This study aimed to investigate the effects of age, period, and cohort on the incidence of psoriasis in Spain from 1990 through 2019 using the Global Burden of Disease (GBD) database and age-period-cohort (A-P-C) analysis.
MethodsWe conducted an ecological trend study to analyze the incidence rates of psoriasis in Spain from 1990 through 2019. Joinpoint Regression Program, Version 5.0.2 - May 2023; Surveillance Research Program, National Cancer Institute and National Cancer Institute A-P-C tools were used to identify trends and assess the effects of age, period, and cohort.
ResultsFrom 1990 through 2019, an estimated 2.99 million cases of psoriasis were diagnosed in Spain, with a mean annual increase of 0.49%. Significant decreases in age-standardized incidence rates (ASIR) were reported for both sexes, with women consistently maintaining a slightly higher ASIR. Joinpoint analysis revealed multiple turning points in the downward trend, indicating periods of stabilization. A-P-C analysis demonstrated significant declines in both net (overall trend) and local drift (age-specific trends), indicating a broad decrease in the incidence of psoriasis across most age groups. While the risk of psoriasis increased with age, peaking in the 50–54 age group, it declined thereafter. Furthermore, the analysis revealed a continuous decline in risk from 1990 through 2019 for both sexes, with individuals born in the early 21st century exhibiting a significantly lower risk vs those born in the early 20th century.
ConclusionThis study observed a slight decline in the reported psoriasis ASIR in Spain, potentially due to reduced exposure to risk factors. However, limitations in data and the complexity of factors influencing the incidence of psoriasis require further research.
Este estudio tuvo como objetivo investigar los efectos de la edad, el periodo y la cohorte de nacimiento en la incidencia de psoriasis en España entre 1990 y 2019, utilizando la base de datos Global Burden of Disease (GBD) y un análisis de edad-periodo-cohorte (A-P-C).
MétodosSe llevó a cabo un estudio de tendencia ecológica, analizando las tasas estandarizadas de incidencia de psoriasis en España de 1990 a 2019. Se utilizaron el software Joinpoint Regression, Versión 5.0.2 - Mayo 2023; Surveillance Research Program, National Cancer Institute y las herramientas A-P-C del Instituto Nacional del Cáncer para identificar tendencias y evaluar los efectos de edad, periodo y cohorte.
ResultadosEntre 1990 y 2019 se diagnosticaron aproximadamente 2,99 millones de casos de psoriasis en España, con un aumento anual promedio del 0,49%. Se observaron disminuciones significativas en las tasas de incidencia estandarizadas por edad (ASIR) para ambos sexos, manteniendo las mujeres consistentemente una ASIR ligeramente más alta. El análisis de Joinpoint reveló múltiples puntos de inflexión en la tendencia descendente, indicando periodos de estabilización. El análisis A-P-C demostró disminuciones significativas tanto en tendencia general como en las tendencias específicas por edad, lo que indica una disminución general en la incidencia de psoriasis en la mayoría de los grupos de edad. Mientras que el riesgo de psoriasis aumentó con la edad, alcanzando su punto máximo en el grupo de edad de 50-54 años, disminuyó después. Además, el análisis reveló una disminución continua en el riesgo desde 1990 hasta 2019 para ambos sexos, con las personas nacidas a principios del siglo XXI mostrando un riesgo significativamente menor en comparación con aquellas nacidas a principios del siglo XX.
ConclusiónEl presente estudio observó una ligera disminución en la ASIR de psoriasis reportada en España, posiblemente debido a una reducción en la exposición a factores de riesgo. Sin embargo, las limitaciones en los datos y la complejidad de los factores que influyen en la incidencia de la psoriasis requieren más investigaciones.
