Real-world evidence of paediatric psoriasis (PsO) is lacking in Spain. The purpose of this study was to identify physician-reported disease burden and current treatment patterns in a real-world paediatric PsO patient cohort in Spain. This will enhance our understanding of the disease and contribute to the development of regional guidelines.
Material and methodThis retrospective analysis of a cross-sectional market research survey assessed the clinical unmet needs and treatment patterns in patients with paediatric PsO in Spain, as reported by their primary care and specialist physicians, using data collected as part of the Adelphi Real World Paediatric PsO Disease-Specific Program (DSP™) between February and October 2020.
ResultsSurvey data from 57 treating physicians were included (71.9% [N=41] dermatologists, 17.6% [N=10] general practitioners/primary care physicians, and 10.5% [N=6] paediatricians); the final analysis included 378 patients. At sampling, 84.1% (318/378) of patients had mild disease, 15.3% (58/378) had moderate disease and 0.5% (2/378) had severe disease. Retrospectively reported physician-judged severity at the time of PsO diagnosis recorded 41.8% (158/378) of patients with mild disease, 51.3% (194/378) with moderate disease and 6.9% (26/378) with severe disease. Overall, 89.3% (335/375) of patients were currently receiving topical PsO therapy, while 8.8% (33/375), 10.4% (39/375) and 14.9% (56/375) of patients were currently receiving phototherapy, conventional systemics and biologics, respectively.
ConclusionsThese real-world data reflect the current burden and treatment landscape of paediatric PsO in Spain. The management of patients with paediatric PsO could be improved by further educating healthcare professionals and developing regional guidelines.
En España se carece de evidencia sobre psoriasis (PsO) pediátrica en el mundo real. El objetivo de este estudio fue identificar la carga de la enfermedad reportada por el facultativo y los patrones actuales de tratamiento en una cohorte de pacientes psoriásicos pediátricos en el mundo real. Ello ampliará nuestra comprensión de la enfermedad y contribuirá al desarrollo de directrices regionales.
Material y métodoEste análisis retrospectivo transversal de una encuesta de investigación mercado evaluó las necesidades clínicas no satisfechas y los patrones de tratamiento en pacientes con PsO pediátrica en España, según lo reportado por sus médicos de atención primaria y especialistas, utilizando datos recopilados como parte del Disease-specific program (DSP™) de Adelphi para PsO pediátrica en el mundo real, entre los meses de febrero y octubre de 2020.
ResultadosSe incluyeron los datos de la encuesta realizada a 57 facultativos médicos tratantes (71,9% [N=41] de dermatólogos, 17,6% [N=10] de médicos generales de atención primaria y 10,5% [N=6] de pediatras); el análisis final incluyó 378 pacientes. En la muestra, el 84,1% (318/378) de los pacientes padeció enfermedad leve, el 15,3% (58/378) enfermedad moderada y el 0,5% (2/378) enfermedad severa. De acuerdo con el reporte retrospectivo, la gravedad juzgada por el facultativo en el momento de diagnosticarse la PsO pediátrica registró un 41,8% (158/378) de pacientes con enfermedad leve, un 51,3% (194/378) con enfermedad moderada y un 6,9% (26/378) con enfermedad severa. En general, el 89,3% (335/375) de los pacientes recibía en la actualidad terapia tópica para PsO pediátrica, mientras que el 8,8% (33/375), el 10,4% (39/375) y el 14,9% (56/375) de los pacientes recibía en la actualidad fototerapia, sistémicos y biológicos convencionales, respectivamente.
ConclusionesEstos datos del mundo real reflejan la carga actual y el panorama de la PsO pediátrica en España. El manejo de los pacientes pediátricos podría mejorar, formando aún más a los profesionales sanitarios y desarrollando directrices regionales.
Psoriasis (PsO) is a chronic, systemic skin disease associated with a physical and psychological burden.1 Approximately one-third of patients experience disease onset before the age of 16, with a mean age of 8–11 years. PsO in children and adolescents affects approximately 0.5–1.2% of the population, and the prevalence increases linearly from 0.1% at age 1 to 1.2% at age 18.2–4 PsO has a profound impact on the quality of life (QoL) of children.5
PsO guidelines from Europe and the United States (US)6,7 recommend multiple measures of assessing disease severity, including body surface area (BSA), as well as Physician's Global Assessment (PGA), Psoriasis Area and Severity Index (PASI) and Children's Dermatology Life Quality Index (DLQI) (CDLQI).6,7 Despite the availability of general guidelines on the management of PsO in Europe and the US, regional guidelines for paediatric PsO in Spain are lacking.
