There is insufficient information on how best to treat moderate to severe psoriasis in difficult clinical circumstances.
Material and methodsWe considered 5 areas where there is conflicting or insufficient evidence: pediatric psoriasis, risk of infection in patients being treated with biologics, psoriasis in difficult locations, biologic drug survival, and impact of disease on quality of life. Following discussion of the issues by an expert panel of dermatologists specialized in the management of psoriasis, participants answered a questionnaire survey according to the Delphi method.
ResultsConsensus was reached on 66 (70.9%) of the 93 items analyzed; the experts agreed with 49 statements and disagreed with 17. It was agreed that body mass index, metabolic comorbidities, and quality of life should be monitored in children with psoriasis. The experts also agreed that the most appropriate systemic treatment for this age group was methotrexate, while the most appropriate biologic treatment was etanercept. Although it was recognized that the available evidence was inconsistent and difficult to extrapolate, the panel agreed that biologic drug survival could be increased by flexible, individualized dosing regimens, continuous treatment, and combination therapies. Finally, consensus was reached on using the Dermatology Quality of Life Index to assess treatment effectiveness and aid decision-making in clinical practice.
ConclusionsThe structured opinion of experts guides decision-making regarding aspects of clinical practice for which there is incomplete or conflicting information.
En situaciones clínicas difíciles de psoriasis moderada y grave la información sobre las estrategias terapéuticas más adecuadas es insuficiente.
Material y métodosSe plantearon 5 escenarios: psoriasis pediátrica, infecciones en pacientes tratados con biológicos, localizaciones difíciles, supervivencia de las terapias biológicas e impacto en la calidad de vida, identificando aquellas cuestiones en las que la evidencia era controvertida o insuficiente. Tras la discusión con un grupo de dermatólogos expertos en el manejo de la psoriasis moderada-grave, se llevó a cabo un cuestionario que fue implementado según la metodología Delphi.
ResultadosSe alcanzó el consenso en 66 de los 93 ítems finales planteados (70,9%) (49 en el acuerdo, 17 en el desacuerdo). Se acordó la conveniencia de incorporar la evaluación del índice de masa corporal, las comorbilidades metabólicas y la medición de la calidad de vida en el seguimiento de la psoriasis infantil. En este grupo de edad, el metotrexato y el etanercept se consideraron las mejores opciones de tratamiento sistémico y biológico, respectivamente. Aunque la evidencia científica se interpretó como heterogénea y de difícil extrapolación, se consensuó que la individualización y la flexibilidad en las dosis, el tratamiento continuo y las combinaciones terapéuticas incrementaban la supervivencia del fármaco. Se acordó la conveniencia de incorporar el índice de calidad de vida en dermatología como marcador de eficacia terapéutica y en la toma de decisiones en la práctica clínica.
ConclusionesLa opinión estructurada de los expertos contribuye en la toma de decisiones en aquellos aspectos en los que la información disponible es incompleta o contradictoria.
In recent years, the availability of new evidence of high quality has led to a qualitative leap forward in the management of psoriasis. However, while the evidence relating to the clinical situations evaluated in pivotal clinical trials is robust and consistent, information on the more complex cases and special circumstance excluded from these studies is deficient. The approach to treatment in such situations differs considerably from the indications specified in the Summary of Product Characteristics and standard recommendations based on structured studies. In this context, articles based on evidence from clinical practice provide data that inform an approach to treatment which is less structured but facilitates practical and strategic conclusions. For example, studies of data relating to everyday clinical practice taken from the BIOBADADERM prospective registry have shown that the proportion of patients receiving biologic therapy increases with longer follow-up1 and that reduction and escalation of treatment are common strategies in clinical practice.2 Consensus recommendations based on expert opinion and drawn up using a rigorous and valid methodology also provide useful guidelines for these situations because they provide clinicians with valuable and specific information that would be difficult to obtain elsewhere. Of interest in this respect are several studies that have used the Delphi method to develop consensus recommendations relating to different aspects of psoriasis and the management of challenging case scenarios.3–10
In a earlier study based on the Delphi method, an expert panel agreed on proposals for managing 5 special clinical scenarios.11 The objective of the present study was to expand that project to include 5 additional situations in which the approach to treatment has not been clearly defined or for which no management protocols based on robust evidence exist.
Material and MethodsScientific CommitteeThe scientific committee was made up of 5 clinical dermatologists with considerable experience in the treatment of moderate to severe psoriasis. The work of the committee was directed by a national coordinator. Each committee member proposed several clinical scenarios for which they considered it would be of interest to develop clinical recommendations because the best approach to treatment in such cases had not been clearly established. Following a meeting of the committee to discuss and analyze these proposals, the following 5 scenarios were chosen: moderate to severe pediatric psoriasis; risk of infection in patients being treated with biologics; psoriasis affecting difficult-to-treat sites; biologic drug survival in psoriasis; and the impact of psoriasis on quality of life.
Literature ReviewThe members of the scientific committee, with the assistance of an independent external methodologist, performed a systematic and exhaustive review of the literature on the following databases published between 2009 and 2014: MEDLINE, Embase, The Cochrane Library, U.S. National Guidelines Clearinghouse, Tripdatabase, and the Spanish Biblioteca de Guías de Práctica Clínica del Sistema Nacional de Salud (GuiaSalud). In the case of clinical trials dealing specifically with the topics of interest, no time limitation was applied. The objective of the literature review was to draw up a set of statements on each of the chosen topics relating to the aspects considered to be of greatest interest in clinical practice for each of the 5 scenarios. The committee members proposed the questions they considered to be of greatest interest and currently not covered by the evidence in the literature, and these were submitted to the consensus process.
The search was performed in December 2014 and included only articles published in Spanish or English. The levels of evidence and grades of recommendation for the articles included were evaluated according to the guidelines established by the Scottish Intercollegiate Guidelines Network (SIGN).12
Development of the Questionnaire and Application of the Delphi MethodAfter critically assessing the evidence, the scientific committee drew up a preliminary questionnaire designed to complement the available evidence with the structured opinion and consensus of a panel of clinicians with expertise in the management of psoriasis. In total, 25 Spanish dermatologists with appropriate experience were invited to participate in the process. These 25 dermatologists, who came from different parts of the country, attended a meeting at which each member of the scientific committee presented one of the proposed scenarios. Ample time was allowed to explain the methodology that would be used in the study and to discuss each scenario in depth. To emphasize the clinical focus of the study, the 5 scenarios were stated in the form of problematic cases and the questions were then formulated on the basis of this starting point.
The final questionnaire, which took the form of a series of statements, was then drawn up on the basis of the initial questions proposed by the committee, the literature review, and the additional questions suggested during the meeting. The aim was to create a survey that would structure the opinion of the experts on the controversial aspects of each of the topics presented. The final questionnaire comprised 93 items and was divided into 5 sections relating to the 5 clinical scenarios.
After the meeting, the final questionnaire was sent, in an electronic format, to the members of the expert panel, who were asked to respond by indicating their degree of agreement with each statement on a scale from 1 to 9, in which 1 indicated strong disagreement and 9 strong agreement. Each statement was accompanied by a blank field where the respondents could include any comments they considered useful. The methodology used was the Delphi process modified as per the RAND/UCLA recommendations.13 After results of the first round of voting had been analyzed, the participants voted on a second-round questionnaire that only included the statements on which consensus had not been reached in the first round. In this second round of voting, each statement was scored using the same criteria as before or could be modified to take into account the responses of other panel members on the first round of voting.
AnalysisAn item (statement) was deemed to have achieved positive consensus if the median score was in the region of 7 to 9, negative consensus if the median score was in the region of 1 to 3, and no consensus if the median score was in the range of 4 to 6. To meet the criteria for consensus, 2 additional criteria also had to be met: a) the number of panelists who scored the item outside the extreme 3-point regions (1-3 or 7-9) had to represent less than 1/3 of the total panel; and b) the interquartile range (IQR) of the median of the responses had to be 4 or lower.
The Delphi methodology used is described in detail in Appendix 1 (additional material).
ResultsThe questionnaire assessed by the panel comprised 93 items. In the first round of voting, the panel agreed on 51 of the 93 statements (54.8%), with positive consensus on 40 items and negative consensus on 11. Overall, after the 2 rounds of voting, the panel agreed on 66 of the 93 statements (70.9%), with positive consensus on 49 items and negative consensus on 17 (Tables 1–5).