Psoriasis – a chronic inflammatory skin disease – represents a significant burden fir global health worldwide, affecting millions of people1 and predisposing individuals to comorbidities such as cardiovascular diseases.2,3
Despite its widespread impact, psoriasis shows considerable geographical variation in prevalence, ranging from 0.14% in East Asia up to 1.99% in Australasia, with particularly high rates in Western Europe, Central Europe, North America and high-income countries in southern Latin America.4
While recent research suggests a stable or slightly decreasing trend in the overall incidence of psoriasis,5–9 prevalence data consistently shows an increase.8,10 Paradoxically, despite a 27% increase in diagnosed cases worldwide from 1990 through 2019, the age-standardised incidence rate (ASIR) showed a slight decline of −0.77% per year, which was consistent across sexes.11 This discrepancy highlights the need for further research into the drivers of this trend, including increased awareness, improved diagnostics, demographic changes or environmental influences.
Former studies have predominantly analyzed the incidence of psoriasis using unadjusted age and rarely examined the effects of age, period and cohort. The declining trend raises questions about its correlation with changes across calendar periods or birth cohorts, regardless of population ageing. Age-period-cohort (A-P-C) analysis is emerging as an important tool to uncover independent effects on the incidence of the disease by considering patient age, period, birth cohort and other influential factors typically omitted in cross-sectional studies.
In Spain, the estimated prevalence of psoriasis increased from 1.4% in 2001 up to 2.3% in 2013, based on telephone surveys.12,13 This increase has been attributed to factors such as evolving diagnostic criteria, increased awareness and improved reporting systems, with the introduction of biologic therapies potentially affecting prevalence and patient outcomes. Unfortunately, the lack of studies on the incidence of psoriasis in Spain complicates understanding these dynamics.4
This study aims to fill this gap by examining the incidence of psoriasis in Spain from 1990 through 2019 using the Global Burden of Disease (GBD) database14,15 and the A-P-C analysis16–18 to disentangle the impact of age, historical period, and birth cohort on the incidence of psoriasis in Spain. This will provide a more comprehensive understanding of the dynamics of psoriasis in this region.
MethodsStudy designWe conducted an ecological trend study to analyze the incidence of psoriasis in Spain from 1990 through 2019. This study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement to improve the quality of reporting and ensure methodological transparency (https://www.equator-network.org/reporting-guidelines/strobe/).
Data sourcesIncidence of psoriasisCases of psoriasis were defined using the International Classification of Diseases (ICD)-10 codes L40 and L41. Data on the incidence of psoriasis, stratified by age group and sex, were obtained from the Institute for Health Metrics and Evaluation GBD Results Tool (https://vizhub.healthdata.org/gbd-results/). For a more in-depth understanding of the GBD methodology, readers are referred to the relevant published materials.14,19
Population dataAge-, sex-, and year-disaggregated population health data for Spain (1990–2019) were obtained from the Spanish National Statistics Institute (INE) (available online: https://www.ine.es/en/).
Statistical analysisJoinpoint software (version 4.9.1.0) was used to estimate rates and identify significant changes (turning points) in trends throughout time (available online: https://surveillance.cancer.gov/joinpoint/). The software default settings were used to calculate the annual percent change (APC) for each identified period. We also obtained the average annual percent change (AAPC) from 1990 through 2019, representing a weighted average of individual APCs. Trend parallelisms between sexes were assessed using the “pairwise comparison” option. All rates are presented per 100,000 persons. The male-to-female ratio was also estimated. We used statistically significant changes (p-value<0.05) to describe trends (e.g., increase, decrease). Non-significant trends were described as “stable” or “no change.”
A-P-C effects were assessed using the National Cancer Institute A-P-C tools (available online: https://analysistools.nci.nih.gov/apc/). We focused on estimable functions, including longitudinal age-specific rates, period and cohort rate ratios, and local drifts with net drift.
The longitudinal age curve provided fitted longitudinal age-specific rates in reference cohorts adjusted for period deviations, while the period (or cohort) relative risk (RR) was adjusted for age and non-linear cohort (or period) effects in a period (or cohort) relative to the reference. Net drift represented the overall log-linear trend by calendar period and birth cohort, indicating the overall annual percent change. Conversely, local drifts reflected the log-linear trend by calendar period and birth cohort for each age group, providing the annual percent change for each age group. All APC analyses used the central age, calendar period and birth cohort as the reference group.
ResultsAn estimated 2.99 million psoriasis cases were diagnosed in Spain from 1990 through 2019. The mean annual increase was minimal (0.49%), with a slightly higher increase in men (0.53%) vs women (0.44%).
