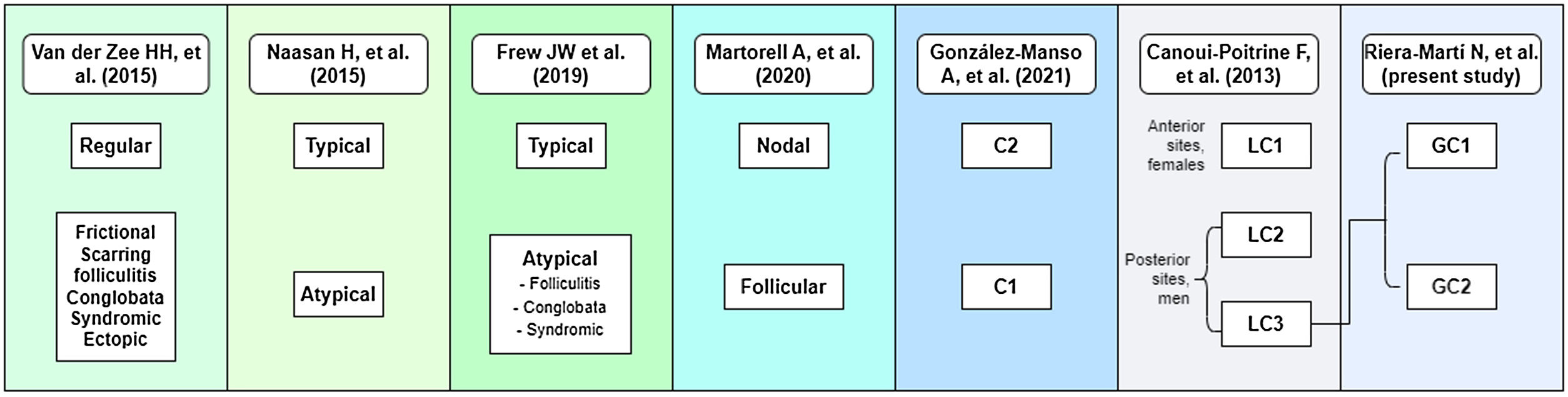
In 2013, Canoui-Poitrine et al. identified three hidradenitis suppurativa (HS) phenotypes by a latent class (LC) analysis, based on anatomical sites of involvement.
ObjectiveTo improve the classification of the gluteal phenotype (LC3) patients given their diverse lesion types and differences in clinical profile.
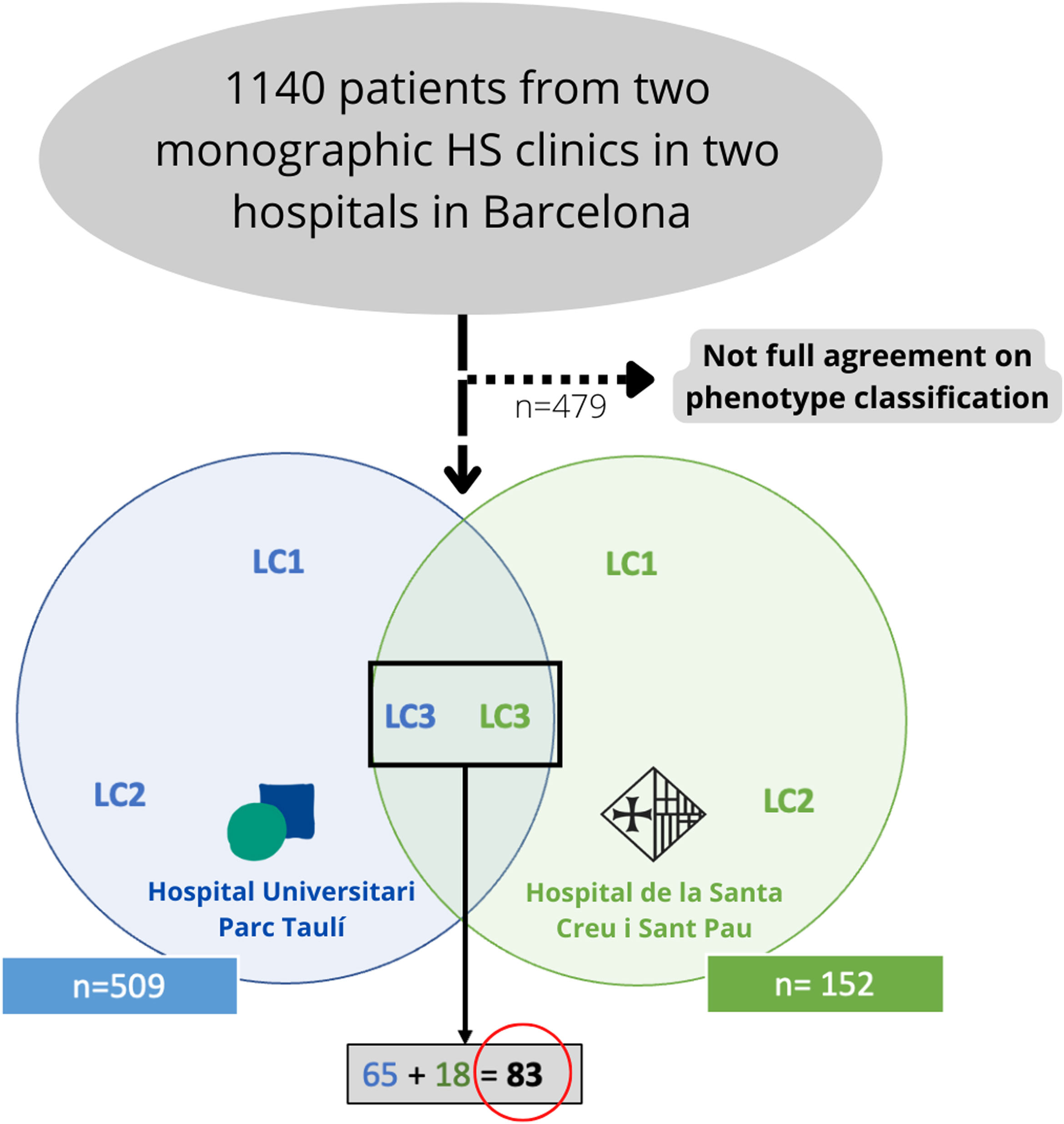
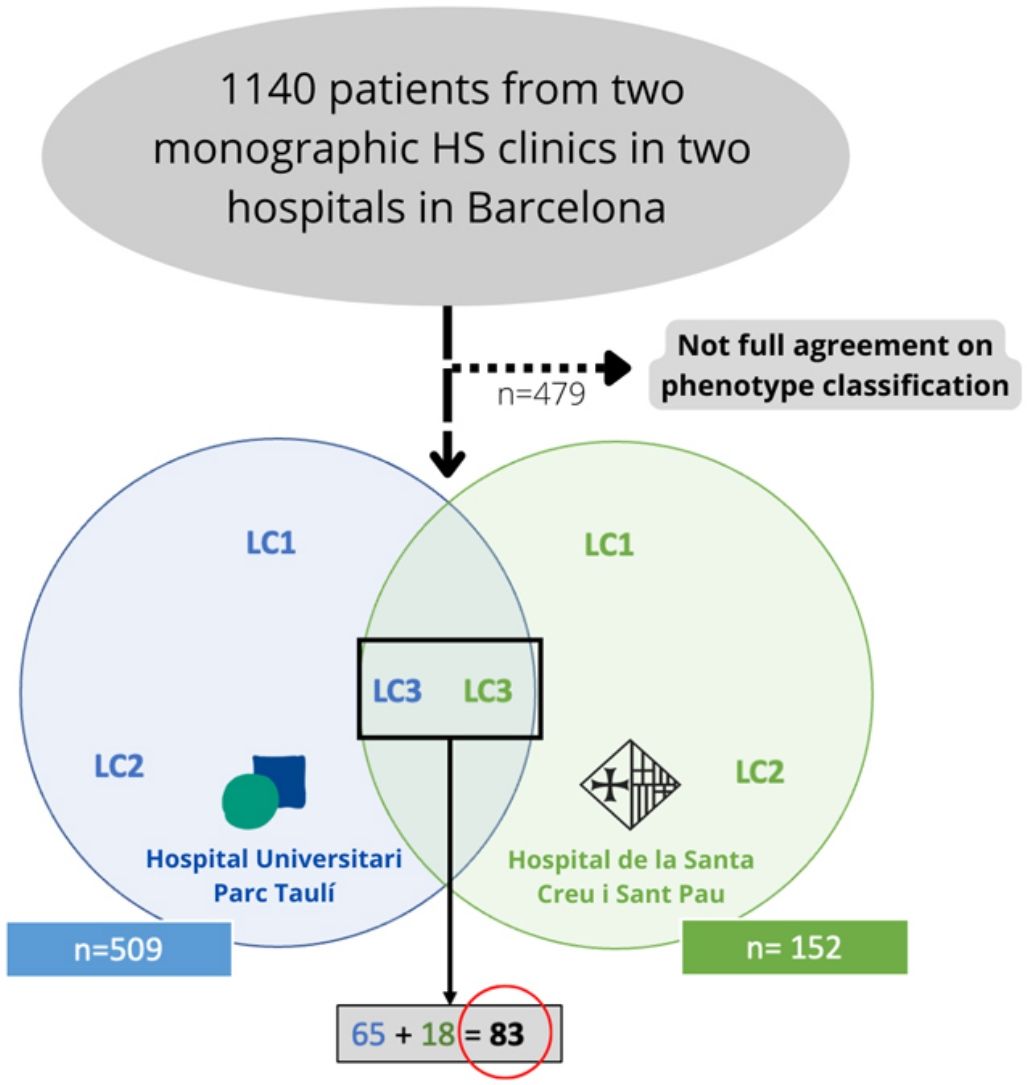
Material and methodsWe designed a bicentric study gathering all LC3 patients (n=83) from two hospitals. We conducted a two-step cluster analysis among them and also compared their characteristics with the rest of the HS patients (n=661).
ResultsCompared with global HS series, LC3 patients were more frequently non-obese men, with smoking habit, an associated arthropathy, and a more frequent history of pilonidal sinus. The analysis of LC3 