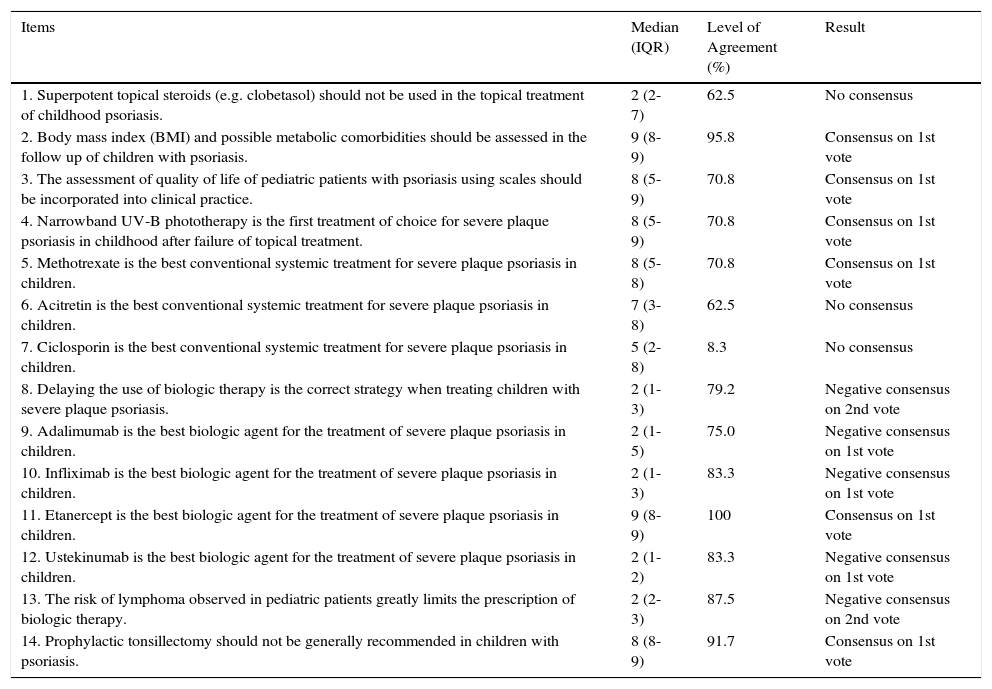
Results for Scenario 1. Moderate to Severe Pediatric Psoriasis. Treatment Controversies.
| Items | Median (IQR) | Level of Agreement (%) | Result |
|---|---|---|---|
| 1. Superpotent topical steroids (e.g. clobetasol) should not be used in the topical treatment of childhood psoriasis. | 2 (2-7) | 62.5 | No consensus |
| 2. Body mass index (BMI) and possible metabolic comorbidities should be assessed in the follow up of children with psoriasis. | 9 (8-9) | 95.8 | Consensus on 1st vote |
| 3. The assessment of quality of life of pediatric patients with psoriasis using scales should be incorporated into clinical practice. | 8 (5-9) | 70.8 | Consensus on 1st vote |
| 4. Narrowband UV-B phototherapy is the first treatment of choice for severe plaque psoriasis in childhood after failure of topical treatment. | 8 (5-9) | 70.8 | Consensus on 1st vote |
| 5. Methotrexate is the best conventional systemic treatment for severe plaque psoriasis in children. | 8 (5-8) | 70.8 | Consensus on 1st vote |
| 6. Acitretin is the best conventional systemic treatment for severe plaque psoriasis in children. | 7 (3-8) | 62.5 | No consensus |
| 7. Ciclosporin is the best conventional systemic treatment for severe plaque psoriasis in children. | 5 (2-8) | 8.3 | No consensus |
| 8. Delaying the use of biologic therapy is the correct strategy when treating children with severe plaque psoriasis. | 2 (1-3) | 79.2 | Negative consensus on 2nd vote |
| 9. Adalimumab is the best biologic agent for the treatment of severe plaque psoriasis in children. | 2 (1-5) | 75.0 | Negative consensus on 1st vote |
| 10. Infliximab is the best biologic agent for the treatment of severe plaque psoriasis in children. | 2 (1-3) | 83.3 | Negative consensus on 1st vote |
| 11. Etanercept is the best biologic agent for the treatment of severe plaque psoriasis in children. | 9 (8-9) | 100 | Consensus on 1st vote |
| 12. Ustekinumab is the best biologic agent for the treatment of severe plaque psoriasis in children. | 2 (1-2) | 83.3 | Negative consensus on 1st vote |
| 13. The risk of lymphoma observed in pediatric patients greatly limits the prescription of biologic therapy. | 2 (2-3) | 87.5 | Negative consensus on 2nd vote |
| 14. Prophylactic tonsillectomy should not be generally recommended in children with psoriasis. | 8 (8-9) | 91.7 | Consensus on 1st vote |
a Adalimumab and ustekinumab had not been approved for use in children at the time of the survey, although results from clinical trials were available.
Abbreviation: IQR, interquartile range.
Results for Scenario 2. Risk of Infections in Patients with Psoriasis on Biologic Therapy.
| Items | Median (IQR) | Level of Agreement (%) | Result |
|---|---|---|---|
| 15. Patients with psoriasis on biologic therapy should be screened annually for latent tuberculosis. | 9 (7-9) | 79 | Consensus on 1st vote |
| 16. The Mantoux test should be performed routinely in patients with psoriasis before conventional systemic therapy is prescribed. | 8 (7-9) | 83 | Consensus on 2nd vote |
| 17. When the initial result for the Mantoux test is negative, the test should be repeated to identify booster reactions before the patient starts biologic therapy. | 9 (9-9) | 88 | Consensus on 1st vote |
| 18. IGRA is the test method of choice for diagnosing latent tuberculosis in patients with psoriasis before starting biologic therapy. | 8 (7-9) | 79 | Consensus on 1st vote |
| 19. The Mantoux test is the method of choice for screening patients for latent tuberculosis infection. | 5 (2-8) | 21 | No consensus |
| 20. It is essential to perform both the Mantoux test and IGRA to diagnose latent tuberculosis. | 6.5 (3-8) | 50 | No consensus |
| 21. Etanercept is the biologic agent associated with the lowest risk of reactivation of latent tuberculosis in patients with psoriasis on biologic therapy. | 8 (6-9) | 71 | Consensus on 2nd vote |
| 22. Infliximab is the biologic agent associated with the lowest risk of reactivation of latent tuberculosis in patients with psoriasis on biologic therapy. | 1 (1-2) | 92 | Negative consensus on 1st vote |
| 23. Adalimumab is the biologic agent associated with the lowest risk of reactivation of latent tuberculosis in patients with psoriasis on biologic therapy. | 1 (1-3) | 88 | Negative consensus on 1st vote |
| 24. Ustekinumab is the biologic agent associated with the lowest risk of reactivation of latent tuberculosis in patients with psoriasis on biologic therapy. | 8 (8-9) | 92 | Consensus on 2nd vote |
| 25. The incidence of opportunistic infections is higher in patients with psoriasis on biologic therapy in clinical practice than in the population in general. | 8 (6-9) | 75 | Consensus on 2nd vote |
| 26. In patients with psoriasis, anti-tumor necrosis factor monoclonal antibodies (infliximab and adalimumab) are associated with a higher incidence of herpes zoster infections than conventional systemic drugs. | 8 (7-8) | 83 | Consensus on 2nd vote |
| 27. In patients with psoriasis, etanercept is associated with a higher incidence of herpes zoster infections than conventional systemic drugs. | 2.5 (2-6) | 58 | No consensus |
| 28. All patients with psoriasis who are candidates for biologic therapy should be vaccinated against the herpes zoster virus (anti-VZV). | 3 (1-7) | 58 | No consensus |
| 29. The risk of serious infection limits the use of biologic agents in the treatment of moderate to severe psoriasis in clinical practice. | 2 (1-3) | 79 | Negative consensus on 1st vote |
| 30. Etanercept is the biologic drug that carries the lowest risk of serious infections in patients with moderate to severe psoriasis. | 8 (5-9) | 71 | Consensus on 1st vote |
| 31. Infliximab is the biologic drug that carries the lowest risk of serious infections in patients with moderate to severe psoriasis. | 1 (1-2) | 92 | Negative consensus on 1st vote |
| 32. Adalimumab is the biologic drug that carries the lowest risk of serious infections in patients with moderate to severe psoriasis. | 2 (1-2) | 83 | Negative consensus on 2nd vote |
| 33. Ustekinumab is the biologic drug that carries the lowest risk of serious infections in patients with moderate to severe psoriasis. | 8 (5-8) | 75 | Consensus on 2nd vote |
| 34. Liver function should be monitored every 3 months in patients with chronic HCV infection who are receiving biologic therapy for psoriasis. | 9 (8-9) | 100 | Consensus on 1st vote |
| 35. Follow up of patients with chronic HCV infection who are being treated with biologic drugs for psoriasis should include an HCV viral load test. | 9 (8-9) | 92 | Consensus on 1st vote |
| 36. Etanercept is the biologic agent that carries the lowest risk of reactivating HCV infection. | 8 (7-9) | 88 | Consensus on 1st vote |
| 37. Infliximab is the biologic agent that carries the lowest risk of reactivating HCV infection. | 1.5 (1-3) | 83 | Negative consensus on 1st vote |
| 38. Adalimumab is the biologic agent that carries the lowest risk of reactivating HCV infection. | 2 (1-3) | 83 | Negative consensus on 2nd vote |
| 39. Ustekinumab is the biologic agent that carries the lowest risk of reactivating HCV infection. | 5 (3-6) | 58 | No consensus |
| 40. Patients receiving biologic therapy for psoriasis who have chronic HBV infection and test negative for active viral replication should receive antiviral treatment. | 8.5 (5-9) | 71 | Consensus on 1st vote |
| 41. Patients with psoriasis who are receiving treatment with conventional systemic or biologic drugs and have chronic HBV infection should be monitored every 3 months. | 9 (8-9) | 92 | Consensus on 1st vote |
| 42. Viral load testing should be included in the monitoring of patients with psoriasis who have chronic HBV infection and are being treated with conventional systemic or biologic drugs. | 9 (8-9) | 96 | Consensus on 1st vote |
| 43. Etanercept is the biologic agent that carries the lowest risk of reactivating HBV infection. | 8 (5-8) | 71 | Consensus on 2nd vote |
| 44. Infliximab is the biologic agent that carries the lowest risk of reactivating HBV infection. | 2 (1-5) | 71 | Negative consensus on 1st vote |
| 45. Adalimumab is the biologic agent that carries the lowest risk of reactivating HBV infection. | 2 (1-5) | 67 | No consensus |
| 46. Ustekinumab is the biologic agent that carries the lowest risk of reactivating HBV infection. | 5 (4-6) | 58 | No consensus |
| 47. Patients with moderate to severe psoriasis should be vaccinated against influenza virus before starting treatment with biologic drugs. | 8 (7-9) | 83 | Consensus on 1st vote |
| 48. Patients with moderate to severe psoriasis should be vaccinated against pneumococcal bacteria before starting treatment with biologic drugs. | 8 (7-9) | 83 | Consensus on 1st vote |
| 49. Patients with moderate to severe psoriasis should be vaccinated against HBV before starting treatment with biologic drugs. | 9 (8-9) | 92 | Consensus on 1st vote |
Abbreviation: HBV, hepatitis B virus; HCV, hepatitis C virus; IGRA, Interferon Gamma Release Assay; IQR, interquartile range.
