A 40-year-old woman was admitted to our hospital with a painful ulcer on her leg and fever of over 38°C. She had been diagnosed with ulcerative colitis (UC) 14 years earlier and was on treatment with mesalazine, 3600mg/d, and prednisolone at doses of up to 40mg/d. Infliximab, 5mg/kg, plus azathioprine, 50mg/d, had been added to her treatment a year prior to admission and had been administered 8 times in total. The patient also reported atopic dermatitis since childhood. One week prior to admission she experienced painful erythema of sudden onset on the lower leg; the lesion became ulcerated 4 days later. She denied any history of trauma or bruising.
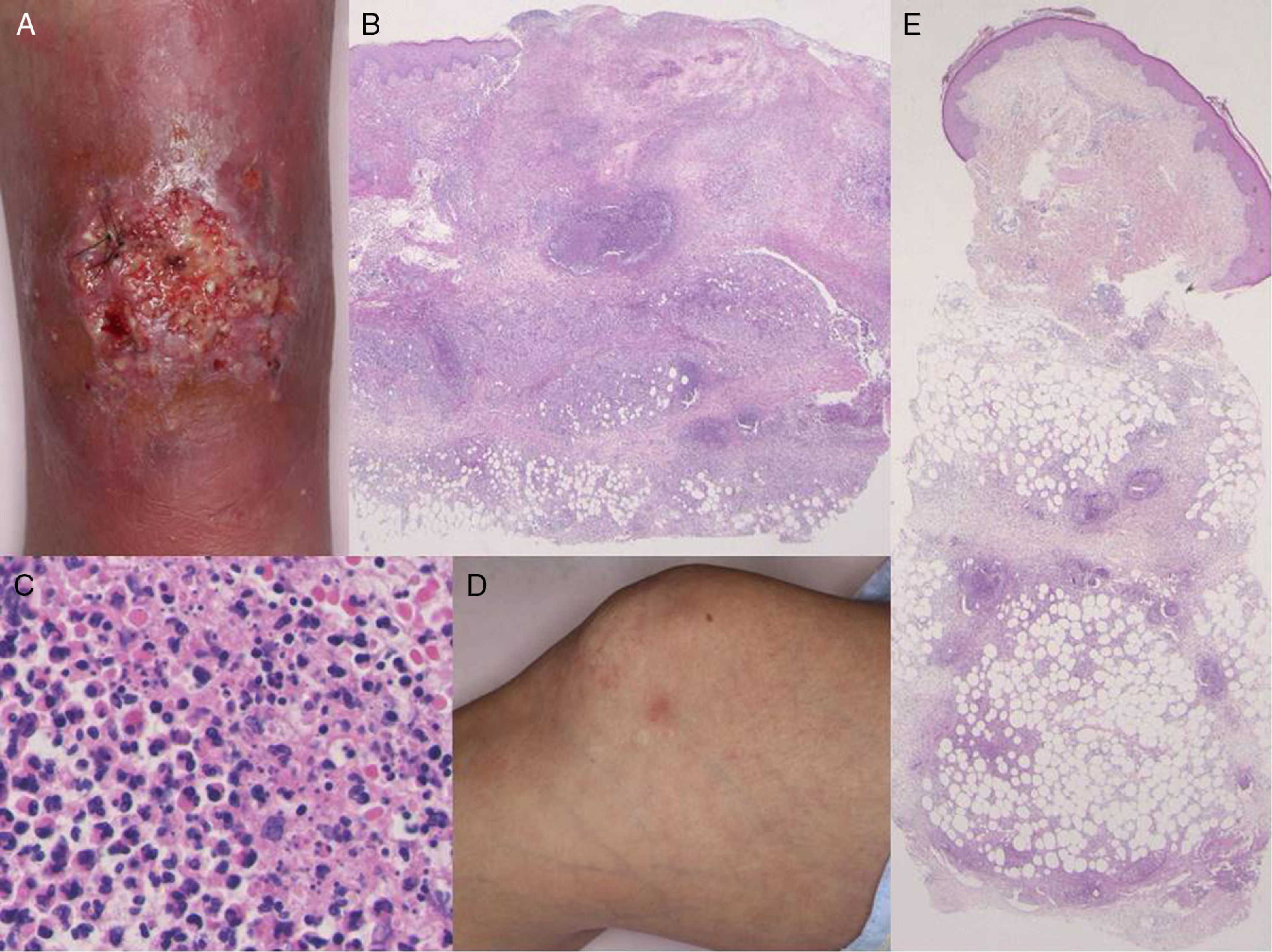
Physical examination revealed a very tender ulcer measuring 3cm×1.5cm on the left lower leg (Fig. 1A). The ulcer had irregular, elevated borders with edema. Laboratory tests revealed a white blood cell count of 7100cells/μL, with 67% neutrophils, and elevation of C-reactive protein levels (9.15mg/dL) and of the erythrocyte sedimentation rate (39mm/h). Serum levels of interleukin (IL) 8 were extremely high (1860pg/mL). Bacterial culture was sterile. A biopsy taken from the border of the ulcer showed a diffuse neutrophil infiltrate in the dermis and subcutaneous adipose tissue (Fig. 1B and C). On the second day of hospitalization, the patient developed tender erythema on both her lower legs and on her right thigh. Physical examination revealed a number of poorly defined, indurated, pale pink-erythematous papules measuring 1cm×1cm on the right knee (Fig. 1D) and on both lower legs. On histology, a neutrophilic infiltrate was observed in the subcutaneous adipose tissue (most intense in the septa) (Fig. 1E). The ulcer had reepithelialized completely 2 months after starting treatment with systemic prednisolone, 25mg/d. The prednisolone dose was then gradually tapered to complete withdrawal 5 months later, with no relapse of the pyoderma gangrenosum (PG). Both infliximab and azathioprine were continued throughout the course of steroid treatment.