patients yielded two clusters: cluster 1 (38.3%) included elderly female patients, with later diagnosis of the disease and more sinus tracts; cluster 2 (61.7%) encompassed more men with earlier disease onset and more nodules and folliculitis lesions.
LimitationsThe study's limitations include its retrospective nature, bicentric design, and small sample size.
ConclusionThe heterogeneous clinical presentation of HS makes it essential to have a good classification of the patients. Gluteal phenotype could actually be classified into two “subphenotypes” with a different clinical profiles and management.
En 2013 Canoui-Poitrine et al. identificaron tres fenotipos de hidradenitis supurativa (HS) mediante un análisis de clases latentes (LC) basado en las regiones anatómicas afectadas.
ObjetivoMejorar la clasificación de los pacientes con fenotipo glúteo (LC3) dados los diversos tipos de lesiones y diferencias en el perfil clínico.
Material y métodosDiseñamos un estudio bicéntrico que incluyó a todos los pacientes con LC3 (n=83) de dos hospitales terciarios españoles. Realizamos un análisis de conglomerados en dos etapas dentro del grupo LC3 y también comparamos sus características con el resto de los pacientes con HS (n=661).
ResultadosEn comparación con la serie global de HS, los pacientes con LC3 fueron más frecuentemente hombres no obesos, fumadores, con una artritis asociada y con mayor prevalencia de sinus pilonidal. El análisis de los pacientes LC3 resultó en dos grupos: el grupo 1 (38,3%), que incluía pacientes mayores, de sexo femenino, con diagnóstico más tardío de la enfermedad y más trayectos sinusales; y el grupo 2 (61,7%), que englobaba a más hombres con inicio temprano de la enfermedad y más nódulos y lesiones de foliculitis.
