Chronic Urticaria (CU) is a debilitating disease whose treatment is mainly symptomatic. UCREX study aimed to identify CU patients’ profile, disease management and quality-of-life (QoL) in daily clinical practice in Spain.
MethodsObservational, 12-months prospective, multicenter study, included de novo or established CU patients attending to dermatology/allergy consultations in 39 Spanish hospitals. Main variables: Urticaria Activity Score (UAS), UAS over 7 days (UAS7). Secondary variables: CU-QoL Questionnaire (CU-Q2oL), EuroQol-5 dimensions (EQ-5D), Medical Outcomes Study Sleep (MOS-Sleep) scale, Hospital Anxiety and Depression Scale (HADS).
Results361 patients included. Of them, 176 (48.8%) considered for the main objective analysis. Mean age (SD) of 46.6 (14.2) years and 71.8% women. The year prior to inclusion, most patients (57.1%) were treated with non-sedating H1-antihistamines (NS-H1AH). At baseline, mean (SD) 3.6 (6.8) visits were registered to primary care. Mean (SD) UAS7 at baseline was 14.3 (11.0) and CU-Q2oL 24.1 (17.0) which tended to improve by 8.6 (9.7) and 13.9 (15.0), respectively, at 12-months. MOS-Sleep and EQ-5D remained steady during the study, except pain/discomfort and anxiety/depression which went from 58.7% and 49.6% to 29.6% and 26.9%, respectively. At baseline, HADS showed a mean (SD) anxiety of 8.7 (4.5) and depression 5.1 (4.4), decreasing to 7.0 (4.3) and 4.7 (4.3), respectively, at 12-months.
ConclusionsAlthough most CU patients are treated with NS-H1AH, disease activity is still important, negatively affecting patients’ QoL, work activity and healthcare resources use. An appropriate disease management could be the basis for symptoms control, QoL improvement and resources optimization.
La urticaria crónica (UC) es una enfermedad debilitante cuyo tratamiento es principalmente sintomático. El estudio UCREX tuvo como objetivo identificar el perfil de los pacientes con UC, el manejo de la enfermedad y la calidad de vida (CdV) en la práctica clínica diaria en España.
MétodosEstudio observacional, prospectivo, multicéntrico de 12 meses, que incluyó pacientes con UC de novo o establecida que acudieron a la consulta de dermatología/alergología de 39 hospitales españoles. Las variables principales fueron: el Urticaria Activity Score (UAS) y el UAS por siete días (UAS7). Las variables secundarias fueron: el cuestionario de CdV de urticaria crónica (CU-Q2oL), el EuroQol-5 Dimensiones (EQ-5D), la escala Medical Outcomes Study Sleep (MOS-Sleep) y la escala hospitalaria de ansiedad y depresión (HADS).
ResultadosSe incluyeron 361 pacientes, de los cuales 176 (48,8%) formaron parte del análisis del objetivo principal. La edad media (DE) fue de 46,6 (14,2) años y el 71,8% eran del sexo femenino. El año anterior al periodo de inclusión de los pacientes, la mayoría de ellos (57,1%) se habían tratado con antihistamínicos H1 no sedantes (AHNS-H1). En la basal, se registró una media (DE) de 3,6 (6,8) de visitas a atención primaria. La media (DE) del UAS7 en la basal fue de 14,3 (11,0) y del CU-Q2oL 24,1 (17,0), observándose una tendencia en la mejoría en 8,6 (9,7) y 13,9 (15,0), respectivamente, a los 12 meses. El MOS-Sleep y el EQ-5D se mantuvieron estables durante el estudio, excepto por el dolor/malestar y la ansiedad/depresión que pasaron de 58,7 y 49,6% a 29,6 y 26,9%, respectivamente. En situación basal, el HADS mostró una ansiedad media (DE) de 8,7 (4,5) y una depresión de 5,1 (4,4), disminuyendo respectivamente a 7,0 (4,3) y 4,7 (4,3) a los 12 meses.
ConclusionesAunque la mayoría de los pacientes son tratados con AHNS-H1, la actividad de la enfermedad sigue siendo importante, afectando negativamente a su CdV, su actividad laboral y repercutiendo negativamente en el uso de recursos sanitarios. Un manejo adecuado de la enfermedad podría ser la base para alcanzar el control de los síntomas, la mejora de la CdV y la optimización de los recursos sanitarios necesarios.
Chronic urticaria (CU) is a cutaneous disease characterized by the presence of wheals with redness, inflammation, pruritus and, in some cases, burning and pain, for at least six weeks, with or without angioedema.1–3 It is estimated that between 8-20% of the population is susceptible to experience at least one episode of urticaria throughout their life.1,2 Based on Gaig et al. the prevalence of CU in Spain is 0.6%, being twice more prevalent in women than in men. The average age of onset for CU is around 40 years.3,4 CU can be classified into chronic spontaneous urticaria (CSU; symptoms persist >6 weeks with no known specific trigger) and chronic inducible urticaria (CINDU; induced by specific triggers).3
Several studies indicate that CU negatively affects patients’ quality-of-life (QoL), as well as work activity,1,2,5 having a considerable impact on direct and indirect health-related costs.6 CU has no cure and therefore, appropriate management of disease symptoms become crucial. Current guidelines recommended non-sedating H1-antihistamines (NS-H1AH) at licensed doses as the first-line of treatment for CU.3 In case of non-respondent patients, NS-H1AH dose could be increased up to four-fold. If a third-line is needed, addition of omalizumab is the recommended option, followed by cyclosporine A as fourth-line of treatment (when omalizumab fails). The use of systemic corticosteroids can be considered for short periods in case of severe exacerbations.1,3,6,7
Although guidelines provide a framework for the management of CU patients, specialists’ experience is the cornerstone for treatment individualization to achieve the most effective pharmacotherapy and healthcare results.8 In this regard, UCREX study was designed to understand CU patients’ profile, clinical management, CU impact in patients’ QoL and healthcare resources use (HRU) of daily clinical practice in Spain.
MATERIALS AND METHODSStudy designUCREX is an observational, multicenter, and prospective study (12 months) conducted in Spain between May 31st, 2013 and January 31st, 2015. 39 hospitals and 42 investigators (21 dermatologists and 21 allergists) participated in the study. Patients were aged ≥18 years, with de novo or established CSU or CINDU diagnosis, and attended at allergy or dermatology services. Patients with acute urticaria, urticarial vasculitis or other type of physical urticaria not associated with CU or angioedema without wheals or pruritus related to cutaneous diseases or participating in any other study were excluded.
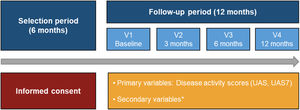
The study was structured in four visits (baseline visit, and at 3, 6 and 12 months). Basic study data was recorded by an ad-hoc electronic Case Report Form (eCRF). Patient reported outcomes (PROs) were recorded through physical questionnaires and transferred to the study database. Data collection referred to daily clinical practice was based on clinical charts and the following completed by study questionnaires: Urticaria Activity Score (UAS) and UAS over 7 days (UAS7) to assess key urticaria signs and symptoms; Chronic Urticaria Quality-of-Life Questionnaire (CU-Q2oL) and EuroQol-5 dimensions (EQ-5D) to evaluate impact on QoL; Medical Outcomes Study Sleep (MOS-Sleep) Scale to assess disease impact on sleep quality and Hospital Anxiety and Depression Scale (HADS) to measure levels of hospital anxiety and depression (Fig. 1).9
Study design from the selection period to the follow-up period.