Most paediatric patients with mild PsO are satisfactorily managed using topical therapies only, but phototherapy and systemic therapies may be necessary to treat moderate to severe PsO.8 Conventional non-biologic systemic treatments are often used off-label in Europe and the US for paediatric PsO.3 In Galicia, Spain, phototherapy, followed by methotrexate, are the most common treatments for moderate to severe PsO in children under 18 years.9
Currently, there are five biologic agents approved by the European Medicines Agency for the treatment of moderate to severe paediatric PsO: adalimumab (≥4 years old), etanercept (≥6 years old), secukinumab (≥6 years old), ustekinumab (≥6 years old) and ixekizumab (≥6 years weighing>25kg).10–13 Results from the BIOBADADERM registry14 show that paediatric patients (up to 21 years) represent only a small number of patients with PsO who are treated with biologics; paediatric patients are more commonly treated with conventional systemics15; however, it was not possible to establish whether this was due to undertreatment or a lower burden of severe disease in this group.15
Seyger et al. reported that, despite receiving treatment for PsO, paediatric patients exhibited a high BSA, PASI and PGA scores, and high numbers of current symptoms and affected areas. While this was most prominent in patients with moderate or severe PsO, a persistent disease burden was observed among patients with mild disease.16 A related study in Europe concluded that older and heavier biologic-treated children with psoriasis predominantly have more severe psoriasis, and prescriptions for biologics are only given after several other treatments have been unsuccessful.17 The current study is a follow-up to Seyger et al., including data collected from the paediatric psoriasis population in Spain.16
Materials and methodsStudy designThis was a retrospective analysis of a cross-sectional market research survey, using data collected as part of the Adelphi Paediatric PsO Disease-Specific Program (DSP™). The methods and study design were previously described by Seyger et al.16 Data were collected between February and October 2020.
The study recruited dermatologists, general or primary care practitioners (GPs/PCPs), and paediatricians actively managing paediatric patients with PsO. Patients were aged 4–17 years receiving treatment for PsO. At least two patients with current or previous biologic use were included per dermatologist. Patients receiving multiple treatment classes in tandem were included (e.g., topical and conventional systemic therapy, or conventional systemic and biologic therapy).
Study objectivesThe primary objective of this study was to describe physician-reported clinical unmet needs among the paediatric PsO population in Spain. The key secondary objective was to describe current treatment patterns among the paediatric PsO population in Spain.16