Results for Scenario 3. Psoriasis in Difficult Sites: Controversies, Impact, and Treatment.
| Items | Median (IQR) | Level of Agreement (%) | Result |
|---|---|---|---|
| 50. Plaque psoriasis affecting difficult-to-treat sites (scalp, nails, palms and soles of the feet) has a greater impact on the quality of life of the patient than disease affecting other sites. | 8.5 (8-9) | 96 | Consensus on 1st vote |
| 51. Measuring the severity of psoriasis using site-specific scales provides an accurate overall picture of the patient's condition. | 8 (7-9) | 83 | Consensus on 2nd vote |
| 52. Topical treatment is the first-line option for psoriasis affecting difficult-to-treat sites. | 2 (1-5) | 75 | Negative consensus on 2nd vote |
| 53. Methotrexate is the best option for the systemic treatment of psoriatic onychopathy. | 4.5 (2-8) | 29 | No consensus |
| 54. Acitretin is the best option for the systemic treatment of psoriatic onychopathy. | 2 (1-5) | 71 | Negative consensus on 2nd vote |
| 55. Ciclosporin is the best option for the systemic treatment of psoriatic onychopathy. | 5 (3-9) | 21 | No consensus |
| 56. Psoralen-UV-A (PUVA) is the best treatment option for plaque psoriasis affecting the nails. | 2 (1-3) | 92 | Negative consensus on 1st vote |
| 57. Narrowband UV-B is the best treatment option for psoriatic onychopathy in the context of moderate to severe disease. | 1.5 (1-2) | 92 | Negative consensus on 1st vote |
| 58. Biologic agents are more effective than conventional systemic drugs in the treatment of psoriatic onychopathy in the context of moderate to severe disease. | 8.5 (8-9) | 88 | Consensus on 1st vote |
| 59. In the long term, all the biologic agents have similar efficacy in the treatment of psoriatic onychopathy in the context of moderate to severe psoriasis. | 8 (7-9) | 83 | Consensus on 1st vote |
| 60. Adalimumab is the best treatment option for plaque psoriasis affecting the scalp. | 5 (5-8) | 50 | No consensus |
| 61. Infliximab is the best treatment option for plaque psoriasis affecting the scalp. | 6 (5-8) | 33 | No consensus |
| 62. Etanercept is the best treatment option for plaque psoriasis affecting the scalp. | 6 (5-8) | 50 | No consensus |
| 63. Ustekinumab is the best treatment option for plaque psoriasis affecting the scalp | 5 (5-7) | 46 | No consensus |
| 64. In the treatment of palmoplantar psoriasis, anti-TNF drugs are the first choice biologic agents in preference to IL12/23 antagonists. | 7 (3-8) | 63 | No consensus |
| 65. Adalimumab is the best biologic therapy for palmoplantar psoriasis. | 5 (5-8) | 42 | No consensus |
| 66. Infliximab is the best biologic therapy for palmoplantar psoriasis. | 5 (5-7) | 46 | No consensus |
| 67. Etanercept is the best biologic therapy for palmoplantar psoriasis. | 6 (5-7) | 46 | No consensus |
| 68. Ustekinumab is the best biologic therapy for palmoplantar psoriasis. | 5 (3-6) | 54 | No consensus |
Abbreviations: IQR, interquartile range; TNF, tumor necrosis factor; IL, interleukin.
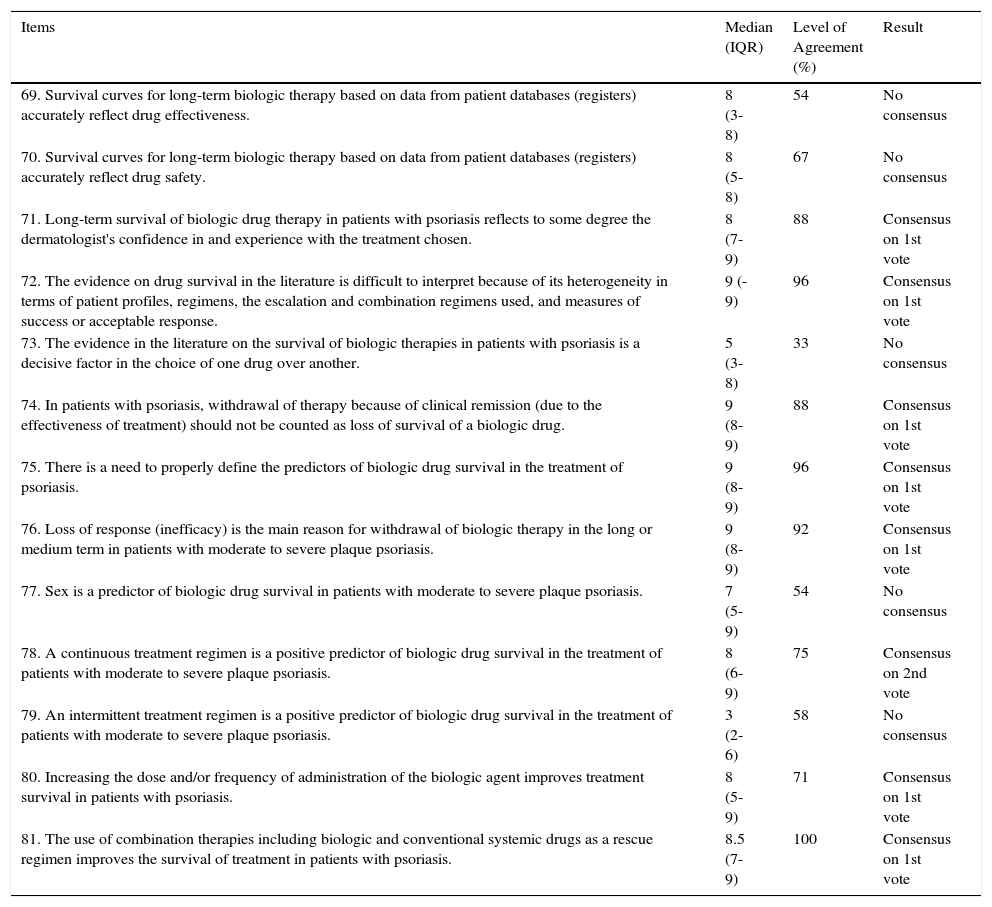
Results for Scenario 4. Survival of Biologic Drug Therapies in Psoriasis.