*HADS, HRQoL (CU-Q2oL, EQ-5D), MOS-Sleep, sociodemographic and anthropometric data, clinical characteristics of urticaria, associated comorbidities, direct resources used due to CU, previous referral circuit, days of work absenteeism, time from the onset of symptoms to diagnosis of CU.
Abbreviations: CU, chronic urticaria; CU-Q2oL, Chronic Urticaria Quality-of-Life Questionnaire; EQ-5D, EuroQol-5 dimensions; HADS, Hospital Anxiety and Depression Scale; HRQoL, Health-Related Quality-of-Life; MOS-Sleep, Medical Outcomes Study Sleep Scale; UAS, Urticaria Activity Score; UAS7, Urticaria Activity Score over 7 days; V, visit.
Secondary variables included: sociodemographic and clinical data, associated comorbidities, disease management, treatment patterns, direct healthcare resources and PROs. CU-Q2oL and EQ-5D questionnaire was used to evaluate symptoms and impact of CU on QoL.10–12 To assess disease impact on sleep quality, MOS-Sleep scale was used.13,14 The HADS questionnaire measured levels of hospital anxiety and depression.15,16
The main study variables were UAS and UAS7. To collect the direct healthcare resources associated with CU, the number of hospital admissions, visits to primary care (PC), emergency, allergology and dermatology services, diagnostic tests performed before and during the study and prescribed treatments were analyzed.
Statistical methodsSample size estimation was based on a 0.6% CU prevalence in Spain4 and an accuracy of 5.5%, estimating a maximum uncertainty proportion and greater variability of 50% and a confidence interval of 95% and considering 5% of losses. An estimated 335 patients were considered as a representative sample size for the study.
Statistical analyses were carried out in evaluable patients and descriptive analyses of all variables were carried out separately, using tables of absolute and relative frequencies for discrete qualitative and quantitative variables, and by means of average statistics, standard deviation (SD), extreme values and quartiles for continuous quantitative variables. For all comparisons a statistical significance level of 0.05 was considered. Statistical package Statistical Analysis System (SAS) version 9.2 or later for Windows was used.
Ethical considerationsThe study was carried out following the ethical principles of the Declaration of Helsinki and the guidelines specified in Order SAS/3470/2009 of the Spanish Agency of Medicines and Medical Devices (AEMPS). Protocol, informed consent form and other information for the patients were approved by the reference Clinical Research Ethics Committee (CEIC) of Hospital del Mar (IMIM of Barcelona), as well as all the CEICs and autonomous communities of the participating hospitals, as needed. All patients signed a written informed consent before being included in the study.
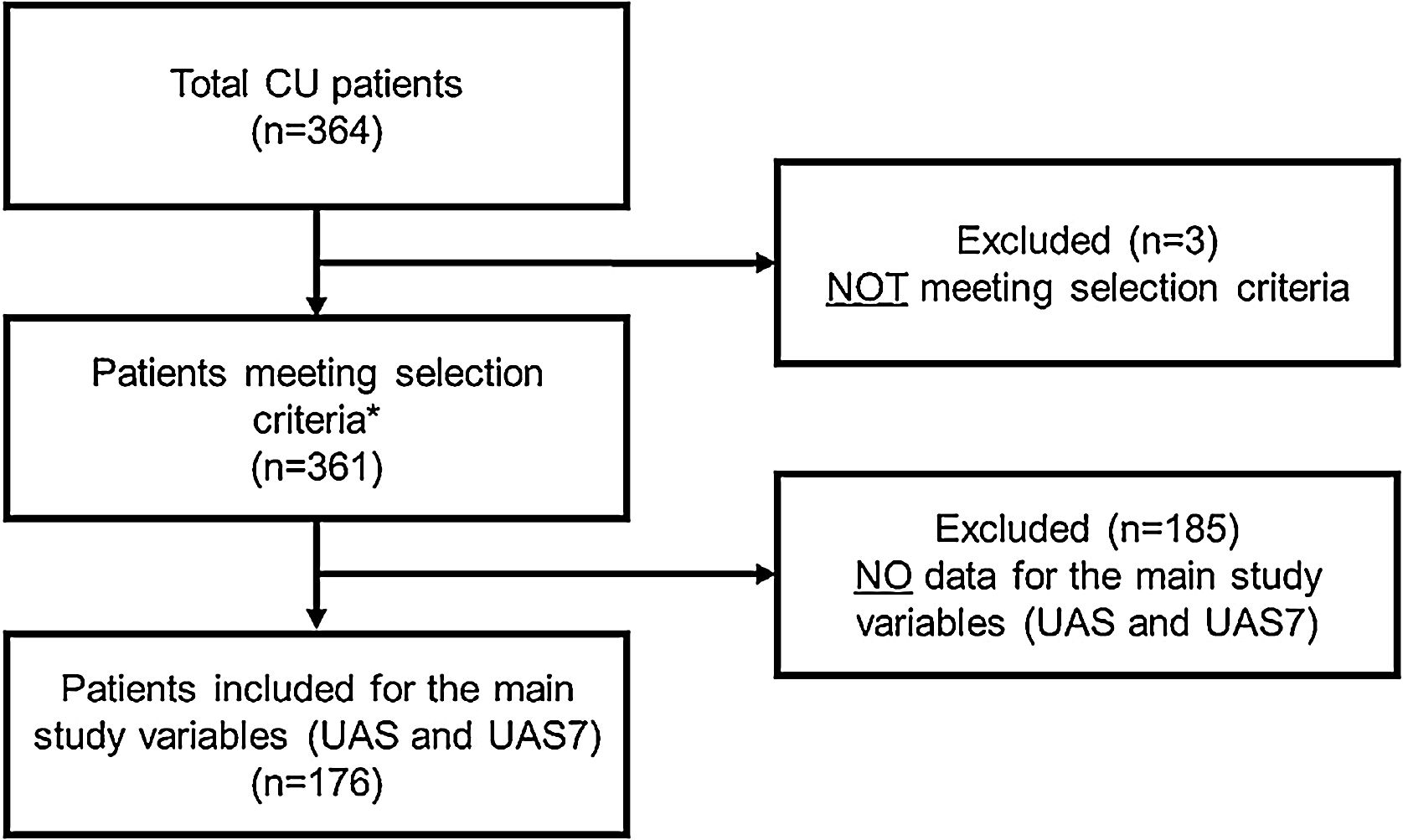
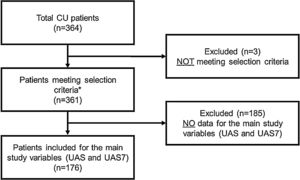
RESULTSA total of 364 CU patients were initially recruited. Baseline sample was formed by 361 patients meeting all the selection criteria. For primary endpoint analysis, 176 patients were evaluable: patients who had data on the primary endpoint (UAS/UAS7) and who received CU treatment at 12 months visit (Fig. 2).
Study flow chart of the study population.