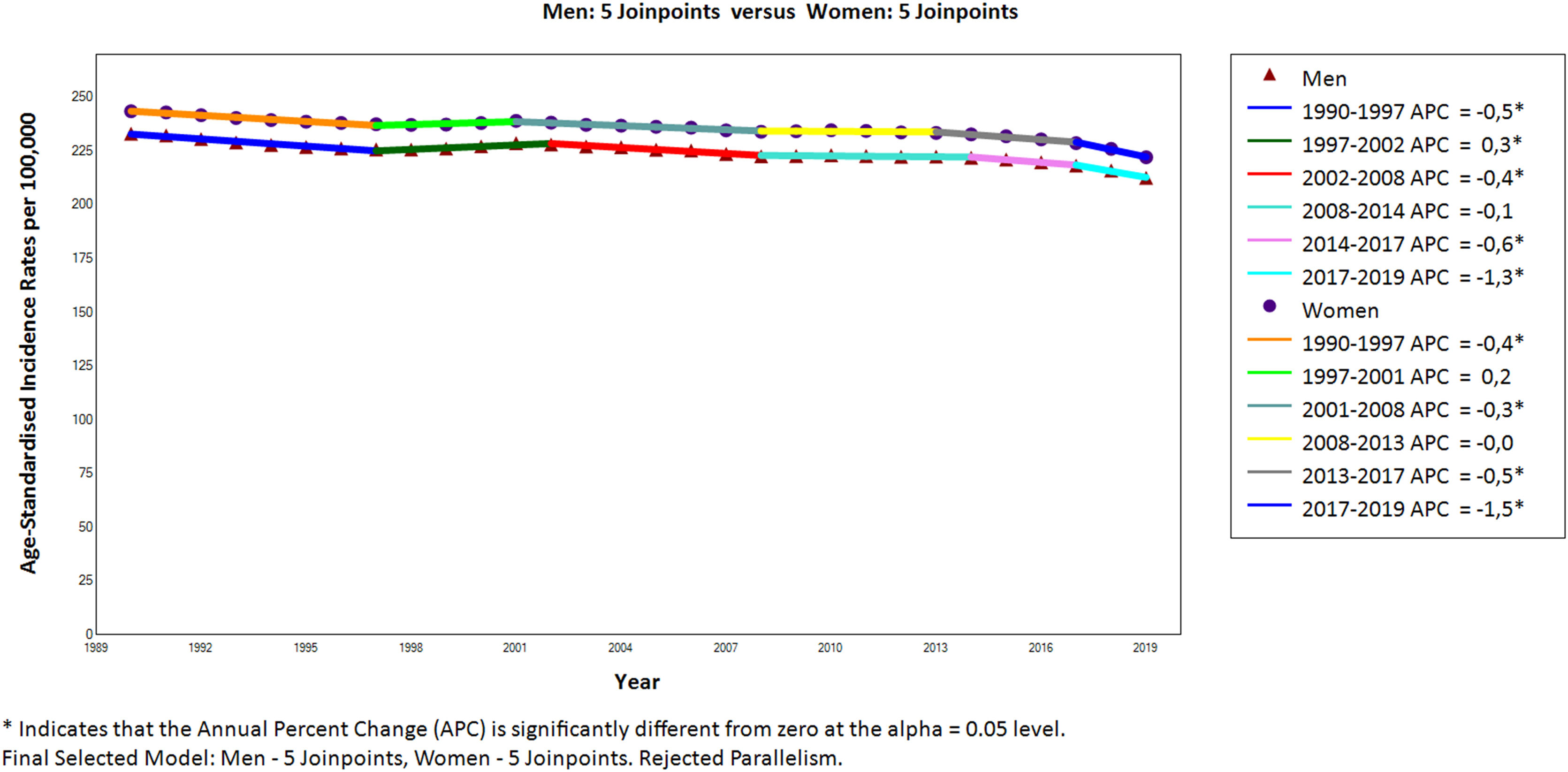
Fig. 1 shows a significant (p<0.05) decrease in the ASIR of psoriasis for both sexes over the study period, with a mean annual decrease of −0.3%. The ASIR for men dropped from 233.2 down to 212.6 per 100,000 and the ASIR for women from 243.6 down to 222.2. Despite the decline, women consistently maintained a slightly higher ASIR (male/female ratio: 0.95). Joinpoint analysis revealed five turning points in the downward trend, dividing the period into six segments with different rates of decline. In particular, the decline stabilised in certain periods for both sexes (men: 2008–2014; women: 1997–2001 & 2008–2013). Interestingly, the most recent period (2017–2019) showed the sharpest decline for both sexes (−1.3%/year for men, −1.5%/year for women).