| Items | Median (IQR) | Level of Agreement (%) | Result |
|---|---|---|---|
| 69. Survival curves for long-term biologic therapy based on data from patient databases (registers) accurately reflect drug effectiveness. | 8 (3-8) | 54 | No consensus |
| 70. Survival curves for long-term biologic therapy based on data from patient databases (registers) accurately reflect drug safety. | 8 (5-8) | 67 | No consensus |
| 71. Long-term survival of biologic drug therapy in patients with psoriasis reflects to some degree the dermatologist's confidence in and experience with the treatment chosen. | 8 (7-9) | 88 | Consensus on 1st vote |
| 72. The evidence on drug survival in the literature is difficult to interpret because of its heterogeneity in terms of patient profiles, regimens, the escalation and combination regimens used, and measures of success or acceptable response. | 9 (-9) | 96 | Consensus on 1st vote |
| 73. The evidence in the literature on the survival of biologic therapies in patients with psoriasis is a decisive factor in the choice of one drug over another. | 5 (3-8) | 33 | No consensus |
| 74. In patients with psoriasis, withdrawal of therapy because of clinical remission (due to the effectiveness of treatment) should not be counted as loss of survival of a biologic drug. | 9 (8-9) | 88 | Consensus on 1st vote |
| 75. There is a need to properly define the predictors of biologic drug survival in the treatment of psoriasis. | 9 (8-9) | 96 | Consensus on 1st vote |
| 76. Loss of response (inefficacy) is the main reason for withdrawal of biologic therapy in the long or medium term in patients with moderate to severe plaque psoriasis. | 9 (8-9) | 92 | Consensus on 1st vote |
| 77. Sex is a predictor of biologic drug survival in patients with moderate to severe plaque psoriasis. | 7 (5-9) | 54 | No consensus |
| 78. A continuous treatment regimen is a positive predictor of biologic drug survival in the treatment of patients with moderate to severe plaque psoriasis. | 8 (6-9) | 75 | Consensus on 2nd vote |
| 79. An intermittent treatment regimen is a positive predictor of biologic drug survival in the treatment of patients with moderate to severe plaque psoriasis. | 3 (2-6) | 58 | No consensus |
| 80. Increasing the dose and/or frequency of administration of the biologic agent improves treatment survival in patients with psoriasis. | 8 (5-9) | 71 | Consensus on 1st vote |
| 81. The use of combination therapies including biologic and conventional systemic drugs as a rescue regimen improves the survival of treatment in patients with psoriasis. | 8.5 (7-9) | 100 | Consensus on 1st vote |
Abbreviation: IQR, interquartile range.
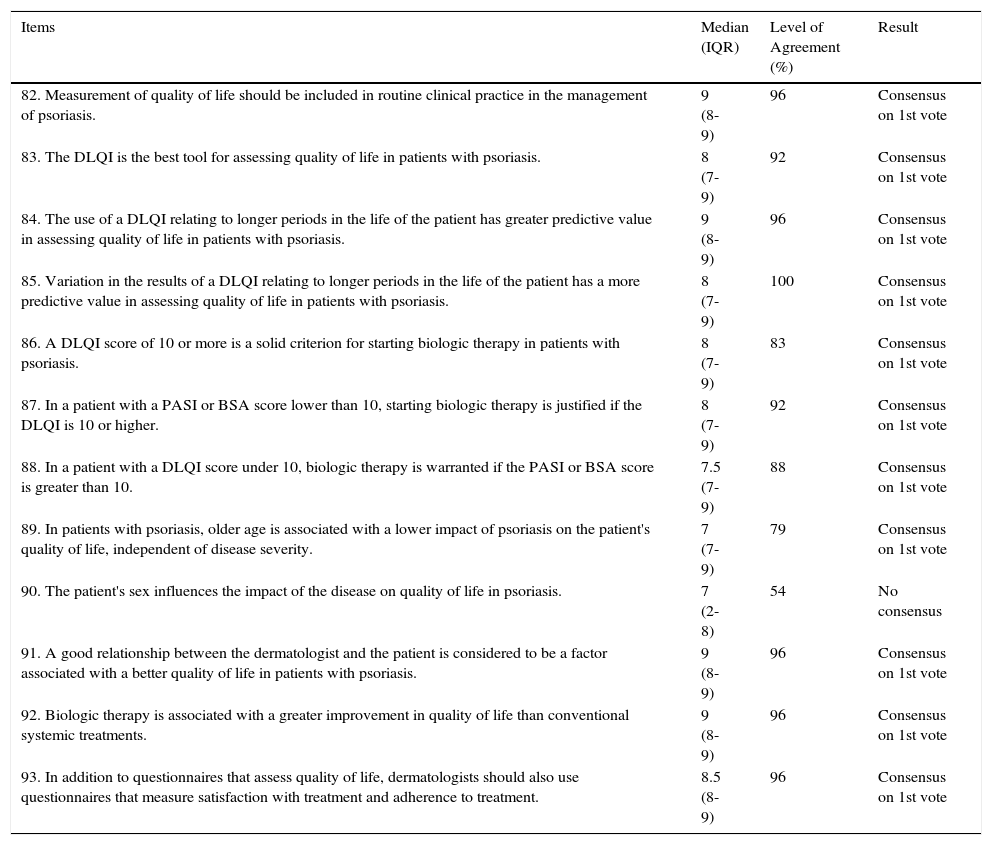
Results for Scenario 5. Assessment of the Impact of Psoriasis on Patient Quality of Life.
| Items | Median (IQR) | Level of Agreement (%) | Result |
|---|---|---|---|
| 82. Measurement of quality of life should be included in routine clinical practice in the management of psoriasis. | 9 (8-9) | 96 | Consensus on 1st vote |
| 83. The DLQI is the best tool for assessing quality of life in patients with psoriasis. | 8 (7-9) | 92 | Consensus on 1st vote |
| 84. The use of a DLQI relating to longer periods in the life of the patient has greater predictive value in assessing quality of life in patients with psoriasis. | 9 (8-9) | 96 | Consensus on 1st vote |
| 85. Variation in the results of a DLQI relating to longer periods in the life of the patient has a more predictive value in assessing quality of life in patients with psoriasis. | 8 (7-9) | 100 | Consensus on 1st vote |
| 86. A DLQI score of 10 or more is a solid criterion for starting biologic therapy in patients with psoriasis. | 8 (7-9) | 83 | Consensus on 1st vote |
| 87. In a patient with a PASI or BSA score lower than 10, starting biologic therapy is justified if the DLQI is 10 or higher. | 8 (7-9) | 92 | Consensus on 1st vote |
| 88. In a patient with a DLQI score under 10, biologic therapy is warranted if the PASI or BSA score is greater than 10. | 7.5 (7-9) | 88 | Consensus on 1st vote |
| 89. In patients with psoriasis, older age is associated with a lower impact of psoriasis on the patient's quality of life, independent of disease severity. | 7 (7-9) | 79 | Consensus on 1st vote |
| 90. The patient's sex influences the impact of the disease on quality of life in psoriasis. | 7 (2-8) | 54 | No consensus |
| 91. A good relationship between the dermatologist and the patient is considered to be a factor associated with a better quality of life in patients with psoriasis. | 9 (8-9) | 96 | Consensus on 1st vote |
| 92. Biologic therapy is associated with a greater improvement in quality of life than conventional systemic treatments. | 9 (8-9) | 96 | Consensus on 1st vote |
| 93. In addition to questionnaires that assess quality of life, dermatologists should also use questionnaires that measure satisfaction with treatment and adherence to treatment. | 8.5 (8-9) | 96 | Consensus on 1st vote |
Abbreviations: BSA, body surface area affected; DLQI, Dermatology Life Quality Index; IQR, interquartile range; PASI, Psoriasis Area and Severity Index.
The patient is a 12-year-old boy with an 8-year history of psoriasis who presents lesions continuously and has occasional exacerbations coinciding with episodes of purulent tonsillitis. He hardly ever leaves home on weekends and is unwilling to participate in sports because he does not want people to see his skin lesions. The patient consults a physician during a flare with a score on the Psoriasis Area and Severity Index (PASI) of 12.6 and an affected body surface area (BSA) of 14.
In children, like in adults, psoriasis is often accompanied by comorbidities such as obesity and cardiovascular disease. In a study of data from 33 981 patients with psoriasis registered on a German statutory health insurance company database containing 1.3 million unselected individuals covering all regions, the overall rate of comorbidities in patients under 20 years of age was double that of patients who did not have psoriasis. In that study, juvenile psoriasis was associated with increased rates of hyperlipidemia, obesity, hypertension, diabetes mellitus, rheumatoid arthritis, and Crohn disease.14 In a multicenter, cross-sectional study of 409 children with psoriasis, 37.9% of the patients with psoriasis were overweight or obese (body mass index [BMI] ≥ 85th percentile) compared to 20.5% of controls.15 The same authors also observed an increase in central adiposity (waist circumference ≥ 90th percentile) in 21.2% of the children with severe psoriasis and only 9.3% of the controls. These findings indicate an increased risk of metabolic syndrome in this population. Other studies have also demonstrated an increase in cardiovascular risk in children with psoriasis.16,17 Consequently, these patients and their parents should be informed about the association between psoriasis and cardiovascular risk and advised of the importance of adopting and fostering a healthy lifestyle.