LimitacionesLas limitaciones del estudio incluyen su naturaleza retrospectiva, el diseño bicéntrico y el tamaño muestral reducido.
ConclusiónLa presentación clínica heterogénea de la HS hace que sea esencial disponer de una buena clasificación clínica de los pacientes. Como hemos visto, parece que el fenotipo glúteo podría clasificarse en dos «subfenotipos» con perfiles clínicos y, consecuentemente, enfoques terapéuticos diferentes.
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by recurrent, painful, and draining lesions in multiple regions. HS lesions include non-inflammatory and inflammatory nodules, abscesses, sinus tracts, and hypertrophic scars. The diagnosis is made clinically according to the modified Dessau definition.1–5 It has recently been suggested that ultrasonography could help in the diagnosis, classification, and treatment monitoring of the lesions by giving information about lesion type, depth, and inflammatory activity.6
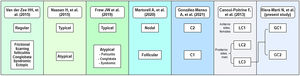
To date, several attempts have been made to classify HS patients according to pathogenesis and clinical manifestations.2,7–17 In 2013 Canoui-Poitrine et al. (C-P)17 identified three HS phenotypes by a latent class analysis based mainly on anatomical sites of involvement, and they published the most accepted classification to date. Lately, in 2015, Naasan et al.10 proposed a “typical/atypical” classification also based on lesion topography. In the same year, van der Zee and Jemec13 suggested another classification comprising six groups following clinical and epidemiological features of the disease. In 2020, Martorell et al.16 published a new classification and some months later, González-Manso et al., from our research group,15 proposed an “endotype” classification based on the patients’ morphologic profiles, and main available biomarkers using a two-step cluster analysis, resulting on a two groups classification (C1 and C2) which combined features of the classification of Naasan et al. and Martorell et al.
Focusing on the C-P classification, one of the groups was defined as “gluteal” (LC3), comprising patients with gluteal involvement with papules and folliculitis. LC3 patients were usually men, less often obese (0.6; 0.3–0.95; p=0.03) and with less severe disease (0.9; 0.7–1.1; p=0.001),17 compared with other HS patients. However, following our clinical practice, we believe that we should try to better classify this group, because we have observed that sinus tracts are also present in some of these patients, and probably LC2 and LC3 patients may overlap (Fig. 1).
