contributed to the conception of the work, the design, the acquisition, and analysis of the data. All authors participated in the interpretation of data for the work, the critical revision of the manuscript and approved the final submitted version.
Conflicts of interestAntonio Torrelo Fernández is member of the advisory board of Eli Lilly and Viatris, has received speaker fees from Eli Lilly, Viatris, Pfizer, Sanofi, and Novartis, and fees for attending meetings from Sanofi and Pierre Fabre.
Asunción Vicente is member of the advisory board of Eli Lilly and Amryt, has received consulting fees from Abbvie, Amgen, Amryt, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novartis, Pierre Fabre and Sanofi Genzyme, speaker fees from Abbvie, Amgen, Amryt, Boehringer Ingelheim, Bristol-Myers Squibb, Ferrer, Galderma, Janssen, Eli Lilly, Novartis, Pierre Fabre, Pfizer, and Sanofi Genzyme and fees for attending meetings from Abbvie, Almirall, Amgen, Amryt, Boehringer Ingelheim, Bristol-Myers Squibb, Ferrer, Galderma, Eli Lilly, Novartis, Pierre Fabre, Pfizer, and Sanofi Genzyme.
Ana Martin-Santiago has received speaker fees from Abbvie, Amgen, Leo-Pharma, Leti, Novartis, Pfizer, and Sanofi, payments for her expert testimony from Abbvie, Amryt, Leo-Pharma, Pfizer, and Sanofi, and fees for attending meetings from Abbvie, Almirall, Janssen, Leo-Pharma, Leti, Eli Lilly, Novartis, Pfizer, Pierre Fabre, Sanofi, UCB and Viatrix.
Raúl de Lucas Laguna is member of the advisory board of Novartis, Abbvie, Lilly, Pfizer, Sanofi, Leo-Pharma, Johnson & Johnson, UCB, Almirall, Galderma, LetiPharma, has received consulting fees from Novartis, Abbvie, Lilly, Pfizer, Sanofi, Leo-Pharma, Johnson & Johnson, UCB, Almirall, Galderma, and LetiPharma, speaker fees from Novartis, Abbvie, Lilly, Pfizer, Sanofi, LetiPharma, Johnson & Johnson, UCB, Almirall, Galderma, and LetiPharma, payments for his expert testimony from Novartis, Abbvie, Lilly, Pfizer, Sanofi, LetiPharma, Johnson & Johnson, UCB, Almirall, Galderma, and LetiPharma, and support for attending meetings from Novartis, Abbvie, Lilly, Pfizer, Sanofi, Leo-Pharma, Johnson & Johnson, UCB, Almirall, Galderma, and LetiPharma.
José Carlos Armario Hita is member of the advisory board of Abbvie, Novartis, Lilly, Sanofi, Galderma, Leo-Pharma, Janssen, Pfizer, UCB farma, and Almirall, has received consulting fees from Abbvie, Novartis, Lilly, Sanofi, Galderma, Leo-Pharma, Janssen, Pfizer, UCB farma, Almirall, speaker fees from Abbvie, Novartis, Lilly, Sanofi, Galderma, Leo-Pharma, Janssen, Pfizer, UCB farma, Almirall, and fees for attending meetings from Abbvie, Novartis, Lilly, Sanofi, Galderma, Leo-Pharma, Janssen, Pfizer, UCB farma, and Almirall.
Rosa Moro, Silvia Díaz-Cerezo and Mercedes Núñez are employees of Eli Lilly. Luis Lizán works as an independent scientific consultant (Outcomes’10) and declared to have received honoraria for conducting the systematic literature review and manuscript drafting tasks.
Assistance in the methodology and editorial preparation of this article was provided by Outcomes’10. The authors hereby wish to thank Patricia Felip, Ana Causanilles and Héctor David de Paz for their help during data analysis and medical drafting tasks.