There is only scant evidence on the relationship between pediatric psoriasis and psychiatric morbidity and on the impact of the condition on quality of life in this age group. However, it has been reported that the risk of depression and anxiety is higher in children with psoriasis than in healthy children.18,19 Psoriasis also has a negative impact on the quality of life of both the patients and their parents. Quality of life is particularly affected by joint involvement and itching.20,21 The authors of one pediatric study observed that quality of life was worse in children with psoriasis than in healthy children, with a negative impact similar to that observed in children with diseases considered to be much more serious, such as arthritis, psychiatric conditions, asthma, and diabetes.22
There are no standardized guidelines for the treatment of psoriasis in children and adolescents. Topical corticosteroids are generally the first-line local treatment.23 A panel of European expert dermatologists and physicians with a special interest in pediatric inflammatory disorders specified corticosteroids and vitamin D analogs as the first-line topical treatments, followed by calcineurin inhibitors.24 The results of a systematic review showed that vitamin D analogs are particularly recommended because of their effectiveness and good safety profile.25
In pediatric patients with more extensive forms of the disease refractory to topical therapy, phototherapy—particularly narrowband UV-B therapy (311-313 nm)—has been cited as the most effective and safest treatment strategy, irrespective of skin type.24
In the case of conventional systemic treatments, there are no randomized clinical trials demonstrating the efficacy of acitretin in children, although systemic treatments have been used successfully and demonstrated an acceptable safety profile in severe plaque psoriasis, pustular psoriasis (palmoplantar and generalized), and erythrodermal psoriasis.26 According to a systematic review of studies on the treatment of pediatric psoriasis published between 1980 and 2008, methotrexate was the systemic treatment of choice for moderate to severe plaque psoriasis in children.25 There is evidence to support the use of ciclosporin in the treatment of recalcitrant juvenile plaque and pustular psoriasis, although psoriasis not one of the approved indications for this drug in pediatric patients.27 Long-term use of ciclosporin in combination with phototherapy is contraindicated because of the possibility of increasing the risk of skin carcinogenesis.24
Finally, in light of the association between streptococcal tonsillitis and psoriasis, tonsillectomy has been proposed as a treatment for recurrent severe psoriasis in childhood. According to a recent systematic review, tonsillectomy may be considered as an option in cases of recalcitrant psoriasis associated with episodes of tonsillitis.28 However, that review was based on data on 410 patients from 20 articles published over the past 53 years. While the authors observed a 71% improvement after surgery, 15 of the articles were case reports or case series with no control group.
There are no specific guidelines for the use of tumor necrosis factor (TNF) inhibitors (infliximab, adalimumab, etanercept) in pediatric patients. The US Food and Drug Administration (FDA) published a series of warnings about the use of these drugs in children because of the possible carcinogenic potential; the warning was based on reports of 48 cases of malignancies (mostly lymphomas) in children treated with TNF inhibitors.29 There are, however, a number of confounding factors, including the risk of malignancy associated with underlying disorders and the concomitant use of immunosuppressive drugs. In fact, at this time, no causal relationship between the use of TNF inhibitors and the onset of malignancy in children has been established on the basis of the accumulated clinical experience.
The European Medicines Agency (EMA) has approved etanercept for the treatment of severe and chronic plaque psoriasis in children aged 6 years or older and in teenagers, provided disease is not adequately controlled by phototherapy or conventional systemic drugs.30 Etanercept is the biologic drug for which the most information is available from randomized clinical trials in children and adolescents,31 and the only one that has been the subject of extension studies.32 Other observational studies have also shown etanercept to be effective in the treatment of erythrodermic and pustular psoriasis in children.33,34 Moreover, 70% of the European experts who participated in a consensus panel considered etanercept to be a first-line treatment for juvenile chronic plaque psoriasis because of its efficacy, tolerability and good safety profile.24 Two studies on juvenile idiopathic arthritis, one with an 8-year follow-up, reported a safety profile for etanercept similar to that observed in pediatric patients with psoriasis.35,36
Recently (25 February 2015), the EMA approved a new indication for adalimumab: the treatment of chronic plaque psoriasis in children and adolescents from 4 years of age. This decision was based on safety and efficacy data from a multicenter, randomized controlled trial (MO4-717) that evaluated 2 doses of adalimumab (0.4mg/kg or 0.8 mg/kg administered subcutaneously up to a maximum of 20 mg and 40 mg, respectively) versus oral methotrexate (0.1 mg/kg increasing to 0.4 mg/kg up to a maximum dose of 25 mg/wk). The results of this study have not yet been published but are available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000481/WC500186769.pdf.
The CADMUS study, a Phase 3 randomized controlled trial, evaluated the efficacy and safety of 2 doses of ustekinumab (standard dose and half-standard dose) versus placebo for 1 year in a group of 110 patients aged between 12 and 18 years who had moderate to severe psoriasis.37 By week 12, 78.4% of the patients on the half dose and 80.6% of those on the standard dose had achieved a reduction of at least 75% in the baseline PASI (PASI 75) and 54.1% and 61.1%, respectively, achieved a PASI 90 response. Most of these patients maintained the response at 52 weeks, with better results in the patients on the standard dose. Treatment was generally well tolerated, with a safety profile similar to that observed with ustekinumab in the adult population.37
Consensus ResultsIn this scenario (Table 1), positive consensus was reached on 6 items, negative consensus on 5, and no consensus on 3. Of note among the items on which the panel agreed were the inclusion of measurement of body mass index (BMI) and assessment of possible metabolic comorbidities in the follow-up of pediatric psoriasis, and the recommendation on evaluating quality of life using instruments designed for this purpose. With respect to treatment, the panel agreed that narrowband UV-B phototherapy was the first choice after failure of topical therapy and that methotrexate and etanercept were the best options for systemic and biologic treatment, respectively, in severe plaque psoriasis in childhood. No consensus was reached on the use of potent topical corticosteroids, acitretin, or ciclosporin. The panelists did not recommend prophylactic tonsillectomy and did not agree that the risk of lymphoma was an important constraint limiting the prescription of biologic agents.
Scenario 2. Risk of Infections in Patients With Psoriasis on Biologic TherapyThe patient is a 52-year old man with a long history of psoriasis who has been treated with etanercept for the last 4 years. At a follow-up visit, the patient has a positive Mantoux test result and a negative Interferon Gamma Release Assay (IGRA) result.
It is well known that treatment with biologic drugs, especially TNF inhibitors, is associated with a risk of reactivation of latent pulmonary tuberculosis (TB). Patients on biologics should, therefore, be screened for TB before treatment and monitored during and after treatment. A meta-analysis of the adverse effects of biologic therapy found that the risk of reactivation of TB was significantly higher in these patients than in controls (odds ratio, 4.68; 95% CI, 1.18-18.60).38 A systematic review of controlled clinical trials found that the risk of reactivation of TB was higher when the biologic agents were used in combination with immunosuppressive agents than when they were used in single-drug therapy.39 In fact, following the implementation of the Spanish Rheumatology Society guidelines in March 2002, which included measures aimed at preventing reactivation of TB infection in patients treated with TNF inhibitors, the relative risk for TB in patients being treated with these agents compared to the general population declined from 19 to 7.40 With regard to diagnostic methods, the debate about which test should be used to diagnose latent TB infection is one of the most controversial issues in the management of the risk of infection in patients on biologic therapy. In countries where TB is endemic and there a high rate of vaccination, IGRA would appear to be the most appropriate test for detecting latent TB.41 The TBNET consensus statement on the risk of TB associated with the use of TNF antagonists states that the agreement between results of IGRAs and Mantoux tests is stronger in countries with low TB prevalence and low BCG vaccination coverage.42 In a pooled analysis of 2282 patients with rheumatic diseases screened for latent TB infection prior to treatment with biologic agents, the IGRA tests had greater specificity (and possibly sensitivity) than the tuberculin skin test.43 However, there is still a lack of clarity about the positive or negative predictive value of QuantiFERON in immunosuppressed patients and in patients with a history of TB who have persistent positive or negative QuantiFERON results. There is also no conclusive evidence or recommendations on the frequency with which the Mantoux test and/or IGRA should be repeated in the follow-up of patients with psoriasis.44
The use of biologic agents in patients with psoriasis has been associated with an increased risk of herpes zoster infection.45,46 According to data from the German RABBIT register, the population treated with adalimumab or infliximab has a 1.82 greater risk of herpes zoster infection than controls; the risk for etanercept was 1.36 greater than controls, which was lower than the risk for anti-TNF therapy as a class (1.63).47 In a review of randomized clinical trials and cohort studies, vaccination was recommended prior to the start of biologic therapy, particularly in the case of infliximab.46 The same recommendation has also been proposed for patients being treated with combination regimens involving biologics and methotrexate, especially when additional risk factors are present.48
In regard to the risk of serious and opportunistic infections in patients with psoriasis on biologic therapy, a recent longitudinal analysis of a cohort of 11 466 patients with psoriasis (the PSOLAR register) showed a cumulative incidence of severe infections of 0.83 per 100 patient-years for ustekinumab, 1.47 for etanercept, 1.97 for adalimumab, and 2.49 for infliximab; pneumonia and cellulitis were the most frequent infections.49 In a 5-year FDA-mandated surveillance registry (OBSERVE-5) of patients with psoriasis treated with etanercept, the cumulative incidence rate of serious infections was 6.5%.50 Treatment with adalimumab, infliximab, and etanercept has been associated with cases of reactivation of hepatitis B virus (HBV) infection and in the case of etanercept reactivation of hepatitis C virus (HCV) infection.51 In patients with hepatitis C, liver function must be assessed before starting treatment with TNF inhibitors. If anti-TNF therapy is started in this setting, periodic liver function monitoring is advised.52 In a multicenter retrospective study that analyzed the safety and efficacy of ustekinumab in patients with psoriasis and hepatitis C or B, it was concluded that biologic therapy was safe and effective in most of the patients; notwithstanding this conclusion, its use should always be restricted to cases where such therapy is justified by the risk-benefit ratio.53,54
Appropriate vaccination is one of the most effective interventions for preventing serious infections in patients on biologic therapy. In fact, once it has been decided that systemic treatment is necessary, patients should be referred to the preventive medicine unit or a specialized infectious diseases unit in order to remedy any deficiencies in their vaccination schedule before starting a therapy with immunosuppressive effects. The vaccination schedule for patients on systemic immunosuppressive or biologic therapies is generally similar to that recommended for the adult population. However, there is some consensus that the vaccinations recommended in all cases are influenza and Streptococcus pneumoniae, and that optional vaccinations (depending on the patient's profile) include type B Haemophilus influenzae, Neisseria meningitidis, HBV, diphtheria, and tetanus.55,56 Ideally, patients should be immunized before starting immunosuppressive therapy. However, although vaccination carried during biologic therapy, may result in lower than usual levels of immunization, the levels achieved are generally sufficient to ensure adequate protection. It should be remembered that vaccination with live vaccines is contraindicated in patients on biologic and conventional systemic immunosuppressive therapy.