*Patients included for secondary variables (evaluable patients): HADS (n = 361), HRQoL (CU-Q2oL: n = 361, EQ-5D; n = 361), MOS-Sleep (n = 361), sociodemographic and anthropometric data (n = 361), clinical characteristics of urticaria (n = 361), associated comorbidities (n = 236), direct resources used due to CU (n = 361), previous referral circuit (n = 351), days of work absenteeism (n = 195 during the year prior to inclusion), time from the onset of symptoms to diagnosis of CU (n = 361).
Abbreviations: CU, chronic urticaria; CU-Q2oL, Chronic Urticaria Quality-of-Life Questionnaire; EQ-5D, EuroQol-5 dimensions; HADS, Hospital Anxiety and Depression Scale; HRQoL, Health-Related Quality-of-Life; MOS-Sleep, Medical Outcomes Study Sleep Scale; UAS, Urticaria Activity Score; UAS7, Urticaria Activity Score over 7 days.
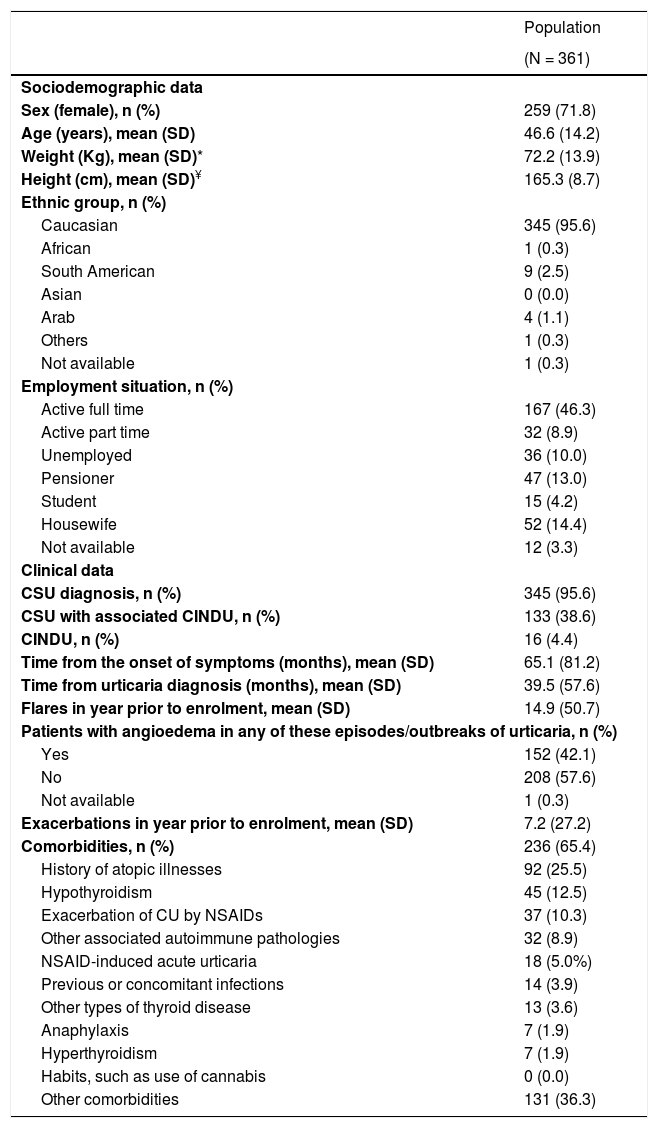
Baseline sociodemographic and clinical characteristics of the study population are collected in Table 1.The patients included in the study had a mean age (SD) of 46.6 (14.2) years, were mainly female (71.8%) and 152 (42.1%) patients showed angioedema in any of the episodes/outbreaks of urticaria. Most patients (n = 345; 95.6%) were diagnosed with CSU, being associated to CINDU in 38.6% of cases. Only 16 patients (4.4%) were diagnosed with CINDU (not associated with CSU). Among the 361 patients included in the study, 199 were actively working (55.1%) (Table 1). Within these active patients, 195 (98.0%) presented absenteeism during the year prior to inclusion and 147 (73.9%) during the 12 months of the study, with a mean (SD) of 2.7 (17.5) and 4.8 (37.4) days, respectively.
Baseline sociodemographic and clinical characteristics of the study population (patients meeting all the selection criteria).
| Population | |
|---|---|
| (N = 361) | |
| Sociodemographic data | |
| Sex (female), n (%) | 259 (71.8) |
| Age (years), mean (SD) | 46.6 (14.2) |
| Weight (Kg), mean (SD)* | 72.2 (13.9) |
| Height (cm), mean (SD)¥ | 165.3 (8.7) |
| Ethnic group, n (%) | |
| Caucasian | 345 (95.6) |
| African | 1 (0.3) |
| South American | 9 (2.5) |
| Asian | 0 (0.0) |
| Arab | 4 (1.1) |
| Others | 1 (0.3) |
| Not available | 1 (0.3) |
| Employment situation, n (%) | |
| Active full time | 167 (46.3) |
| Active part time | 32 (8.9) |
| Unemployed | 36 (10.0) |
| Pensioner | 47 (13.0) |
| Student | 15 (4.2) |
| Housewife | 52 (14.4) |
| Not available | 12 (3.3) |
| Clinical data | |
| CSU diagnosis, n (%) | 345 (95.6) |
| CSU with associated CINDU, n (%) | 133 (38.6) |
| CINDU, n (%) | 16 (4.4) |
| Time from the onset of symptoms (months), mean (SD) | 65.1 (81.2) |
| Time from urticaria diagnosis (months), mean (SD) | 39.5 (57.6) |
| Flares in year prior to enrolment, mean (SD) | 14.9 (50.7) |
| Patients with angioedema in any of these episodes/outbreaks of urticaria, n (%) | |
| Yes | 152 (42.1) |
| No | 208 (57.6) |
| Not available | 1 (0.3) |
| Exacerbations in year prior to enrolment, mean (SD) | 7.2 (27.2) |
| Comorbidities, n (%) | 236 (65.4) |
| History of atopic illnesses | 92 (25.5) |
| Hypothyroidism | 45 (12.5) |
| Exacerbation of CU by NSAIDs | 37 (10.3) |
| Other associated autoimmune pathologies | 32 (8.9) |
| NSAID-induced acute urticaria | 18 (5.0%) |
| Previous or concomitant infections | 14 (3.9) |
| Other types of thyroid disease | 13 (3.6) |
| Anaphylaxis | 7 (1.9) |
| Hyperthyroidism | 7 (1.9) |
| Habits, such as use of cannabis | 0 (0.0) |
| Other comorbidities | 131 (36.3) |
Abbreviations: CINDU, chronic inducible urticaria; CSU, chronic spontaneous urticaria; CU, chronic urticaria; NSAID, non-steroidal anti-inflammatory drugs; SD, standard deviation.