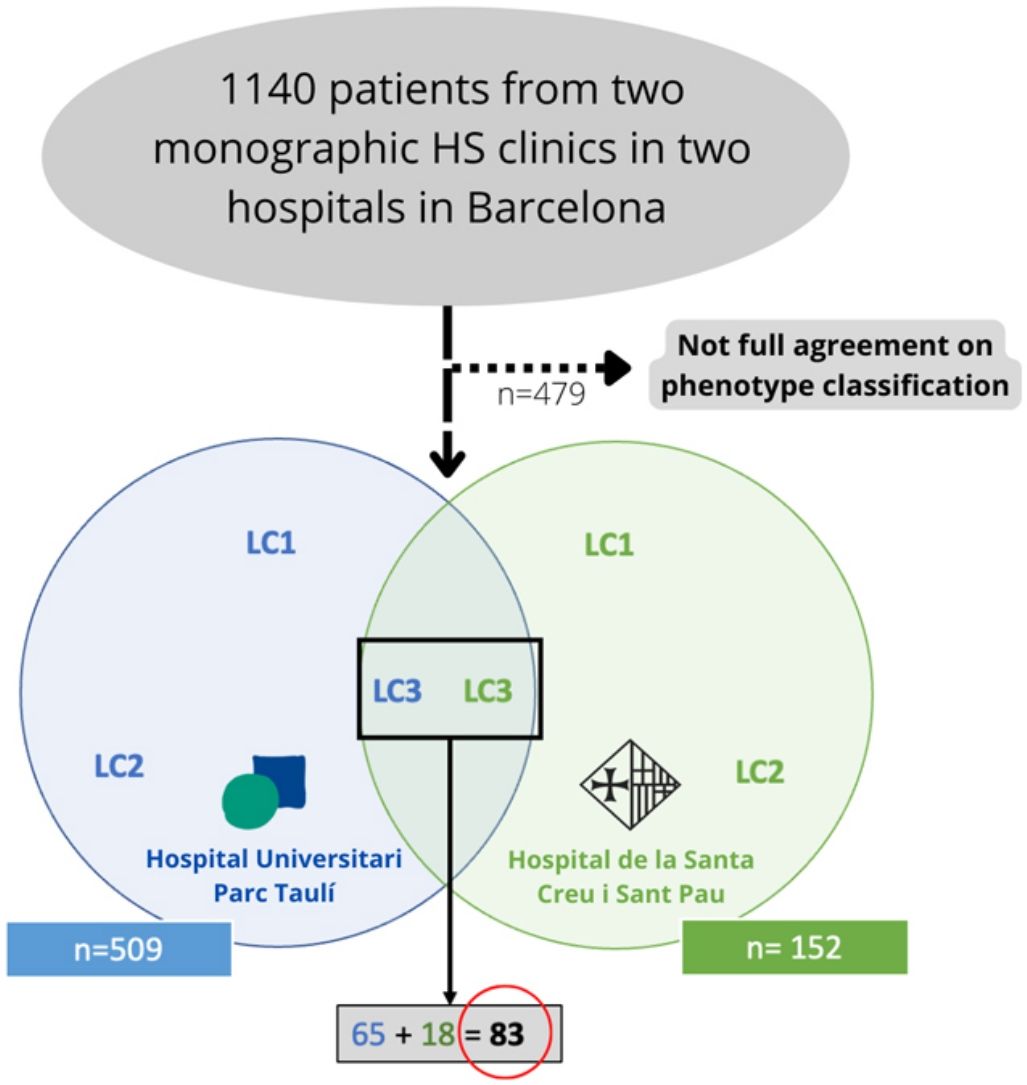
Materials and methodsWe conducted a bicentric retrospective study gathering all the patients classified as LC3 following the C-P classification criteria17 (n=83) from two monographic HS clinics in two tertiary hospitals in Barcelona, Spain (Fig. 2). All patients were included from May 2014 to January 2022. To better classify the phenotypes, all patients were examined by a first trained dermatologist from one of the aforementioned hospitals (according to clinical aspects and complementary tests such as ultrasound or RMI), and the diagnosis was validated by a second dermatologist (from the other hospital), based on clinical photographs. Only those patients who achieved full agreement in the validation were included. The study protocol was accepted by the ethics committee of our hospital. Written informed consent was obtained from all study participants. From all the included patients we collected the following demographic and clinical data: age, gender, age of onset, BMI, Hurley stage, tobacco habit, presence of pilonidal sinus and HS family history. We also gathered information about the received treatments (dapsone, antibiotics, biological treatment, and surgery).
Datasets were tested for normality. For comparison of LC3 patients with the rest of the HS patients, a Chi2-test was applied to dichotomous variables, and one-way ANOVA was applied to continuous variables. Univariate and multivariate analysis with logistic regression was also carried out. All p-values were two-sided and p<0.05 was considered statistically significant. Results are reported as mean±standard deviation. We also conducted a two-step cluster analysis among patients classified as having “gluteal” or LC3 phenotype (IBM SPSS statistics v22®).
ResultsFrom an initial number of 1140 patients, we achieved full agreement on the phenotypes in 661 patients. Among the global series of HS patients from the two mentioned hospitals, the LC3 group represented 12.55% (83/661), showing their features in Table 1.
