This is the case of a 50-year-old man, with no drug allergies known to date, hypercholesterolemia, asthma, and allergic rhinoconjunctivitis, controlled with simvastatin, inhalers, and antihistamines. He has been suffering from atopic dermatitis (AD) since childhood. Back in 2010, he underwent a protected penetrating keratoplasty (corneal transplant) in his left eye due to herpetic keratitis complicated with corneal perforation and endophthalmitis. Two years later, he was treated with cataract surgery in the same eye, and 5 years later he developed a fungal corneal ulcer. At the time of the skin examination, his condition was stable, and he was not on any ophthalmological treatment.
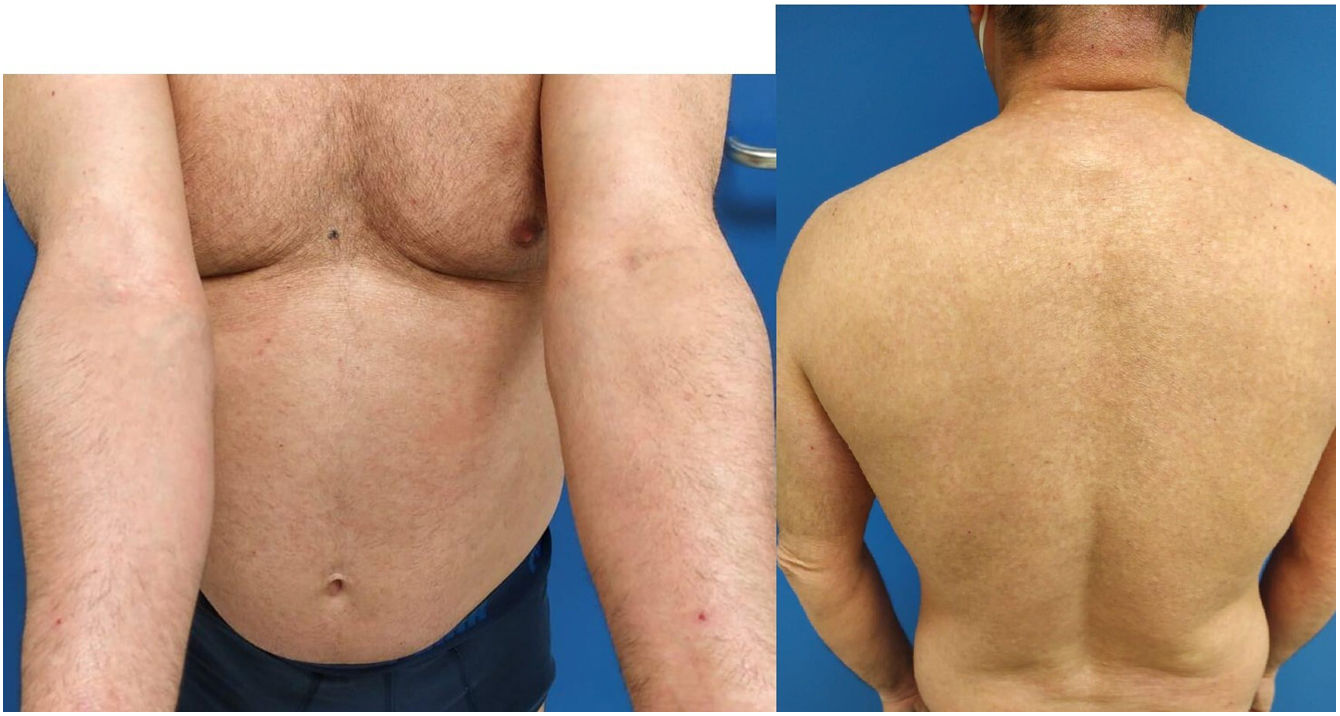
The patient's AD had been treated with topical corticosteroids and immunomodulators until 2013, when he required the use of systemic treatments. He underwent numerous cycles of oral corticosteroid therapy, 2 14- and 16-month cycles of cyclosporine at maximum doses of 300mg/day from 2013 to 2016. These cycles proved effective but were followed by relapses upon discontinuation. The patient also received a 14-month course of 12.5mg/week of methotrexate (2016–2017), which was discontinued due to ineffectiveness. Additionally, he received 17 sessions of narrowband ultraviolet B phototherapy in 2017 and mycophenolate mofetil [2g/day for 25 months (2017–2019)], which were discontinued due to a partial response. In 2020, a new cycle of cyclosporine therapy (200mg/day) was initiated. After 15 months on therapy, the patient's Eczema Area Severity Index (EASI) score was 27; the Scoring Atopic Dermatitis (SCORAD), 62.1%; and the Body Surface Area (BSA), 65.5% (Fig. 1), which prompted the discontinuation of cyclosporine and the on-labe initiation of dupilumab.