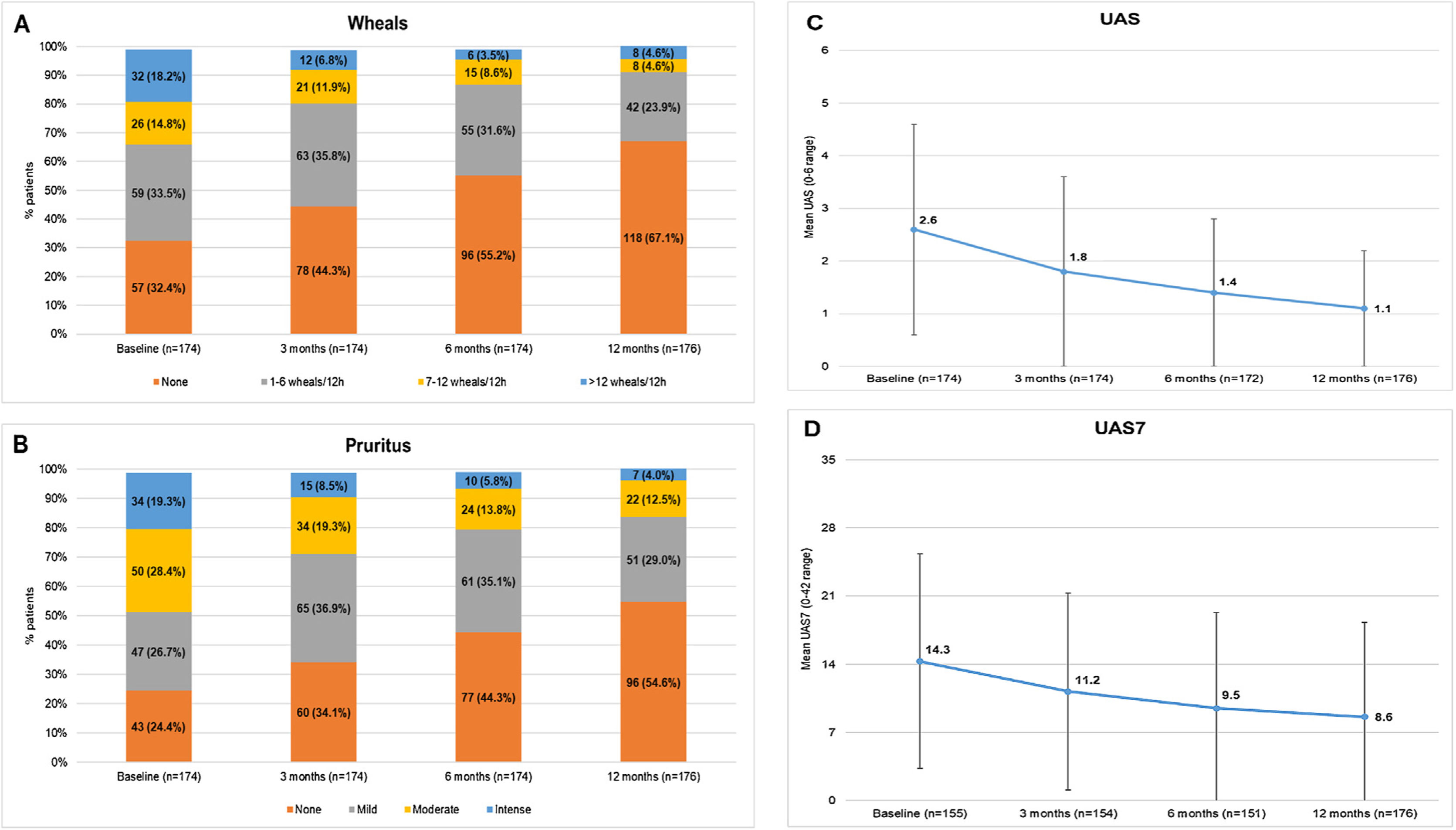
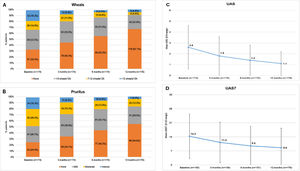
In the study, 75.6% of the CU patients were symptomatic (UAS7 >0) at the 12-months visit despite being treated according to daily clinical practice. Among patients who responded to the questionnaires (N = 176), the incidence of wheals and/or pruritus was tended to reduce according to the UAS scale between baseline and the 12-months visit (117 [66.5%] and 131 [74.4%] patients with wheals and pruritus, respectively, at baseline vs 58 [33.0%] and 80 [45.5%] patients at the 12-months visit, respectively). Mean (SD) UAS and UAS7 scores also tended to decrease throughout the study (2.6 [2.0] and 14.3 [11.0] at baseline vs 1.1 [1.5] and 8.6 [9.7] at 12 months, respectively). Although UAS and UAS7 score reductions were related to symptom improvement, the severity of the disease in these patients at the 12-months visit was categorized as moderate for 22 patients (12.5%) and as intense for seven patients (4.0%) (Fig. 3)
Descriptive of CSU activity by visit stratified by wheals, pruritus, UAS and UAS7 mean scores by visit.
Wheals (A), pruritus (B), UAS (C) and UAS7 (D) mean scores by visit.
Evaluable population (N = 176).
Mild pruritus: pruritus present but not irritating or annoying; moderate pruritus: annoying itching but without interfering with patient’s daily activities or with patient’s sleep; intense pruritus: severe itching, annoying enough to interfere with patient’s daily activities and with patient’s sleep.
Abbreviations: CSU, chronic spontaneous urticaria; h, hours; UAS, Urticaria Activity Score; UAS7, Urticaria Activity Score over 7 days.
Disease management of the CU patients was considered from the year prior to study inclusion until the 12-months visit. During the year prior to inclusion, emergency services had a mean (SD) of 1.6 (2.8) visits due to CU (n = 355), tended to reduce by 0.3 (1.3) during the 12 months of the study (n = 268). Hospitalization was rarely needed during the year prior to study inclusion (n = 357; mean [SD] of 0.0 [0.1]) and during the 12 months of the study (n = 268; mean [SD] of 0.0 [0.2]). PC was the management reference during the year prior to inclusion (n = 340), with a mean (SD) of 3.6 (6.8) visits (vs allergology and dermatology [n = 358 in both cases], with 2.4 [4.3] and 2.6 [4.2] visits, respectively). CU patients were initially identified in PC centers (294 patients, 81.9%) and subsequently tendency of patients being referred to dermatology services (155 patients, 42.9%) was higher than allergy services (139 patients, 38.5%) for diagnosis confirmation and monitoring. After being referred to specialists, CU patients had a mean (SD) average of 1.7 (3.3) follow-up visits to allergy services and 1.4 (2.8) to dermatology services per year.