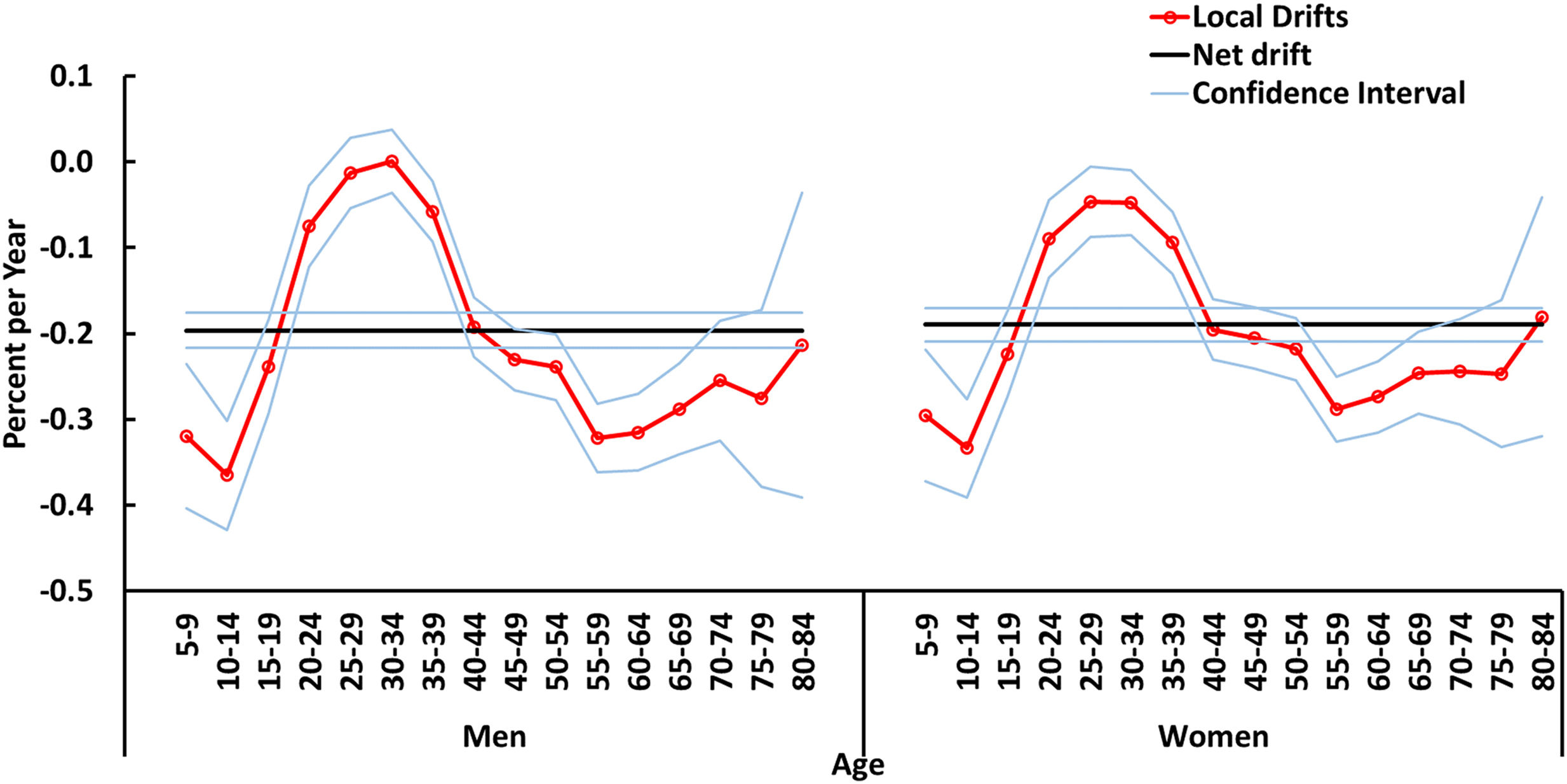
Fig. 2 shows the change in the psoriasis incidence rates in Spain from 1990 through 2019, examining both the overall trend (net drift) and the variation between different age groups (local drift). The overall net drift, representing the mean annual percent change in age-adjusted rates, showed a statistically significant decline for both men (−0.2% with 95% CI: −0.22% to −0.18%) and women (−0.19% with 95% CI: −0.21% to −0.17%). Local drift refers to age-specific changes in incidence rates over time. Notably, a consistent downward trend was observed in most age groups for both genders, suggesting a broad decrease in the incidence of psoriasis across the population. However, an exception was found in the 25–34 age group for men, in whom local drift did not exhibit a statistically significant decline. Interestingly, the most substantial declines in local drift were observed in younger age groups. Among men, local drift went from 0% in the 30–34 age group up to −0.37% in the 10–14 age group. Women exhibited the steepest decline in the 5–14 age group, with local variations ranging from −0.05% in the 25–34 age group up to −0.33% in the 10–14 age group.
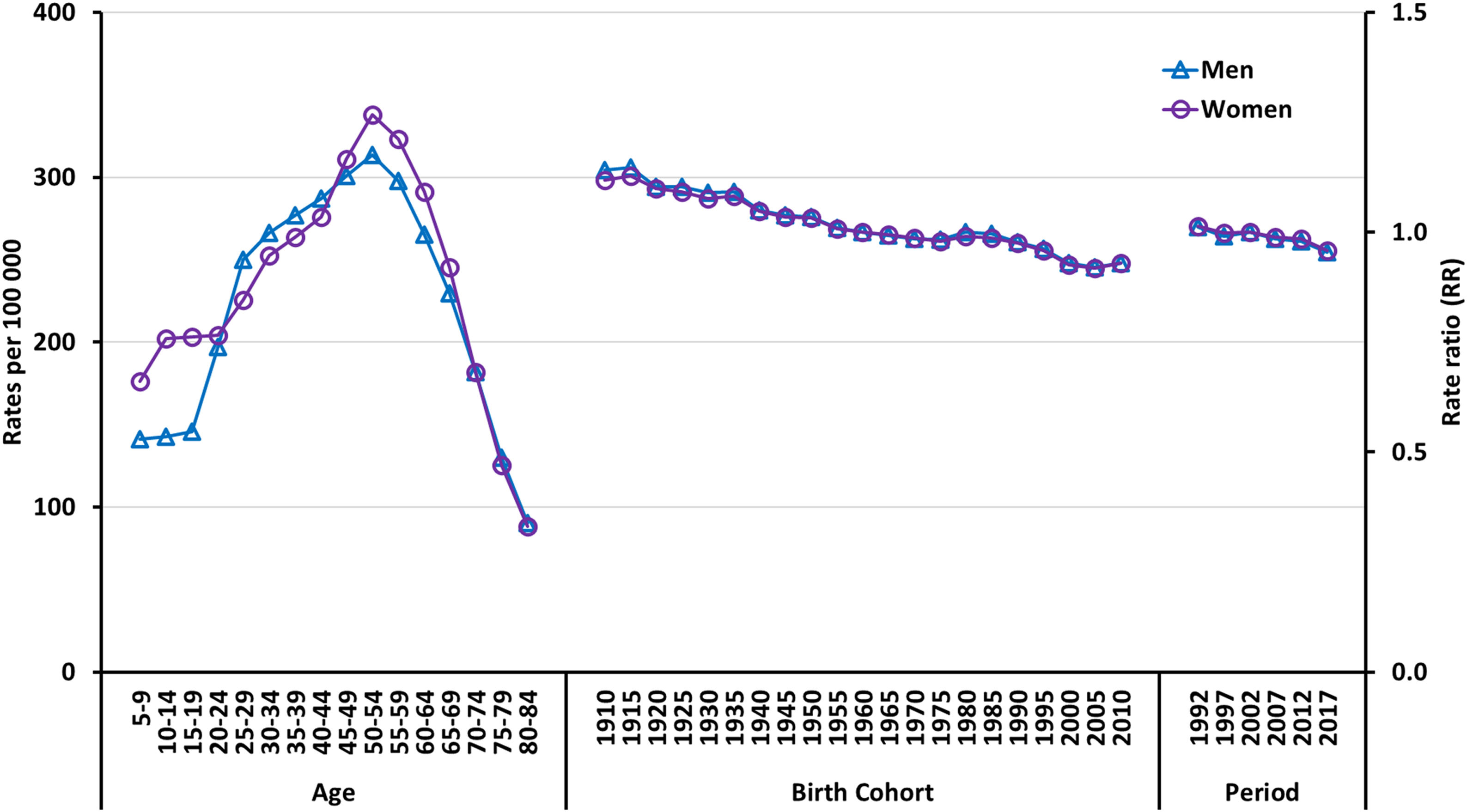