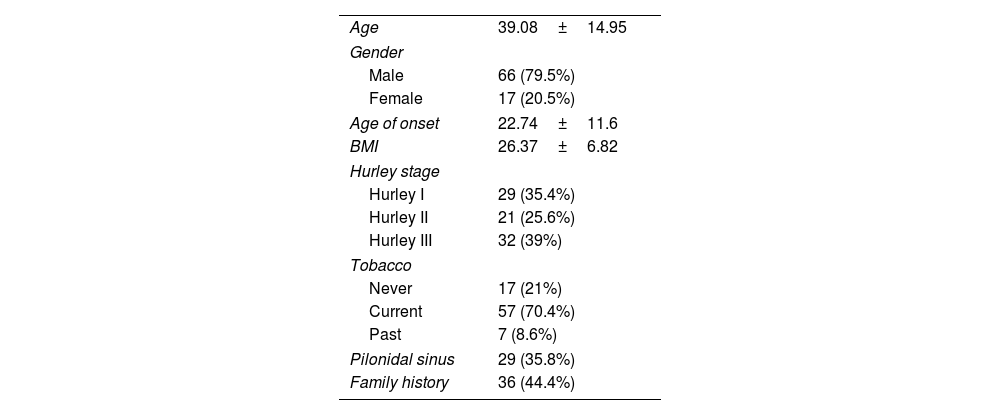
Basal features of analyzed patients.
| Age | 39.08±14.95 |
| Gender | |
| Male | 66 (79.5%) |
| Female | 17 (20.5%) |
| Age of onset | 22.74±11.6 |
| BMI | 26.37±6.82 |
| Hurley stage | |
| Hurley I | 29 (35.4%) |
| Hurley II | 21 (25.6%) |
| Hurley III | 32 (39%) |
| Tobacco | |
| Never | 17 (21%) |
| Current | 57 (70.4%) |
| Past | 7 (8.6%) |
| Pilonidal sinus | 29 (35.8%) |
| Family history | 36 (44.4%) |
In the bivariate analysis between the selected LC3 patients and the rest of the HS patients, we obtained that the LC3 phenotype patients were more frequently men (p<0.001), smokers (p=0.024), with a lower BMI (p<0.001), had more associated arthropathy (p=0.003), more presence of a pilonidal sinus (p=0.004) and suffered from a more severe disease (Hurley III, p=0.014).
Furthermore, the multivariate analyses showed that LC3 group was more dependent on male gender (p=0.007), low BMI (p=0.015), tobacco use (p=0.018) and arthropathy (p=0.02).
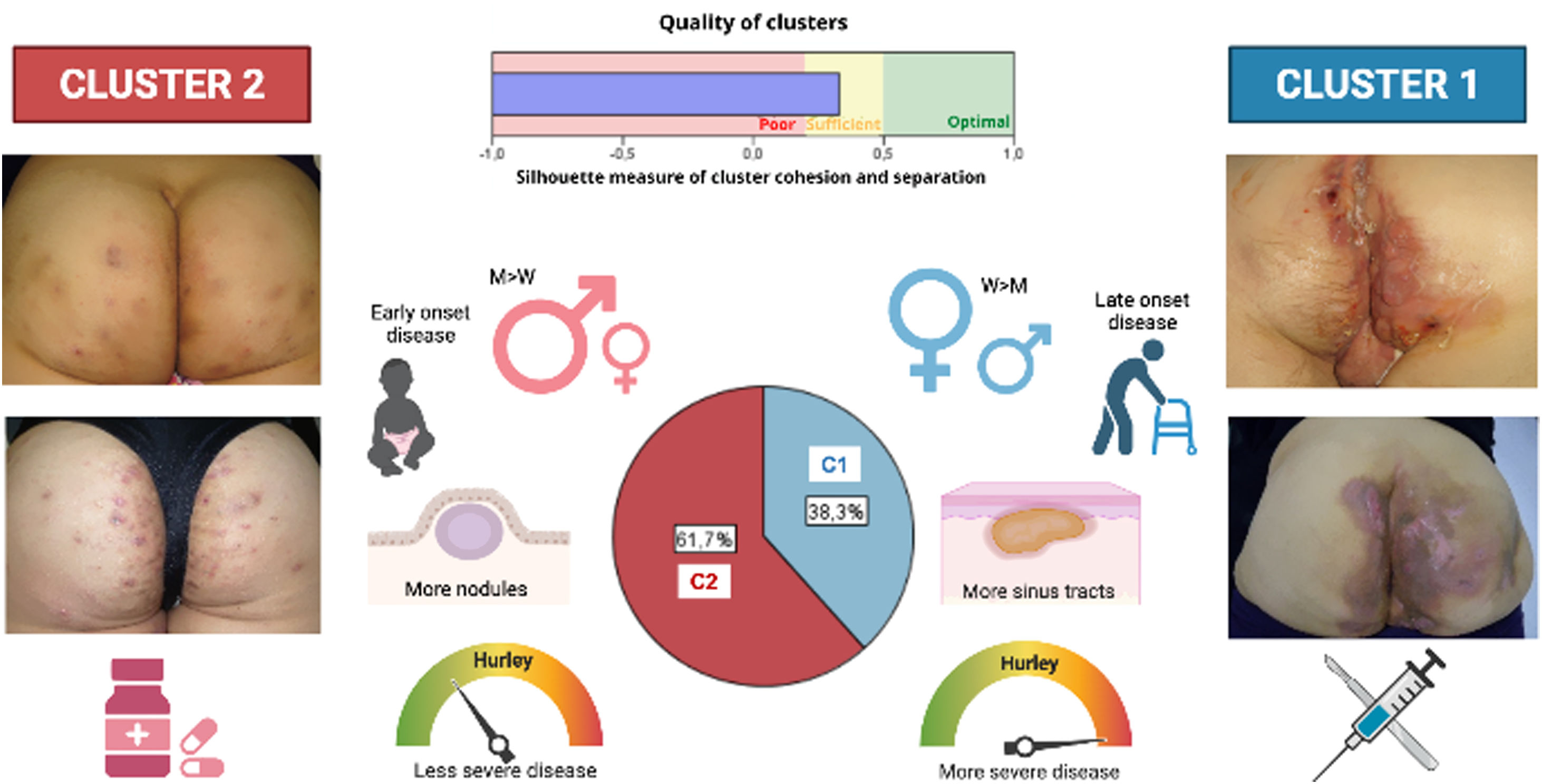
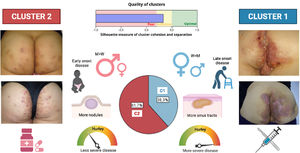
A two-step cluster analysis among all LC3 HS patients (n=83) yielded the following two groups, with fair cohesion: cluster 1 (38.3% of the LC3 patients), including elderly female patients, with later diagnosis of the disease and more severe (more Hurley III patients), less sinus pilonidal lesions, and more sinus tracts, instead of nodules and folliculitis; and cluster 2 (61.7%), encompassing more men with less evolved and severe disease, more sinus pilonidal lesions, and more nodules and folliculitis lesions.
Finally, referring to treatment, we found that therapeutic interventions differed, depending on the patient's phenotype. We analyzed the use of oral and intravenous antibiotics, dapsone, biological treatments, retinoids, and surgery (not including simple incision and drainage). Oral antibiotics were widely used in both groups. Retinoids were more used in those patients presenting with nodules but without sinus tracts, whereas biological treatment, intravenous antibiotics, and surgery were more used in patients with sinus tracts. Likewise, these last three treatments were more frequent as disease severity increased (Hurley III).
DiscussionHerein, we present a novel study with a large cohort of HS patients (n=661), focusing on the third phenotype described by C-P, the LC3 or “gluteal” phenotype. The percentage obtained for the LC3 phenotype (12.55%) was less than the 26% obtained by C-P,17 being the least frequent of the three HS phenotypes described. The predominance of male sex and smoking habit in our “LC3” patients, was comparable to the original description by Canoui-Poitrine et al.,17 but, contrary, the severity of the disease was greater than that observed in the overall HS series. However, we found two subgroups that could be differentiated by lesion types. Not all of them presented with “papules and folliculitis” as in the original description. Since LC3 patients present with different types of lesions and clinical features, this would permit to re-classify this group into two new groups or clusters that might be named “subphenotypes” (Fig. 3).