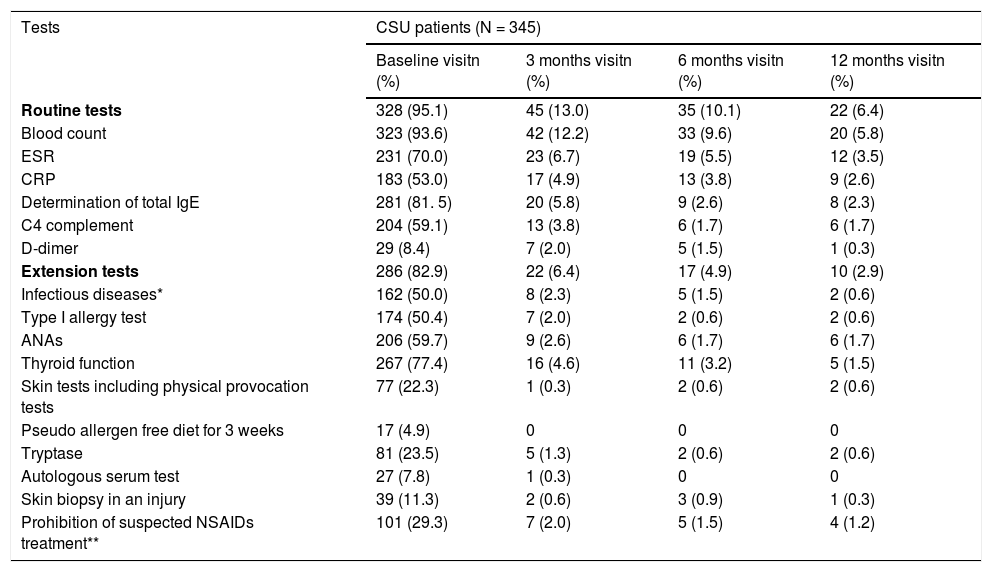
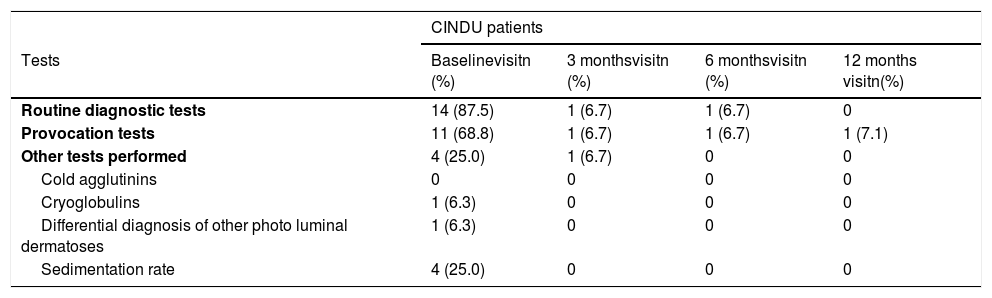
Diagnostic tests were conducted in 328 (95.1%) of CSU patients, being blood count the most commonly used (323 patients, 93.6%). Extension tests were also performed in 286 patients (82.9%) (Table 2). Regarding CINDU patients (n = 16), 14 patients (87.5%) had undergone diagnostic tests at inclusion, and provocation tests has the tendency to be the most frequents (11 patients, 68.8%) (Table 3).
Diagnostic tests performed to CSU patients throughout UCREX study
| Tests | CSU patients (N = 345) | |||
|---|---|---|---|---|
| Baseline visitn (%) | 3 months visitn (%) | 6 months visitn (%) | 12 months visitn (%) | |
| Routine tests | 328 (95.1) | 45 (13.0) | 35 (10.1) | 22 (6.4) |
| Blood count | 323 (93.6) | 42 (12.2) | 33 (9.6) | 20 (5.8) |
| ESR | 231 (70.0) | 23 (6.7) | 19 (5.5) | 12 (3.5) |
| CRP | 183 (53.0) | 17 (4.9) | 13 (3.8) | 9 (2.6) |
| Determination of total IgE | 281 (81. 5) | 20 (5.8) | 9 (2.6) | 8 (2.3) |
| C4 complement | 204 (59.1) | 13 (3.8) | 6 (1.7) | 6 (1.7) |
| D-dimer | 29 (8.4) | 7 (2.0) | 5 (1.5) | 1 (0.3) |
| Extension tests | 286 (82.9) | 22 (6.4) | 17 (4.9) | 10 (2.9) |
| Infectious diseases* | 162 (50.0) | 8 (2.3) | 5 (1.5) | 2 (0.6) |
| Type I allergy test | 174 (50.4) | 7 (2.0) | 2 (0.6) | 2 (0.6) |
| ANAs | 206 (59.7) | 9 (2.6) | 6 (1.7) | 6 (1.7) |
| Thyroid function | 267 (77.4) | 16 (4.6) | 11 (3.2) | 5 (1.5) |
| Skin tests including physical provocation tests | 77 (22.3) | 1 (0.3) | 2 (0.6) | 2 (0.6) |
| Pseudo allergen free diet for 3 weeks | 17 (4.9) | 0 | 0 | 0 |
| Tryptase | 81 (23.5) | 5 (1.3) | 2 (0.6) | 2 (0.6) |
| Autologous serum test | 27 (7.8) | 1 (0.3) | 0 | 0 |
| Skin biopsy in an injury | 39 (11.3) | 2 (0.6) | 3 (0.9) | 1 (0.3) |
| Prohibition of suspected NSAIDs treatment** | 101 (29.3) | 7 (2.0) | 5 (1.5) | 4 (1.2) |
Evaluable population (N = 345), * Infectious disease: Helicobacter pylori, Anisakis, and others ** Medical indication.
Abbreviation - ANA, antinuclear antibodies; CRP, C-reactive protein; CSU, chronic spontaneous urticaria; ESR, Erythrocytes Sedimentation rate; IgE, immunoglobulin E; NSAID, non-steroidal anti-inflammatory drugs.
Diagnostic tests performed to CINDU patients throughout UCREX study
| CINDU patients | ||||
|---|---|---|---|---|
| Tests | Baselinevisitn (%) | 3 monthsvisitn (%) | 6 monthsvisitn (%) | 12 months visitn(%) |
| Routine diagnostic tests | 14 (87.5) | 1 (6.7) | 1 (6.7) | 0 |
| Provocation tests | 11 (68.8) | 1 (6.7) | 1 (6.7) | 1 (7.1) |
| Other tests performed | 4 (25.0) | 1 (6.7) | 0 | 0 |
| Cold agglutinins | 0 | 0 | 0 | 0 |
| Cryoglobulins | 1 (6.3) | 0 | 0 | 0 |
| Differential diagnosis of other photo luminal dermatoses | 1 (6.3) | 0 | 0 | 0 |
| Sedimentation rate | 4 (25.0) | 0 | 0 | 0 |
Evaluable population (N = 16 at baseline, N = 15 at 3 months, N = 15 at 6 months visit, N = 14 at 12 months). Number of tests for CINDU populations is calculated in the evaluable population.
Abbreviations: CINDU, chronic inducible urticarial.
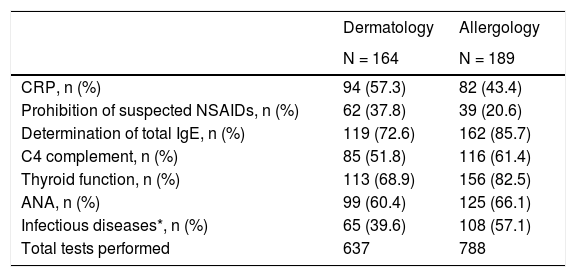
Trends of dermatology and allergology specialists (n = 164 and n = 189 patients, respectively) were detected in the routine tests of patients with CSU performed in the study. At baseline visit, more number of diagnostic tests were tended to be performed by allergology services (n = 788, 55.3%) than dermatology (n = 637, 44.7%) (Table 4). Among CINDU patients, routine tests and provocation tests were carried out for 26 (15.9%) and 28 (17.1%) patients in dermatology services, respectively, and 47 (24.9%) and 26 (13.8%) patients in allergy services at baseline visit, respectively.