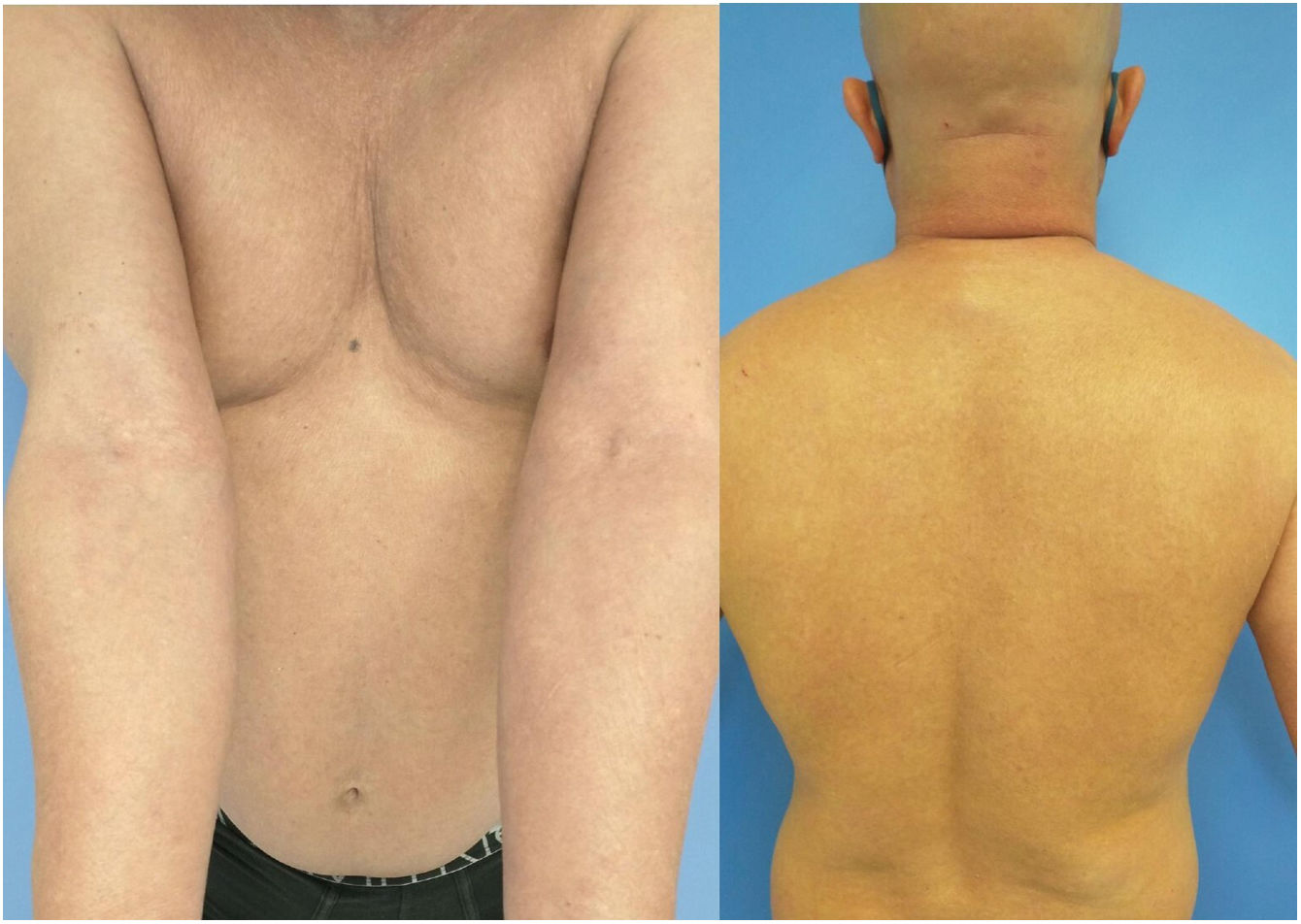
On week 12 of the treatment regimen, the patient reported improvement in itching and eczema to the nurse but mentioned hair loss, which he attributed to stress. By week 16, his EASI, SCORAD, and BSA scores were 1, 13%, and 12%, respectively, and he exhibited clinical and trichoscopic evidence of alopecia areata universalis (AAU), which the patient preferred not to treat (Fig. 2). On week 24, the EASI, SCORAD, and BSA scores were 1, 7%, and 12%. It was then that the patient experienced redness, pain, and blurred vision in his left eye. The ophthalmological evaluation confirmed the presence of conjunctival hyperemia with ciliary injection, a leukomatous graft, and fine keratic precipitates, along with +++ Tyndall effect reaction and +++ particulate reaction, yet no hypopyon or hyphema. There was focal thinning between donor and recipient, indicating corneal rejection. The patient received topical and oral corticosteroids, herpetic prophylaxis. Dupilumab was discontinued. The patient almost completely recovered his hair after 6 months, and his ophthalmological disease improved without additional treatment. For the management of his AD, he received methotrexate, which was discontinued after 4 months due to elevated hypertransaminasemia, and eventually started treatment with baricitinib, a Janus kinase inhibitor. The patient's status at the time of the last follow-up is unknown.
Dupilumab is a recombinant human monoclonal antibody that inhibits interleukin (IL) 4 and 13 signaling pathways, thus reducing Th2 immune response.1–5 It was approved by the European Medicines Agency to treat moderate-to-severe AD back in 2017.1–5
Dupilumab can induce ocular surface diseases (DIOSD: dupilumab-induced ocular surface disease.) These are mostly mild-to-moderate conjunctivitis that can often be controlled with topical treatment without having to discontinue the drug.1–3,5 Other ocular side effects reported include keratitis, blepharitis, ocular itching, dry eye, corneal ulcers, conjunctival scarring, and madarosis.2,4 No cases of dupilumab-related corneal transplant rejection have been documented to date. An ophthalmological evaluation should be performed before starting dupilumab treatment in patients with a previous ophthalmological history.1,2,4,5 Our patient was not involved in regular check-ups, or was referred to the ophthalmology consultation prior to therapy given his stability.