Fig. 3 shows longitudinal age curves, estimated cohort relative risk (RR) trends, and estimated period RR trends. Longitudinal trends showed a slightly increased risk in women in certain age groups (<25 years and 45–69 years). The risk of psoriasis increased steadily with age in both sexes, peaking in the 50–54 age group and decreasing in the 80–84 age group. In Spain, a continuous decrease in the risk of psoriasis was observed from 1990 through 2019, affecting both men and women. People born at the beginning of the 21st century had a significantly lower risk vs those born at the beginning of the 20th century.
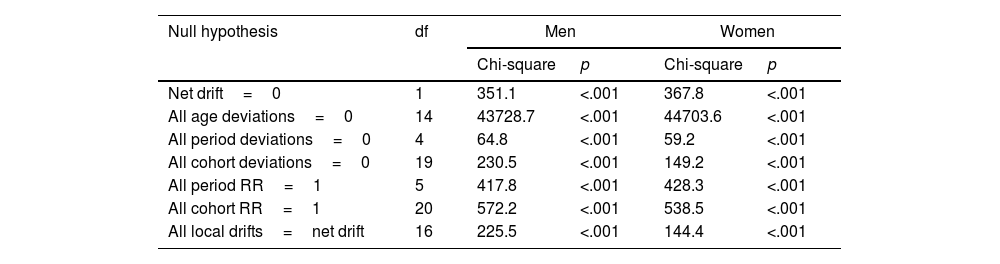
Wald tests for both sexes showed statistical significance for all net drifts, local drifts, cohort effects and period effects (Table 1). These results highlight a significant difference in the incidence of psoriasis concerning local and net drifts, as well as age, period and cohort effects.
Wald chi-square test for estimable parameters in the age-period-cohort model.
| Null hypothesis | df | Men | Women | ||
|---|---|---|---|---|---|
| Chi-square | p | Chi-square | p | ||
| Net drift=0 | 1 | 351.1 | <.001 | 367.8 | <.001 |
| All age deviations=0 | 14 | 43728.7 | <.001 | 44703.6 | <.001 |
| All period deviations=0 | 4 | 64.8 | <.001 | 59.2 | <.001 |
| All cohort deviations=0 | 19 | 230.5 | <.001 | 149.2 | <.001 |
| All period RR=1 | 5 | 417.8 | <.001 | 428.3 | <.001 |
| All cohort RR=1 | 20 | 572.2 | <.001 | 538.5 | <.001 |
| All local drifts=net drift | 16 | 225.5 | <.001 | 144.4 | <.001 |
Our study is consistent with overall observations of a slight decline in the reported incidence of psoriasis.20 However, interpreting these trends can be challenging. Increased awareness among health care professionals and the public, along with improved diagnostic tools, could lead to more diagnosed cases, even if the actual prevalence remains stable.7,14,21 The influence of socioeconomic factors on access to health care can also impact the observed trends in the incidence of psoriasis.22,23
Our study found a slightly higher incidence of psoriasis in women vs men, yet the difference was minimal (male-to-female ratio of 0.95). This is consistent with other researches that report similar rates between sexes.8,20,24,25 Worldwide, the incidence of psoriasis seems similar in women and men (1.00 ratio for 1990–2019).20 While some studies show variations in incidence and severity by sex,7,18 these variations are often small, and their clinical significance remains unclear.