(A) Deep ulcerative lesions on the lower leg. (B) Histological features showing a dense neutrophil infiltrate in the upper to mid-dermis. Hematoxylin and eosin (H&E), original magnification ×40. (C) Higher magnification reveals prominent neutrophil infiltration and extravasation of red blood cells. H&E, original magnification ×400. (D) Painful, subcutaneous erythematous nodules on the knee. (E) Histology shows cellular infiltrates most intense in the subcutaneous septa. H&E, original magnification ×40.
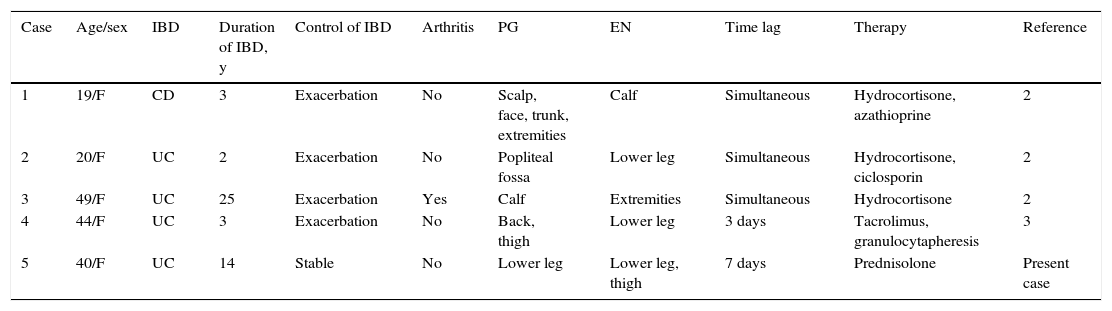
PG and erythema nodosum (EN) are skin lesions associated with UC.1 In general, such manifestations appear to be related to activity of the intestinal disease; however, some patients develop skin lesions despite remission of their bowel condition. Our patient developed a deep ulcer on her lower leg, followed by painful erythematous subcutaneous nodules at an interval of several days, despite control of her intestinal disease. To date, counting our patient, only 5 five cases of concomitant PG and EN have been reported.2,3 The details of these patients, all women aged between 19 and 49 years, are summarized in Table 1. One patient had Crohn disease, the other 4 had UC. Skin symptoms appeared simultaneously with an exacerbation of the intestinal disease in all cases except in our patient. Arthritis was observed in 1 case. Systemic steroids were used in 4 cases, with an adequate response, and 1 case was successfully treated using tacrolimus and granulocytapheresis.3 Very rarely, PG can be induced by tumor necrosis factor (TNF) inhibitors4; however, we ruled out the possibility of biologics-induced PG in our patient because the administration of infliximab to control her intestinal symptoms after the onset of her PG and EN did not exacerbate either the skin or the joint manifestations. Furthermore, azathioprine was continued throughout the course of steroid treatment, indicating that PG and EN were not related to azathioprine.
Clinical features of patients with inflammatory bowel disease and concomitant pyoderma gangrenosum and erythema nodosum.
| Case | Age/sex | IBD | Duration of IBD, y | Control of IBD | Arthritis | PG | EN | Time lag | Therapy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19/F | CD | 3 | Exacerbation | No | Scalp, face, trunk, extremities | Calf | Simultaneous | Hydrocortisone, azathioprine | 2 |
| 2 | 20/F | UC | 2 | Exacerbation | No | Popliteal fossa | Lower leg | Simultaneous | Hydrocortisone, ciclosporin | 2 |
| 3 | 49/F | UC | 25 | Exacerbation | Yes | Calf | Extremities | Simultaneous | Hydrocortisone | 2 |
| 4 | 44/F | UC | 3 | Exacerbation | No | Back, thigh | Lower leg | 3 days | Tacrolimus, granulocytapheresis | 3 |
| 5 | 40/F | UC | 14 | Stable | No | Lower leg | Lower leg, thigh | 7 days | Prednisolone | Present case |
Abbreviations: CD, Crohn disease; EN, erythema nodosum; F, female; IBD, inflammatory bowel disease; M, male; PG, pyoderma gangrenosum; UC, ulcerative colitis.
Our patient complained of flu-like symptoms, such as rhinitis and fever, which may have triggered neutrophil activation and recruitment to the skin via chemokines such as IL-8, a potent neutrophil chemoattractant. Neutrophil priming can occur in response to a number of stimuli, including TNF-α, IL-8, and granulocyte macrophage-colony stimulating factor. In the present case, serum IL-8 was very significantly elevated; this interleukin is also able to induce the production of reactive oxygen species, leading to skin damage.5
A further factor to be taken into account in our patient was that she had had atopic dermatitis since childhood. Previous studies have shown an association between atopic dermatitis and UC,6 and an increased risk of inflammatory bowel disease among patients with atopic dermatitis.7 However, other studies have not observed a statistically significant increase in the prevalence of atopic dermatitis among patients with UC compared with healthy controls.8 Possible mechanisms may include an impaired barrier function, potential sharing of type 2 T-helper cell cytokines, and thymic stromal lymphopoietin (TSLP).9
In summary, we have described the rare case of a patient with UC who developed concurrent PG and EN not related to the activity of her intestinal disease. Reporting of similar cases will help to clarify the mechanisms of the aseptic neutrophilic disorders, such as the so-called aseptic abscess syndrome.
Conflict of interestsThe authors declare no conflict of interests.