Vaccination rates continue to be low among patients with inflammatory autoimmune diseases, including psoriasis. Consequently, dermatologists should advise their patients about the need to update their vaccinations in line with current schedules to prevent infections and increase the safety of immunomodulatory therapies.57
Consensus ResultsThe panel reached positive consensus on 20 (57.1%) and negative consensus on 8 of the 35 items in this section of the questionnaire; no consensus was reached on the other 7 items (Table 2). Notably, the panel agreed on the importance of performing a Mantoux test before starting treatment with either biologic agents or conventional systemic drugs having immunosuppressive properties, the need to repeat the Mantoux test in patients who have a negative result, and on the use of an IGRA technique to diagnose latent TB before the starting biologic therapy. They also agreed that etanercept is the biologic agent with the lowest risk of reactivating latent TB, of causing serious infections, and of reactivating HCV or HBV infection. There was also a high level of agreement on the need to monitor liver function and viral load in patients with HCV or HBV infection being treated with conventional systemic drugs or biologic therapy. The panel considered that patients should be vaccinated against the influenza virus, pneumococcal bacteria, and HBV before starting biologic therapy.
Scenario 3. Psoriasis in Difficult-to-Treat Sites: Controversies, Impact, and TreatmentThe patient is a 36-year-old woman whose psoriasis is not severe (BSA 5) but who presents marked involvement of the scalp and of the nails (most of which are affected by psoriatic onychopathy). Consequently the impact of the disease on the patient's quality of life is high (DLQI 15).
Psoriasis in difficult-to-treat sites is a term that has been used to denote psoriasis affecting the nails, scalp, palms of the hands, and soles of the feet. Psoriasis affecting these sites is often associated with considerable physical, emotional, and functional impact, and most topical treatments are inconvenient, uncomfortable, and ineffective in these locations.58–60 There are very few controlled clinical trials comparing the safety and efficacy of systemic treatments, phototherapy, and biologic therapy in this setting.
In the case of nail psoriasis, the authors of a systematic Cochrane review, which included 18 randomized controlled clinical trials published between 1946 and 1982 involving 1266 participants, noted that the heterogeneity of the studies prevented them from drawing conclusive results.61 While systemic treatments have been shown to be beneficial, their use is limited by potentially serious adverse effects. Consequently, these drugs do not appear to be a realistic option in the treatment of nail psoriasis unless they are specifically indicated because of concomitant skin psoriasis or psoriatic arthritis or in the case of severe nail psoriasis refractory to other treatments or a condition that is having a major impact on the patient's quality of life. Evidence supporting the use of topical therapies is inconclusive and of poor quality.61 A post hoc analysis of the BELIEVE clinical trial included a sub-analysis of the effects of treatment with adalimumab in 733 patients with psoriasis of the nails and/or scalp. In all of the variables studied (PASI 75, a visual analog scale, and DLQI), the positive effect of adalimumab was greater in scalp psoriasis than in nail psoriasis.62 The US National Psoriasis Foundation recently published recommendations for the treatment of nail psoriasis based on a review of the relevant literature in the PubMed database, which was searched from 1947 to 2014 without language restrictions.63 The resulting recommendations indicated that all patients should be evaluated for fungal infection of the nails, which may complicate treatment. For disease confined to the nails, they recommended high-potency topical corticosteroids with or without vitamin D analogs as the first-line treatment, followed by biologic therapy (adalimumab, etanercept, or ustekinumab) or conventional systemic treatment (methotrexate, acitretin) when topical treatment is not effective. In the case of nail psoriasis associated with skin and/or joint involvement, they recommended treatment with adalimumab, etanercept, ustekinumab, acitretin, or infliximab, with the choice depending on the characteristics of the disease in each case and the criteria of the physician.63 No significant differences were observed between the treatment groups in a retrospective study that compared the efficacy of infliximab, adalimumab, etanercept, and ustekinumab in nail psoriasis, as measured by improvement in the Nail Psoriasis Severity Index (NAPSI) score at 12, 24, and 36 weeks.64
In the case of scalp psoriasis, a systematic review of the topical treatments used in 26 randomized controlled trials involving 8020 patients showed that potent topical corticosteroids were more effective than vitamin D analogs in this setting, although there is no data on the safety of long-term treatment.65 The findings of a network meta-analysis also supported the use of potent topical corticosteroids with or without vitamin D analogs (calcipotriol or betamethasone).66,67 Recommendations based on expert consensus suggest that phototherapy and biologic therapy should be reserved for patients with severe and recalcitrant disease68 and for cases in which scalp involvement is accompanied by generalized skin psoriasis.69
Although there is evidence supporting the efficacy of infliximab, adalimumab, and etanercept in difficult locations—including the palms and soles—no comparative, prospective studies have been published that justify the preferential use of any particular biologic drug. In a study of 15 patients with pustular palmoplantar psoriasis randomized to treatment with etanercept 50 mg or placebo twice weekly for 3 months (after the first 3 months all the patients were treated with etanercept for 3 additional months), the decline in median Palmoplantar Pustulosis Area and Severity Index score from baseline to 24 weeks was statistically significant (P = 0.038) for the etanercept group but not for the controls.70Patients with chronic palmoplantar psoriasis who took part in the REACH study were randomized to receive adalimumab or placebo for the first 16 weeks.71 In a post-hoc analysis of REACH, the authors found that the adalimumab-treated patients who achieved the primary endpoint (a reduction of at least 50% in NAPSI score) also improved in terms of clearing of disease as measured by the Physician's Global Assessment of hands and/or feet. The efficacy of adalimumab was not affected by age, sex, weight, PASI score, duration of disease, history of psoriatic arthritis, prior systemic treatment, smoking history, or nail disease.71
According to the evidence-based guidelines of the Spanish Psoriasis Working Group,72 treatment of psoriasis affecting difficult-to-treat sites with biologic agents is indicated when any of the following conditions are fulfilled: a) disease uncontrolled with topical or conventional systemic treatment; b) extensive lesions (NAPSI>10); c) rapid worsening of disease; d) subjective perception of severity (DLQI > 10); or e) concomitant psoriatic arthritis. Overall, only about 1 in 3 patients treated achieved complete or almost complete remission (for example, PGA 0/1) in the studies of biologic therapy in such sites. The methods used to assess results in those studies are extremely heterogeneous.
Consensus ResultsThis was the scenario on which there was the least agreement, with consensus being achieved on only 11 of the 19 items (Table 3). The panel agreed that plaque psoriasis affecting difficult sites (scalp, nails, palms, and soles) has a greater impact on quality of life than disease affecting other sites. They also agreed that the measurement of severity using scales specifically designed to evaluate these sites adequately reflected the severity and impact of disease and that biologic agents were more effective than conventional systemic treatment in the treatment of psoriatic onychopathy. No consensus was achieved on the best biologic agent for the treatment of palmoplantar psoriasis.