Some studies,14,16 including one conducted with the population of Lleida, Spain,26 highlight a peak incidence of psoriasis that usually occurs in the 50–54 age group, followed by a decline later in life, reflecting our observations for Spain as a whole. Conversely, other areas show a bimodal distribution, characterized by two distinct incidence peaks in different age groups.7,8,27 The first peak tends to manifest itself in early adulthood, between the ages of 30 and 39, followed by a decline before a resurgence in later years, typically around 60–69 years of age. This is entirely consistent with the accepted classification of chronic plaque psoriasis as ‘type I’ (early onset) and ‘type II’ (late onset), which is defined as occurring at ≤40 and >40 years of age, respectively.28 Our findings and existing research8 suggest a higher prevalence of early-onset psoriasis in women, peaking in their late teens and early twenties, vs men who experience a later peak in their thirties. Hormonal fluctuations during puberty and perimenopause are thought to be contributing factors to this difference.29 Furthermore, exposure to certain environmental triggers, such as specific drugs or endocrine-disrupting chemicals found in some cosmetics, could contribute to the observed gender-based differences in the reported incidence rates.29
Notably, a recent Israeli study reported an earlier onset, with the first peak being observed even earlier, at 25–34 years of age.9 These findings highlight the importance of considering age-related variations in the onset of psoriasis.30
Our study revealed a significant decline in the incidence of psoriasis among younger age groups (Fig. 2). Interestingly, the overall burden of psoriasis in young adults also decreased worldwide during this period, but at varying rates.21 Notably, North Africa, the Middle East, Western sub-Saharan Africa, and East Asia witnessed the most rapid decline, whereas regions like Tropical Latin America, Western Europe, and the high-income Asia Pacific regions showed the slowest decrease. The latter observation might be partially explained by the rising prevalence of obesity – a known risk factor for psoriasis – in these areas. Furthermore, countries such as Japan, Sweden, and Somalia exhibited the most significant changes in the age-adjusted incidence rates of psoriasis among young people. This could potentially be associated with factors such as economic development, advancements in medical technology, and improved preventative measures and treatments for psoriasis implemented in these countries during the study period.
When considering factors influencing trends over time (period effect) and across different birth years (cohort effect), our findings show a decrease in relative risks similar to what has been observed in other countries.16 This decline suggests a potential reduction in exposure to risk factors or triggers over the observed period.
While genetics plays a significant role in psoriasis susceptibility, environmental factors act as triggers, initiating the disease process.31,32 Well-established triggers include stress, infections, and specific drugs. Beyond these, several factors contribute to the onset and severity of psoriasis.
Both genetic and environmental factors influence psoriasis development and progression. Genetic risks involve variations in genes such as the HLA-Cw6 and CARD14. Environmental factors include infectious diseases (particularly streptococcal pharyngitis, which can trigger flare-ups), use of medication (beta-blockers and lithium, have been associated with the development or worsening of psoriasis), and lifestyle choices (obesity,33,34 smoking35 and alcohol36 contribute to the risk and severity of psoriasis).