Diagnostic tests performed to CSU patients at baseline by dermatology and allergology services.
| Dermatology | Allergology | |
|---|---|---|
| N = 164 | N = 189 | |
| CRP, n (%) | 94 (57.3) | 82 (43.4) |
| Prohibition of suspected NSAIDs, n (%) | 62 (37.8) | 39 (20.6) |
| Determination of total IgE, n (%) | 119 (72.6) | 162 (85.7) |
| C4 complement, n (%) | 85 (51.8) | 116 (61.4) |
| Thyroid function, n (%) | 113 (68.9) | 156 (82.5) |
| ANA, n (%) | 99 (60.4) | 125 (66.1) |
| Infectious diseases*, n (%) | 65 (39.6) | 108 (57.1) |
| Total tests performed | 637 | 788 |
Abbreviation: ANA, antinuclear antibodies; CRP, C-reactive protein; IgE, immunoglobulin E; NSAID, non-steroidal anti-inflammatory drugs.
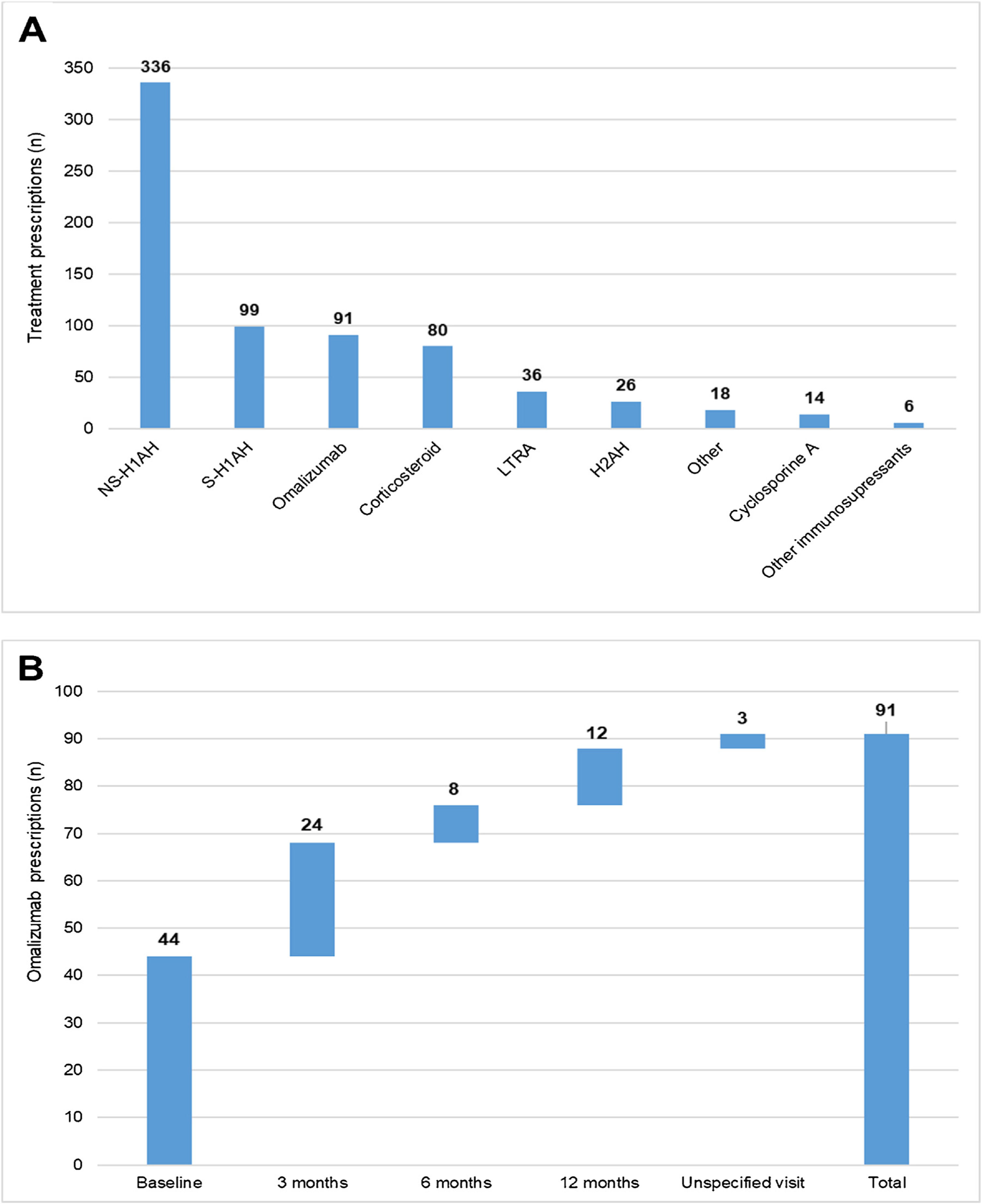
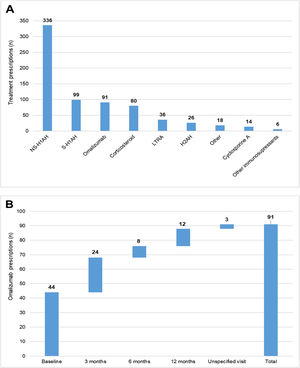
During the 1-year UCREX study in Spain, NS-H1AH (336 patients, 47.6%) was tended to be the most frequently prescribed treatments for CU patients (n = 706), followed by sedating H1-antihistamines (S-H1AH) (99 patients, 14.0%), omalizumab (91 patients, 12.9%) and corticosteroids (80 patients, 11.3%) (Fig. 4). Omalizumab prescription was present along the 1-year UCREX study. The trend of omalizumab prescriptions were mostly at the beginning of the study (44 prescriptions at baseline), although they also occurred in subsequent visits (24, 8 and 12 prescriptions at 3-, 6- and 12-months visits, respectively) (Fig. 4).
Treatments prescribed to CU patients in Spain throughout UCREX study and Omalizumab prescription during the study.
Treatments prescribed to CU patients (A), Omalizumab prescription (B).
Evaluable population (N = 361). A patient may have taken treatments from different treatment families. Treatments could be concomitants and/or sequential.
Abbreviations: H2AH, -antihistamines; LTRA, leukotriene receptor antagonists; NSAID, non-steroidal anti-inflammatory drugs; NS-H1AH, non-sedating H1-antihistamines; S-H1AH, sedating H1-antihistamines.