Scenario 4. Survival of Biologic Drug Therapy in PsoriasisThe patient is a 47-year-old man with a BMI of 32 kg/m2. He has a long history of psoriasis and has received prior treatment with methotrexate, ciclosporin, and phototherapy. Biologic therapy with adalimumab initially improved his condition, but the response was lost after 1 year of treatment. He was then treated with infliximab, which never achieved complete response but was continued for 18 months. At that time, a relapse occurred despite combined treatment with methotrexate and a lower dose of infliximab. The available treatment options and the prospects of achieving a sustained response over time are evaluated.
Drug survival is defined as the period during which a given drug continues to be an adequate treatment for a specific patient. It is difficult to measure the survival of biologic drugs in psoriasis because of the heterogeneity of the studies undertaken and limitations in study design. Drug survival is a parameter that reflects the safety, efficacy, and convenience of the therapy. Safety data indicate that, at least during the initial months of treatment, biologic agents have a good safety profile, even better than conventional systemic treatments. In a meta-analysis of 24 randomized controlled trials involving 9384 patients, in which the primary endpoint was the proportion of patients who achieved a PASI-75 response, infliximab was the most effective drug (risk difference [RD], 77%) followed by adalimumab (RD, 44%), and etanercept (RD, 30%).73In a recent observational study that investigated the clinical efficacy and survival of drugs after 12 months of treatment, the survival rate was 96.7% for ustekinumab, 79.7% for adalimumab, and 73.3% for infliximab.74 A study that determined differences in biologic drug survival in a group of patients with psoriasis registered in the database of a medical center in Amsterdam reported similar drug survival in treatment-naïve patients at 1 and 4 years (etanercept, 85% and 64%, respectively; adalimumab, 77% and 77%; infliximab, 75% and 75%); survival was somewhat lower in non-naïve patients (etanercept, 86% and 42%, respectively; adalimumab, 84% and 56%; infliximab 68% and 43%; ustekinumab 84% and 57%).75 In the DERMBIO prospective registry, which includes all the patients with psoriasis treated with biologic agents in teaching hospitals in Denmark (1867 treatments in 1277 patients over 10 years), mean survival was 47 months and 67% of withdrawals were attributed to a lack of efficacy and 9.7% to adverse effects.76 In that registry, estimated mean survival was 59 months for adalimumab, 44 for infliximab, and 30 for etanercept; survival was better in men and patients not previously treated with biologic agents.
Another study analyzed data from the BioCAPTURE prospective registry of biologic treatments started after January 2010 (when all the biologic agents were available).77 A course of treatment was deemed continuous if the withdrawal period did not exceed 90 days (because treatment is often interrupted for surgeries, vacations, infections, etc.). The authors of this 1-year study reported drug survival and the percentage of “happy” drug survival, that is, continued treatment in conjunction with a DLQI of 5 or less. Ustekinumab showed better survival than etanercept and a trend towards better survival than adalimumab. The percentage of patients assessed as happy increased during the course of the study: 27%, 64%, 69%, 72%, and 79% at baseline and 3, 6, 9, and 12 months, respectively. Lack of response and adverse events, or both, were the main reasons for withdrawal of treatment. On the first round of voting, the panelists reached consensus (level of agreement, 92%) on the role of inefficacy (loss of response) as the main cause of withdrawal of treatment.
A 3-year study of the long-term safety and efficacy of etanercept and adalimumab in 85 patients aged 65 years or older with psoriasis and psoriatic arthritis found better drug survival rates for etanercept (74.5%) than adalimumab (60.7%) and reported that the majority of withdrawals were due to inefficacy.78 The OSCAR I study reported the following biologic drug survival rates in treatment-naive patients with psoriasis in routine clinical practice after follow-up of up to 6 years: etanercept 80.7%, infliximab 61.9%, and adalimumab 59.8%.79 In most cases, withdrawal of treatment was due to a lack of efficacy (etanercept 14.4%, infliximab 27.9%, adalimumab 27.2%) and in a few it was due to adverse events (etanercept 2.8%, infliximab 8.8%, adalimumab 4.4%).79 The Phase 4 OSCAR II study analyzed long-term treatment survival in patients with psoriasis on etanercept in routine clinical practice.80 Of the 367 patients studied, 152 had plaque psoriasis. These patients were treated with etanercept on either an intermittent (110 patients, 72.4%) or continuous (42 patients, 27.6%) regimen. Mean duration of treatment was 1706 days in the group of patients on the intermittent regimen and 1249 in the group on continuous treatment.80
It is importance to stress that lack of adherence to therapy is one of the main factors that increases the cost of treatment. Loss of response to biologic therapy often leads to the decision to switch the patient to another agent, involving another induction phase, which is more costly than a maintenance regime.81 Besides the inherent properties of each drug, authors of different studies have cited the following predictors of biologic drug survival: dose increase, frequency of administration, the use of combination regimens, antidrug antibody production, a higher catabolic rate, and the clinical factors that give rise to the decision to switch from one drug to another.
Consensus ResultsThe expert panelists reached consensus on 8 of the 13 items relating to this scenario (Table 4). They agreed that the evidence in the literature is heterogeneous and difficult to interpret and that there is a need to adequately define the predictors of drug survival in biologic therapy. They also agreed that increasing the dose and/or frequency of administration of the biologic agent improves survival of treatment and that cessation should not be considered as a loss of drug survival when the decision to withdraw the treatment is due to a clinical remission attributed to therapeutic efficacy. A continuous treatment regime was considered by the panel to be a positive predictor of drug survival. The use of rescue regimens involving a combination of biologic and conventional systemic drugs was also considered to improve treatment survival in patients with psoriasis.
Scenario 5. Assessment of the Impact of Psoriasis on Patient Quality of LifeThe patient is a 22-year-old woman with plaque psoriasis who presents a PASI score of 5 and a BSA of 3. Her lesions, which are located in several different sites, are highly infiltrated, erythematous, and reported to be very itchy. Despite the moderate extent of her lesions, the patient's DLQI has been high—over 10—in successive visits.
The challenge in the management of psoriasis is not only to achieve long-term remission of the signs and symptoms of the disease, but also to improve the patients’ quality of life. Given the considerable impact of the disease on multiple dimensions of these patients’ lives, validated questionnaires are an essential tool in the evaluation of quality of life in this setting. In actual practice, however, the systematic use of such tools is not well established.
In an expert consensus study based on evidence from 154 articles selected from among 10 642 studies published between 1980 and 2009, 44 dermatologists formulated 10 recommendations for clinical practice, including the use of the DLQI, the SF-36, and Skindex-29 before starting systemic treatment and during follow-up.82 That study also highlighted the fact that these questionnaires are incomplete and do not effectively assess certain factors, such as mood, stress, impact on sleep or the family environment, level of fatigue, and the patient's acceptance of and satisfaction with their current treatment regimen. The findings of a recent study showed that the Lifetime DLQI (LT DLQI), which measures chronic quality of life, is a better predictor of quality of life than the DLQI scales relating to the previous week or year.83
All the biologic drugs (etanercept, infliximab, adalimumab, and ustekinumab) have been shown to be effective in improving quality of life in patients with psoriasis as measured by several different quality-of-life scales.
Itching is an important symptom in psoriasis and one that has a major impact on quality of life. The PRISTINE randomized controlled trial assessed 2 etanercept treatment regimens84: 50 mg subcutaneously once weekly versus 50mg subcutaneously twice weekly. In that study, quality-of-life scores correlated with severity of pruritus: the more severe the pruritus, the greater the negative impact on the patients’ quality of life. After 24 weeks of treatment, itching levels decreased in both treatment groups, with concurrent improvement in all of the quality of life scores. Further evidence on the influence of different regimens on quality of life comes from the 54-week CRYSTEL trial, in which patients were randomized to either a continuous or an intermittent etanercept regimen.85 The group on the continuous regimen received etanercept 25 mg subcutaneously twice weekly for 54 weeks. The group on the intermittent regimen received etanercept 50 mg subcutaneously twice weekly for no more than 12 weeks (until a PGA of 2 or less was reached); upon relapse, etanercept was resumed at a dose of 25 mg twice weekly. The continuous regimen was evaluated in 352 patients and the intermittent regimen in 359. At 54 weeks, both groups showed significant improvement on the DLQI, the EuroQOL 5-dimension questionnaire (EQ-5D), the Hospital Anxiety and Depression, and the SF-36-Vitality scales. The continuous group showed significantly greater improvement in the DLQI and EQ-5D, but the different was small.