The survival rate of corneal grafts reaches 90% within the first year, but drops to 55% at 15 years.6 Risk factors for graft rejection mainly depend on the technique used, the immunological mechanisms involved, and the characteristics of the recipient.6,7 In our case, the close temporal relationship and the already known DIOSD suggest a possible association of dupilumab with this event. The discontinuation of immunosuppressive treatments for AD cannot be ruled out as an additional mechanism. However, systemic immunosuppressants are not often used in corneal transplant rejection prevention, as they have moderate efficacy and low scientific evidence regarding their use.6,7
On the possible impact of dupilumab on AAU, there is still controversy surrounding the scientific medical literature currently available.8–10 Some have suggested that the dupilumab-induced inhibition of Th2 lymphocytes could promote the amplification of other pathways (Th1 and Th17) involved in AAU, while cases of improvement could be justified by the inhibition of inflammatory mediators common to both AAU and AD.8 Others propose that the induction or improvement of AAU are random processes, and its immunity is primarily mediated by pathways different from Th2. In conclusion, it is unknown how the blockade of the Th2 pathway can affect Th1 and Th17 responses, or why dupilumab causes paradoxical reactions regarding AAU.8–10
We described the very first case of corneal transplant rejection and concomitant AAU in a patient with AD on dupilumab. However, more studies are still needed to understand how dupilumab is involved in both phenomena. Also, we should mention the importance of close ophthalmological monitoring in patients with previous ophthalmological history.
Conflict of interestsThe authors declare that they have no conflict of interest.