It is well known that smoking influences the onset of the disease as well as its progression.37 Smoking habits in Spain have changed in recent years, with a decrease in the number of smokers.38 This decline is consistent with the decrease in the incidence rate of psoriasis in our findings. While a cause-and-effect relationship cannot be definitively established from this data alone, research suggests that smoking can worsen psoriasis and increase the risk of health-related problems.35 This highlights the potential benefits of smoking cessation for both managing psoriasis and reducing the risk of associated comorbidities. Raising awareness of psoriasis risk factors through health education can empower young adults to adopt healthier habits, potentially reducing their risk of developing the condition.21,39
Obesity is a well-established risk factor for psoriasis, and its rising prevalence could hinder efforts to decrease the burden of psoriasis, especially in certain regions.10 Studies in Spain have shown a higher prevalence of obesity among individuals with psoriasis,40 and a confirmed association between metabolic syndrome (a cluster of conditions often linked to obesity) and psoriasis has been established.41 The obesity rates reported in Spain have continuously increased since 1987, remaining >15% in recent years.42 This concerning trend is consistent with the observed rise in psoriasis cases, suggesting that increasing obesity rates in Spain might be a contributing factor, similar to what has been reported in other countries.4,10
Public education and awareness campaigns are essential to improve our understanding of psoriasis, including triggers and management strategies.31 This empowers individuals to take steps to avoid triggers, such as skin damage and certain drug use and maintain a healthy lifestyle.
While our study used robust A-P-C analysis and the comprehensive GBD database to investigate psoriasis trends in Spain, the GBD data per se has limitations to be able to establish definitive causal relationships between observed trends and potential influencing factors.14 These data, compiled from a variety of sources including research and government reports, vary in quality and completeness across regions, which may affect the accuracy of estimates14. The reliance on reported diagnoses introduces bias influenced by differences in access to health care and coding practices over time. The GBD reliance on modelled data, compounded by variations in the quality of raw data may affect the reliability of the results. In particular, the GBD datasets do not distinguish between early- and late-onset psoriasis, which limits the scope of our analysis.
In addition to these GBD-related limitations, our ecological study design – which analyses data at population level – raises the possibility of ecological bias. This means that population-level associations may not reflect the true cause–effect relationships at individual level. Changes in diagnostic practices, access to health care, or public health policies could also influence the observed trends. For example, stricter diagnostic criteria or limited access to dermatologists could lead to an apparent decrease in the reported incidence rates even if true prevalence remains stable. Conversely, increased awareness or shorter waiting times could lead to a perceived increase. We were not able to adjust for these potential confounders in this study.
More research is needed to explore the complex interplay between genetic and environmental factors contributing to psoriasis. Studies using data at individual level can examine potential contributing factors in more detail, such as changes in diagnostic coding practices, patterns of health care use and environmental exposures. In addition, future studies should use data sources that differentiate between early- and late-onset psoriasis to gain deeper insights into this important distinction. This comprehensive approach will help elucidate the reasons for the observed decline in the reported incidence rates of psoriasis and inform the development of effective prevention and management strategies.
Further research is needed to explore the complex interplay of genetic and environmental factors and to establish definitive causal relationships between observed trends and potential influencing factors. Additionally, future studies should consider utilizing data sources that differentiate between early- and late-onset psoriasis to gain deeper insights into this important distinction.
Our study observed a slight decline in reported psoriasis ASIR in Spain, which is consistent with global trends. This decline might be due to reduced exposure to risk factors over time. However, interpreting these trends is complex due to potential biases and limitations in the data used. Further research is needed to explore the underlying mechanisms behind these observations and gain a deeper understanding of the factors influencing the incidence of psoriasis in Spain.
Ethical approvalThis study employed anonymized data from the Global Burden of Disease (GBD) study, adhering to the principles of good clinical practice (GCP) and the Declaration of Helsinki. As the data were anonymized and no personal information was accessed, informed consent from participants or approval from an ethics committee was not required. Additionally, adherence to the Guidelines for Accurate and Transparent Health Estimation Reporting for Population Health Research (GATHER) safeguards the credibility and integrity of the findings.
FundingThis research did not receive any specific grants from funding agencies in the public, commercial or nonprofit sectors.
Authors’ contributionsAll authors contributed to the conception and design of the work; the acquisition, analysis, and interpretation of data; drafting the work and revising it critically for important intellectual content; approved the version to be published; and are responsible for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are properly investigated and resolved.
Conflict of interestThe authors did not declare conflicts of interest in relation to the contents of this manuscript.
Data availabilityThe data that support the findings of this study are openly available at: https://vizhub.healthdata.org/gbd-results/ and https://www.ine.es/en/.