The authors of a meta-analysis on the comparative effects of biological therapies on quality of life in patients with plaque psoriasis concluded that all the biologic agents improved quality of life (DLQI) more than placebo.86 A recent systematic review demonstrated that the discomfort and dissatisfaction caused by psoriasis (measured using the EQ-5D) were comparable to that produced by other chronic diseases (cardiovascular disease, diabetes, end-stage renal diseases, liver diseases, cancer, and visual disorders).87 A survey of 5604 patients with psoriasis or psoriatic arthritis carried out by the US National Psoriasis Foundation between 2003 and 2011 showed that 92% of the unemployed respondents cited their disease as the cause of their unemployment. Among the working respondents, 49% reported regularly missing work because of psoriasis. After adjusting for age and sex, the patients with severe psoriasis were 1.8 more likely to be unemployed than those with mild psoriasis.88
Finally, other factors that affect the quality of life of patients with psoriasis include the extent of the skin lesions, consultation of numerous physicians, younger age, the perception on the part of the patient of not being supported by medical professionals, a feeling that the care they are being offered is deficient because the medical facilities are inadequate or information on side effects and alternative treatment options is not provided. Finally, while the decision to prescribe biologic therapy is influenced by both the results of DLQI and the PASI score, in clinical practice the PASI score appears to have a greater influence on this decision.89
Consensus ResultsOf the 12 statements relating to this scenario, all but 1 (the assertion that the impact of psoriasis on quality of life is conditioned by the patient's sex) achieved positive consensus on the first round of the Delphi process (Table 5). This result underscores the importance of incorporating the measurement of quality of life into everyday clinical practice, preferably using the DLQI. A score of 10 or more on the DLQI was considered to be a reference criterion for starting therapy with biologic agents. The other items that achieved consensus were the greater improvement in quality of life with biologic therapy than with conventional systemic treatments, the positive influence of a good relationship between the dermatologist and the patient, the importance of variations in DLQI as a marker of the efficacy of biologic therapy, and the need to incorporate questionnaires relating to patient satisfaction into treatment protocols, and the measurement of adherence to treatment.
DiscussionPediatric psoriasis is a relatively unexplored area. This may be due to numerous probable causes, including the lower prevalence of the disease in children, the difficulty of conducting clinical trials in children, and the high priority of safety issues in this age group. However, the advances in our knowledge about psoriasis in general have also improved the management of the disease in children and have highlighted the importance of comorbidities and the impact of psoriasis on quality of life among children as well as adults.14,15 This was an area in which the members of the expert panel agreed and, as a result, recommended that physicians should evaluate affected children for comorbidities and take into account the results of an objective assessment of the impact of disease on the patient's quality of life in the decision-making process. The panel's conclusions on the subject of treatment prioritized safety, a focus reflected in the designation of narrowband UV-B therapy as the first choice for treating severe cases. Methotrexate—a drug for which a growing body of experience in dermatology has emerged in recent years—was chosen as the first-line option among conventional systemic drugs. In the opinion of the panel, the efficacy and safety described by studies of biologic agents could support the decision to prioritize such therapies over conventional systemic treatments in the more severe forms of psoriasis. Moreover, in the case of many of the non-biologic systemic therapies, the evidence available on efficacy and safety in this setting is often scant.31–34 Based on the cumulative experience at the time of the consensus, etanercept was considered to be the biologic treatment of choice. The recent publication of studies on newer biologic agents, such as ustekinumab and adalimumab (recently approved by the EMA for the treatment of chronic plaque psoriasis in children and adolescents from 4 years of age) could increase the range of treatment options available.37
The risk of common or opportunistic infections has been considered to be one of the limitations and dangers of biologic therapy in various inflammatory diseases since these therapies were introduced.38 However, the growing experience in dermatology—which differs from the experience in patients with other chronic inflammatory diseases—would appear to indicate that, overall, the risk of infection may be much lower in patients with psoriasis than in patients with other inflammatory diseases; this different may be attributable to the patient profile and the absence of other immunosuppressive therapies. The risk may be somewhat greater with infliximab and adalimumab than with etanercept and ustekinumab.49 These conclusions, which are based mainly on data from epidemiological studies and registries, are reflected by the panelists’ prioritization of the various treatment options bearing in mind the risk of opportunistic infections, serious infections, and the reactivation of latent infections. While the debate on the best method for latent TB screening (IGRA or the skin tuberculin test) may be in the process of being resolved in favor of IGRA, the lack of any solid evidence is also reflected in the experts’ preference for retaining both tests as suitable, and even complementary, options.43
The complexity of managing psoriasis in difficult-to-treat sites (scalp, nails, palms and soles) and the lack of structured and high quality evidence relating to this topic are reflected in the panel members’ lack of agreement on the best treatments in each case.58–60 They did, however, agree that biologic therapy is a more effective option in some of these variants, such as psoriatic onychopathy, and recognize the significant impact on the patient's quality of life of such involvement. However, no particular biologic agent was prioritized.
As more experience is gained in managing biologic therapy in psoriasis, it is becoming increasingly clear that drug survival, that is, the duration of a course of treatment with a particular drug, is one of the important factors that should be taken into account when making therapeutic decisions. Drug survival is important not only because of the impact of a relapse on the patients’ quality of life when psoriasis has been controlled during the initial months of treatment, it is also important in terms of safety and because of the additional cost involved in the prescription of an induction regimen for a new biologic agent.78 The panelists agreed, however, that while the body of data on this subject is growing, it is also heterogeneous and difficult to interpret because, for example, registers may erroneously attribute a pause in a course of biologic to treatment to treatment failure when it is in fact due to a reason unrelated to the course of treatment, such as the patient's desire to stop treatment once remission has been achieved or an intercurrent condition. In any case, based on their own experience, the panelists favored the use of continuous regimens, a flexible approach to treatment, and individualized dosage regimens as opposed to rigid adherence to the specifications of the Summary of Product Characteristics. They were also in favor of combination regimens, which they saw as a strategy that could improve outcomes and prolong therapeutic effect. The consensus appears to indicate that approaches derived from the accumulated experience of routine clinical practice, although difficult to structure as solid evidence, can be very useful in optimizing treatment outcomes.
There is a growing awareness of the importance of incorporating quality-of-life parameters into the pre-treatment assessment and subsequent follow up of these patients. This type of assessment is needed not only because improved quality of life is a primary treatment goal but also because of the differences between the priorities of the physician and those of the patient and because the course of disease as measured by objective severity indices often differs considerably from the patient's subjective perception of their condition, when the areas of involvement are small but visible, for example.81 The opinion of the panelists was very consistent on this point and they agreed on the need to bear in mind, and even to prioritize, quality-of-life parameters when taking decisions about treatment in moderate to severe psoriasis. However, these opinions contrast with the infrequent use of these parameters outside of clinical trials and the fact that the available questionnaires are limited in terms of covering all the relevant aspects of the patient's lives in a complete and satisfactory manner.80
In short, this article discusses some of the most important and controversial issues in the treatment of psoriasis in special circumstances. Since the evidence currently available on these issues is incomplete and even contradictory, the structured opinion of experts may be of use to clinical dermatologists experienced in the management of psoriasis and serve as an aid to decision-making in everyday practice.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no private patient data are disclosed in this article.
Privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
FundingThis Delphi consensus study was supported by Pfizer España, which financed the electronic survey, the face-to-face meetings, and teleconferences. No Pfizer employees took part in any of the discussions of the expert panel or intervened in any way in the drafting of the text.
Conflicts of InterestJ.M. Carrascosa has received honoraria as a consultant or for participation in conferences from Abbvie, Janssen, MSD, Pfizer-Wyeth, Amgen and Lilly.
M. Ribera has received honoraria as a consultant and/or investigator and for participation in conferences from Abbvie, Gebro, Leo-Pharma, MSD, Novartis, Pfizer, and Janssen.
M. Galán has taken part in studies and served as a speaker for Pfizer, Janssen, Abbvie, MSD, Novartis, Leo-Pharma, and Gebro-Pharma.
A. Pérez Ferriols has received honoraria for research, as an expert adviser, for taking part in clinical trials, and/or for speaking from Abbvie, Almirall, Avéne, Biogen, Cantabria, Ferrer, Isdin Johnson & Johnson, Leo-Pharma, MSD, Novartis, Pfizer, and Viñas.
I. Yanguas has received honoraria for consultancy from Abbvie, Janssen, Pfizer, and Schering-Plough.
R. Lucas declares no conflicts of interest.
The authors gratefully acknowledge the contribution of the all the dermatologists who participated in the Delphi process (without whom this study could not have been carried out), the editorial support of Nature Publishing Group Iberoamérica, and the contribution of Dr. Marta Pulido, to writing the manuscript.
Please cite this article as: Carrascosa JM, Galán M, de Lucas R, Pérez-Ferriols A, Ribera M, Yanguas I. Recomendaciones de expertos para el tratamiento de la psoriasis en situaciones especiales (II). Actas Dermosifiliogr. 2016;107:712–729.