 16 severe AD; dSCORAD takes into account the extent and intensity of the lesions, as well as the symptoms (pruritus and loss of sleep) it causes; extent (A): the body surface is divided into four segments (head and neck, trunk, upper and lower extremities) to which a percentage is assigned based on the extent represented; intensity (B); eThe Clinical Global Impression – Severity scale (CGI-S) is a valuable tool used by clinicians to assess the severity of a patient's illness vs their past experience with similar diagnoses. It involves rating the patient's current condition on a 7-point scale, reflecting different levels of illness severity: (1) normal, not at all ill, (2) borderline mentally ill, (3) mildly ill, (4) moderately ill, (5) markedly ill, (6) severely ill, and (7) extremely ill.' title='AD severity. (A) Studies including mild, moderate and severe patients measured with IGA, PtGA and POEM. (B) Studies restricted to moderate-to-severe patients measured with SCORAD, IGA, CGI-SI, IGA and medical history. NA, not available; aIGA has six categories of response and its score ranges from 0 (no illness, with no inflammatory signs of AD) up to 5 (very serious illness, with intense erythema and intense papule/infiltration with crusts/exudation). Barroso et al. ranged severity from 0 up to 4, considering scores of 3 as moderate and scores of 4 as severe AD; bPatient Global Assessment (PtGA), which asks, “Please check one answer that best describes the severity of your or your child's eczema over the past week,” with responses of clear, mild, moderate, or severe; cPatient-Oriented Eczema Measure (POEM), with a total score ranging from 0 (lower severity) up to 28 (higher severity); severity groupings have been defined as bands of 0–7 indicating mild AD, 8–16 moderate AD, and >16 severe AD; dSCORAD takes into account the extent and intensity of the lesions, as well as the symptoms (pruritus and loss of sleep) it causes; extent (A): the body surface is divided into four segments (head and neck, trunk, upper and lower extremities) to which a percentage is assigned based on the extent represented; intensity (B); eThe Clinical Global Impression – Severity scale (CGI-S) is a valuable tool used by clinicians to assess the severity of a patient's illness vs their past experience with similar diagnoses. It involves rating the patient's current condition on a 7-point scale, reflecting different levels of illness severity: (1) normal, not at all ill, (2) borderline mentally ill, (3) mildly ill, (4) moderately ill, (5) markedly ill, (6) severely ill, and (7) extremely ill.'/>
16 severe AD; dSCORAD takes into account the extent and intensity of the lesions, as well as the symptoms (pruritus and loss of sleep) it causes; extent (A): the body surface is divided into four segments (head and neck, trunk, upper and lower extremities) to which a percentage is assigned based on the extent represented; intensity (B); eThe Clinical Global Impression – Severity scale (CGI-S) is a valuable tool used by clinicians to assess the severity of a patient's illness vs their past experience with similar diagnoses. It involves rating the patient's current condition on a 7-point scale, reflecting different levels of illness severity: (1) normal, not at all ill, (2) borderline mentally ill, (3) mildly ill, (4) moderately ill, (5) markedly ill, (6) severely ill, and (7) extremely ill.' title='AD severity. (A) Studies including mild, moderate and severe patients measured with IGA, PtGA and POEM. (B) Studies restricted to moderate-to-severe patients measured with SCORAD, IGA, CGI-SI, IGA and medical history. NA, not available; aIGA has six categories of response and its score ranges from 0 (no illness, with no inflammatory signs of AD) up to 5 (very serious illness, with intense erythema and intense papule/infiltration with crusts/exudation). Barroso et al. ranged severity from 0 up to 4, considering scores of 3 as moderate and scores of 4 as severe AD; bPatient Global Assessment (PtGA), which asks, “Please check one answer that best describes the severity of your or your child's eczema over the past week,” with responses of clear, mild, moderate, or severe; cPatient-Oriented Eczema Measure (POEM), with a total score ranging from 0 (lower severity) up to 28 (higher severity); severity groupings have been defined as bands of 0–7 indicating mild AD, 8–16 moderate AD, and >16 severe AD; dSCORAD takes into account the extent and intensity of the lesions, as well as the symptoms (pruritus and loss of sleep) it causes; extent (A): the body surface is divided into four segments (head and neck, trunk, upper and lower extremities) to which a percentage is assigned based on the extent represented; intensity (B); eThe Clinical Global Impression – Severity scale (CGI-S) is a valuable tool used by clinicians to assess the severity of a patient's illness vs their past experience with similar diagnoses. It involves rating the patient's current condition on a 7-point scale, reflecting different levels of illness severity: (1) normal, not at all ill, (2) borderline mentally ill, (3) mildly ill, (4) moderately ill, (5) markedly ill, (6) severely ill, and (7) extremely ill.'/>