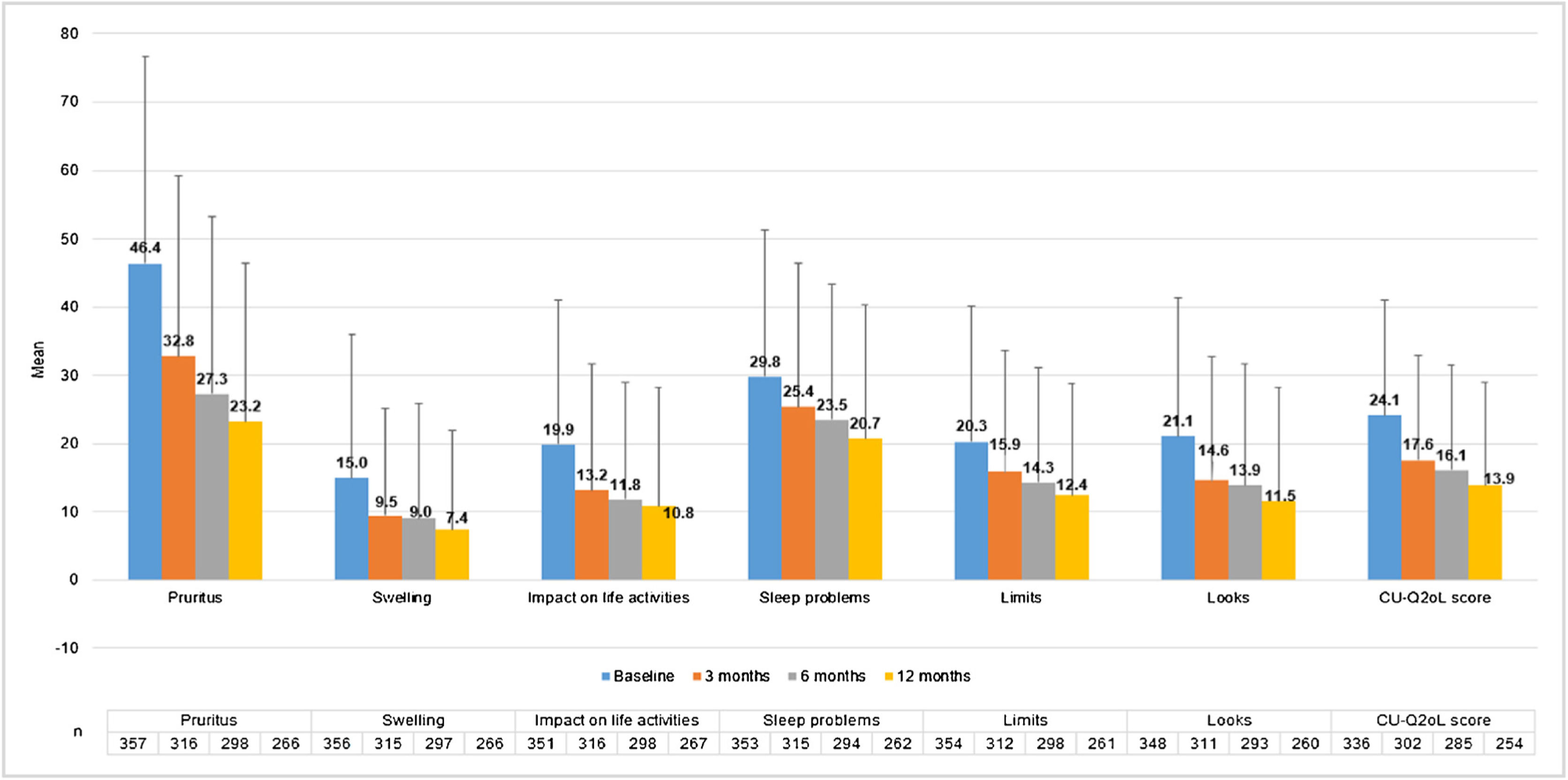
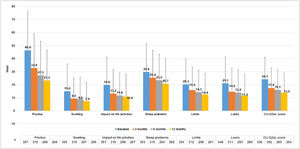
CU patients reported a CU-Q2oL mean score (SD) of 24.1 (17.0) at baseline visit (n = 336) and 13.9 (15.0) at 12-months visit (n = 254), showing pruritus as the main negative impact (46.4 (30.3) and 23.2 (23.2), respectively) (Fig. 5). According to EQ-5D questionnaire, at baseline visit (n = 357) 212 CU patients (58.7%) referred pain/discomfort, 179 patients (49.6%) anxiety/depression, 90 patients (24.9%) impact on daily activities and 61 patients (16.9%) referred mobility problems. These percentages remained stable, except for pain/discomfort and anxiety/depression, which tended to decrease at 12-months visit (n = 266: 107 [29.6%] and 97 [26.9%] patients, respectively). Mean VAS score (SD) was 68.4 (20.4) at baseline visit (n = 355), which tended to increase by 73.6 (19.6) at 12-months visit (n = 266). Index score remained stable during the study (0.8 [0.2]) (n = 357 at baseline and n = 266 at 12-months visit) (Appendix A1).
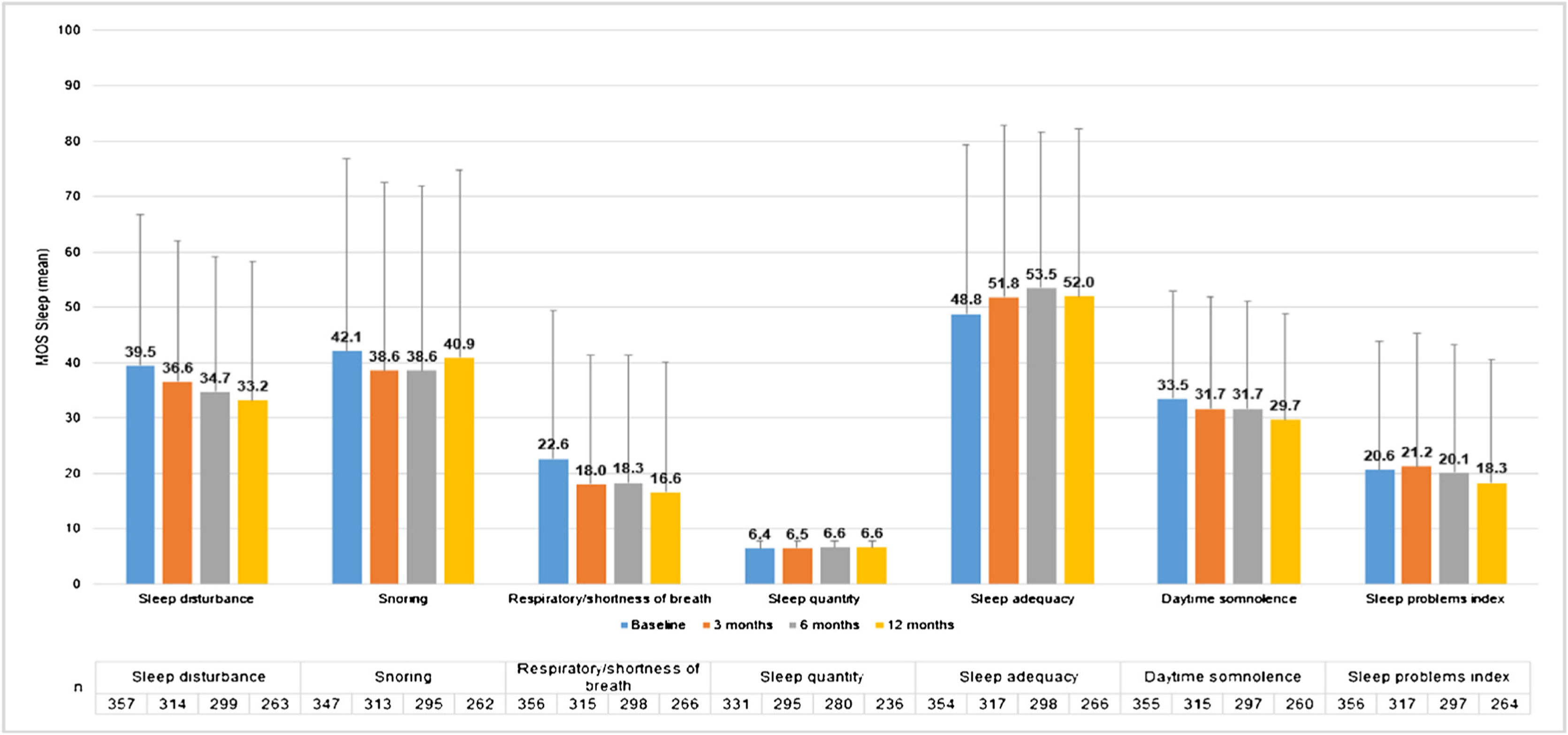
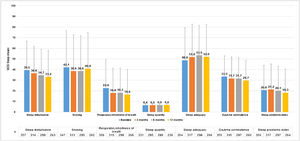
MOS-Sleep questionnaire established that CU had a negative impact in sleep quality, and this trend was barely improved with the treatment. Patients slept a mean (SD) of 6.4 (1.4) hours per night at baseline visit (n = 331) and 6.6 (1.2) at 12-months visit (n = 236). The trend of mean scores for the different dimensions hardly improved throughout the study (Fig. 6).