Diagram showing the two-step cluster model resulting in two groups. Cluster 1 (C1), (38.3% of the LC3 patients), including more female patients, with later diagnosis, more severe disease, and more sinus tracts; and cluster 2 (C2), (61.7%), encompassing more men with less evolved and severe disease and more nodules and folliculitis lesions. Furthermore, therapeutic interventions differed depending on the patient's phenotype. Surgery and biological treatments were more used among C1 patients, whereas retinoids and dapsone were more frequent in C2 patients. M, men; W, women.
One of them (cluster 1 or “fistulous”) seems to be closely related to the “Inflammatory” group described by Martorell et al.,16 with a less genetic burden which fits with later disease onset, and less keratinization disorder or follicular occlusion, as they present with fewer pilonidal sinus lesions and fewer comedos and nodular/folliculitis lesions.
The other group (cluster 2 or “nodular”) seems to be closer to the “Follicular” phenotype16 because in them we find more signs of keratinization disorder (such as more pilonidal sinus), more nodular lesions, and fewer sinus tracts. Also, they have an earlier disease onset, translating a probable major genetic burden linked to gamma-secretase mutations, previously described.12,18,19
However, these clusters are probably not static but dynamic, and as severity increases, one cluster can evolve into another. In our clinical experience, this means that sometimes a “nodular” subphenotype (a patient who until that moment had only presented with some nodules and folliculitis lesions) evolves into a “fistulous” patient (suddenly presents with a sinus tract involving the gluteal area), but not vice versa.
In the same way, some of these patients who initially only present gluteal involvement may end up presenting lesions in other anatomical regions, evolving to LC1 or LC2 phenotypes.
Another question that may arise is if these two clusters also differ from the treatment. From our experience, and after reviewing all the treatments received by our 83 gluteal patients, we usually treated cluster 1 patients with intravenous antibiotics and biological treatments, whereas dapsone and retinoids are more used among cluster two patients. As described recently by López-Llunell et al.,20 sinus tracts could be a marker of non-response to these drugs.
Regarding treatment with surgery, this tends to be more frequent among patients in cluster 1, since, as described in HS guidelines, it is the first choice for patients not responding to medical treatment and presenting in the late stages.21–24
However, we have not detected differences in the use of oral antibiotics between the two clusters, since regardless of the “maintenance” treatment that all patients receive, we frequently used short antibiotic courses during the flares.24
Recently, Cuenca-Barrales et al.25 published a retrospective study with 233 patients about the implications of HS phenotypes (inflammatory (IP) and follicular (FP)) in treatment decisions, and they reported that IP patients were more likely to be treated with systemic corticosteroids or adalimumab, whereas the FP patients were more likely to be treated with combined oral contraceptives. They also pointed out that surgery, especially wide excisions, was mainly indicated for IP patients. The results are similar to those observed by our group.
Our study has some methodological limitations. First, the sample size was small, which limits its generalizability. Secondly, the bicentric and retrospective design of the study adds difficulty to the patient's phenotype classification; and even though all were examined by a trained dermatologist in a monographic consultation, the validation of the diagnosis by a second dermatologist, was only based on clinical photographs. Additionally, disease management was also retrospectively evaluated.
ConclusionsIn conclusion, our study provided evidence that gluteal phenotype could be subclassified into two “subphenotypes” with a different clinical profile and probably different pathogenesis, which in turn may lead to different therapeutic management. So, maybe, to simplify and equalize all the existing classifications, we could say that all patients classified as LC3 are, actually, “typical/atypical” patients with restricted gluteal involvement. All these supports the idea that in HS, given its heterogeneous clinical presentation, it is essential to have a good classification of the patients, to continue advancing in the understanding of the disease and improving its therapeutic management. More studies, with larger populations, would be necessary in the future to reach a definite classification.
Conflict of interestJorge Romaní has received honoraria from Abbvie, Novartis, Almirall, Janssen, LEO Pharma, UCB, and Celgene for participation on advisory boards, conferences, and as an investigator in clinical trials. Eva Vilarrasa has received honoraria from Amgen, MSD, Gebro, Pfizer, UCB, Lilly, Galderma, Biofrontera, Abbvie, Novartis, Almirall, Janssen, LEO Pharma, and Celgene for participation on advisory boards, conferences, and as an investigator in clinical trials.