Anxiety and depression levels according to HADS scale remained steady during the 1-year UCREX study. Anxiety mean (SD) value went from 8.7 (4.5) at baseline (n = 349) to 7.0 (4.3) at 12-months visit (n = 262), while mean (SD) depression value went from 5.1 (4.4) (n = 345) to 4.7 (4.3) (n = 261), respectively.
DISCUSSIONUCREX study was designed to identify the percentage of CU patients that remained symptomatic despite standard treatment established according to daily clinical practice in Spain. The study results suggest that although there is a positive trend in symptom control throughout the treatment, there is still a high proportion of uncontrolled patients at the end of the study.
There are several scales validated for Spanish population focused in the CU symptoms monitoring. In this study we used UAS/UAS7 scale, being the most common tool to evaluate CU activity1,2,5,17,18 and recommended by clinical consensus in the European clinical guide of urticaria.3
Most CU patients were treated according to the standard guidelines at the time of the beginning of the study, decreasing the proportion of patients with wheals and/or pruritus by 33.5% and 28.9%, respectively, at the 12-months visit. However, 75.6% of CU patients were symptomatic at the 12-months visit, showing a moderate activity of the disease. According to previous studies carried out in daily clinical practice, less than 50% of CSU patients respond to treatment with authorized doses of NS-H1AH,3 possibly due to a considerable delay between diagnosis and specialized referral, as well as a low follow-up of the urticaria clinical guide by specialists5 or to a lack of effectiveness of the H1-antihistamines. Additionally, suboptimal control of CU symptoms could be explained by low therapeutic adherence, as was shown for S-H1AH, and attributed to their anticholinergic and sedative effects.19
A limitation of this study is lack of data regarding adherence, discontinuation and treatment changes in non-responders, which could have provided an explanation for the high rate of uncontrolled patients. Our results show that CU disease is still active according to UAS7 score despite the use of recommended treatments, which might point toward the lack of effectiveness of the available therapies.
According to the clinical guidelines during the UCREX study, CU-Q2oL and EQ-5D were the recommended questionnaires to assess patients’ health-related QoL (HRQoL).1,3,5,20 Consistent with previous studies, our results show that CU negatively impacts patient’s QoL, although there is a positive trend of the QoL throughout the study.5,21–24 Pain and discomfort were evidenced from disease onset, with VAS values above 60, indicating a moderate-to-severe pain.25 Regarding sleep quality, results of the MOS-Sleep questionnaire revealed that the disease showed a negative impact at baseline, consistent with previous studies.20
In line with the used guidelines at the time of the study, during the 12-months of the UCREX study the most frequently prescribed treatments were NS-H1AH (47.6% of patients). Omalizumab, was launched for CSU during the period of inclusion of patients and was mostly prescribed at the beginning of the study. The results of a phase III clinical trial analyzing of omalizumab in CSU showed that a three-fold higher number of patients receiving omalizumab achieved a good control of the disease versus placebo, improving patient’s QoL.26,27 In this sense, updated European Urticaria clinical guidelines3 recommend ad-hoc treatment with biologics in patients who remain symptomatic after receiving up to four-fold dose of NS-H1AH from one to four weeks.
The lack of control of the CU symptoms despite using the standard treatment based on NS-H1AH3,5 leads to daily life disability for the patient, a low QoL and work absenteeism. The inability to control CU activity, negatively impacts on HRU, including PC, specialists and/or emergency visits.1,5 This points toward the need for a better management of the patients and optimization of the use of resources associated to the disease, as well as improved treatments that would allow a better control of the CU symptoms. The development of new biological therapies such as omalizumab is a promising strategy to tackle CSU symptoms and improve the patients’ QoL.
CONCLUSIONSCU is an insidious long-term disease that mostly affects actively working population (women and men), leading to a negative impact in their QoL, labor, and HRU. Data collected from the UCREX study showed that in Spain, during the studied period, 75.6% of patients still have moderate CU activity despite being treated according to routine clinical practice. An appropriated management from the physicians could be the basis to reduce the burden of disease and improve patients’ symptoms and QoL.
Funding statementFunding for the UCREX Study was provided by Novartis Pharmaceuticals, Spain.
Declaration of authorshipAll the authors participated actively both in the conception and design of the work. All the authors have equally contributed to the development of the current manuscript, have performed an exhaustive review of its content and have approved the final version.
Data availability statementUpon confidentiality agreement data might be shared
Conflict of interestsJ. Bartra, M. Labrador-Horrillo, J. Miquel-Miquel, J. Ortiz and A. Valero have collaborated with Novartis and other pharmaceutical industries. Pau Terradas and Marian Vidal are Novartis employees.
M. Ferrer: is on the scientific advisory board and is a speaker for Novartis and FAES Farma, has served on the scientific advisory board for Genentech, has received research grants from Novartis, and has received speaker honorarium from Menarini.
J.F. Silvestre: has performed consultancies and has lectured at educational events for Sanofi-Genzyme, Regeneron, Abbvie and Novartis. He has been principal investigator in clinical trials sponsored by AbbVie, Amgen, Eli-Lilly, Leo Pharma, Novartis and Pfizer.
A. Giménez-Arnau: Medical Advisor for Uriach Pharma, Genentech, Novartis, FAES, GSK, Sanofi. Research Grants supported by Uriach Pharma, Novartis, Grants from Instituto Carlos III FEDER. Educational activities for Uriach Pharma, Novartis, Genentech, Menarini, LEO- PHARMA, GSK, MSD, Almirall, Sanofi.
J. Sastre: has served as a consultant to Thermofisher, MSD, Novartis, Sanofi, Leti, Roche, ALK, FAES FARMA, Mundipharma, and GSK; having been paid lecture fees by Novartis, GSK, Stallergenes, LETI, and FAES FARMA; as well as having received grant support for research from Thermofisher, ALK and Sanofi.
I. Jáuregui: has acted as a paid member of advisory boards for Novartis Spain, Sanofi-Genzyme and Abbvie, has served as a consultant to FAES Farma, has received educational grants from Leti Pharma and Menarini, and has been paid for lecture fees by Novartis, Sanofi-Genzyme, MSD, Roxall Laboratories, and FAES Farma.
We thank to all the centers and investigators that have collaborated in the UCREX study (see the Appendix A2). Also, we thank IQVIA, Carmen Barrull, Mireia Sumalla, and Sukanya Ghildiyal for providing medical editorial assistance with this manuscript.