Disease severityAs reported previously,16 disease severity was physician-judged, with no clinical definition previously applied. Physicians may have considered several factors when subjectively defining a patient's disease severity (e.g., BSA, PASI, current symptoms, and areas affected). While this study was a cross-sectional survey, with data related to the time of sampling (or ‘currently’), physician-judged severity classifications were also collected retrospectively at diagnosis.
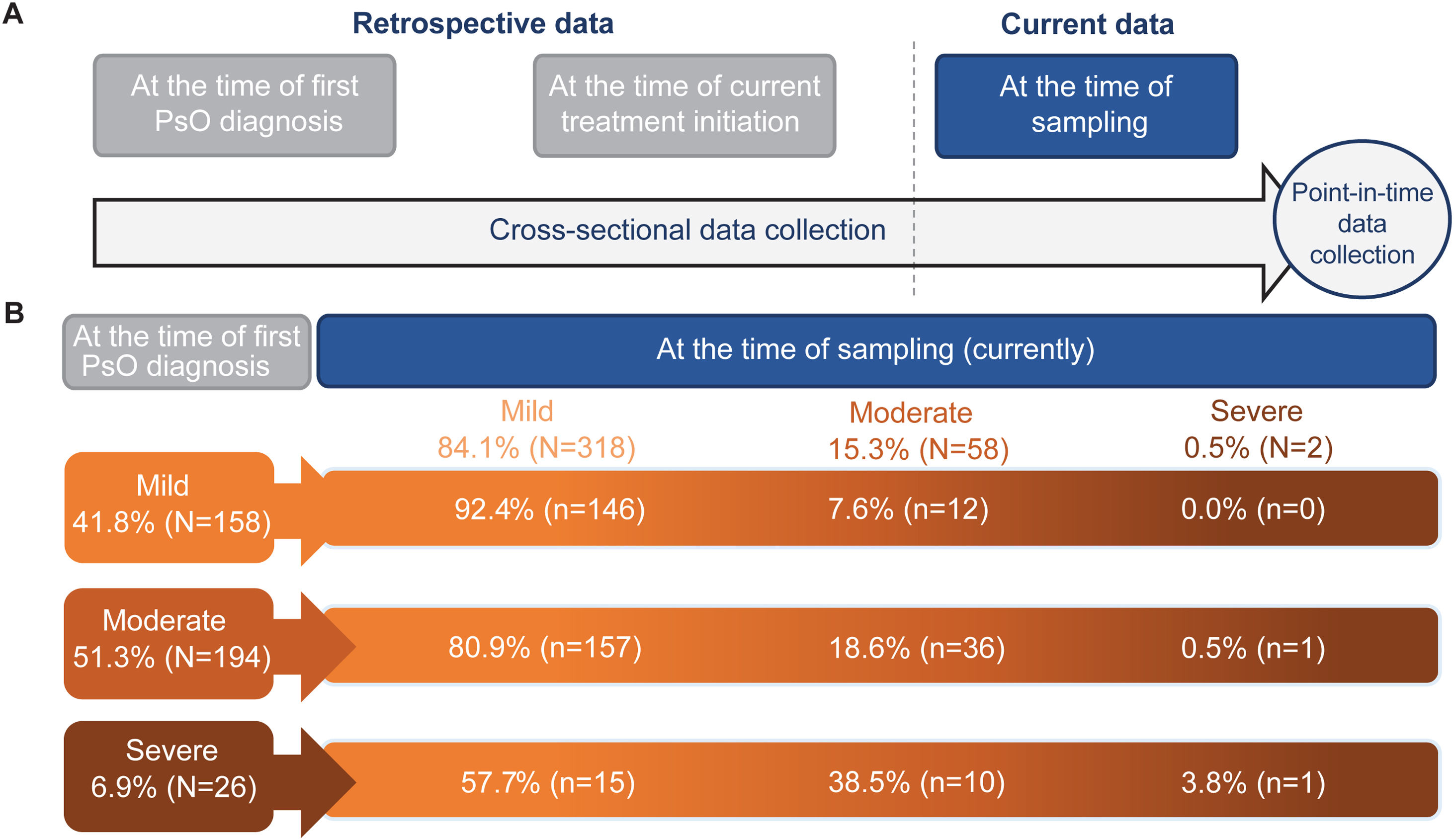
Data are presented with severity grouped at the following points (Fig. 1A): At time of sampling (‘currently’; Analysis 1), at time of first PsO diagnosis (Analysis 2) and at time of current treatment initiation (Analysis 3). BSA, PASI and physician-judged severity outcomes were also recorded both retrospectively (Analyses 2/3) and at sampling (Analysis 1); all other outcomes were recorded at sampling only. For specific analyses regarding treatment patterns, patients with moderate or severe disease were grouped together (termed ‘moderate to severe disease’), since both categories are eligible for treatment escalation with systemic agents.
Change in patient disease severity and data collection. (A) Schematic illustrating the collection of severity data both retrospectively (at the time of diagnosis, at the time of current treatment initiation and at the time of sampling [currently]). (B) Schematic showing the frequency of patients with mild, moderate, and severe PsO based on categorisation at the time of first PsO diagnosis (retrospective) versus at the time of sampling (currently). n, number of patients with outcome; N, total number of patients in the group; PsO, psoriasis.
Data were also filtered to accurately determine clinical unmet needs in the study population. The following filters were used:
- •
To exclude patients with a treatment duration<4 weeks for topical therapy and/or <12 weeks for conventional systemic and/or biologic therapy.
- •
A further analysis of the subset of patients who were currently not experiencing PsO flares.
Continuous data are reported as mean±standard deviation (SD) unless otherwise stated, and categorical data are presented as a percentage and as n/N (where n=number of patients with outcome and N=number of patients with available data).
Regulatory and ethics considerationsThe survey was conducted in compliance with the European Pharmaceutical Market Research Association and in full accordance with the US Health Insurance Portability and Accountability Act 1996. Ethical approval was granted by the Western Copernicus Group Institutional Review Board.
ResultsPopulationData from 57 treating physicians in Spain were included in the study (71.9% [N=41] dermatologists, 17.6% [N=10] GPs/PCPs and 10.5% [N=6] paediatricians), completing a total of 477 patient records. Each patient record represented a single patient with paediatric PsO. To ensure sufficient treatment response time, patients with a treatment time<4 weeks for topical therapy and/or <12 weeks for conventional systemic and/or biologic therapy were removed from the overall population for all subsequent analyses, leaving a total population of 378 patients (Table 1).
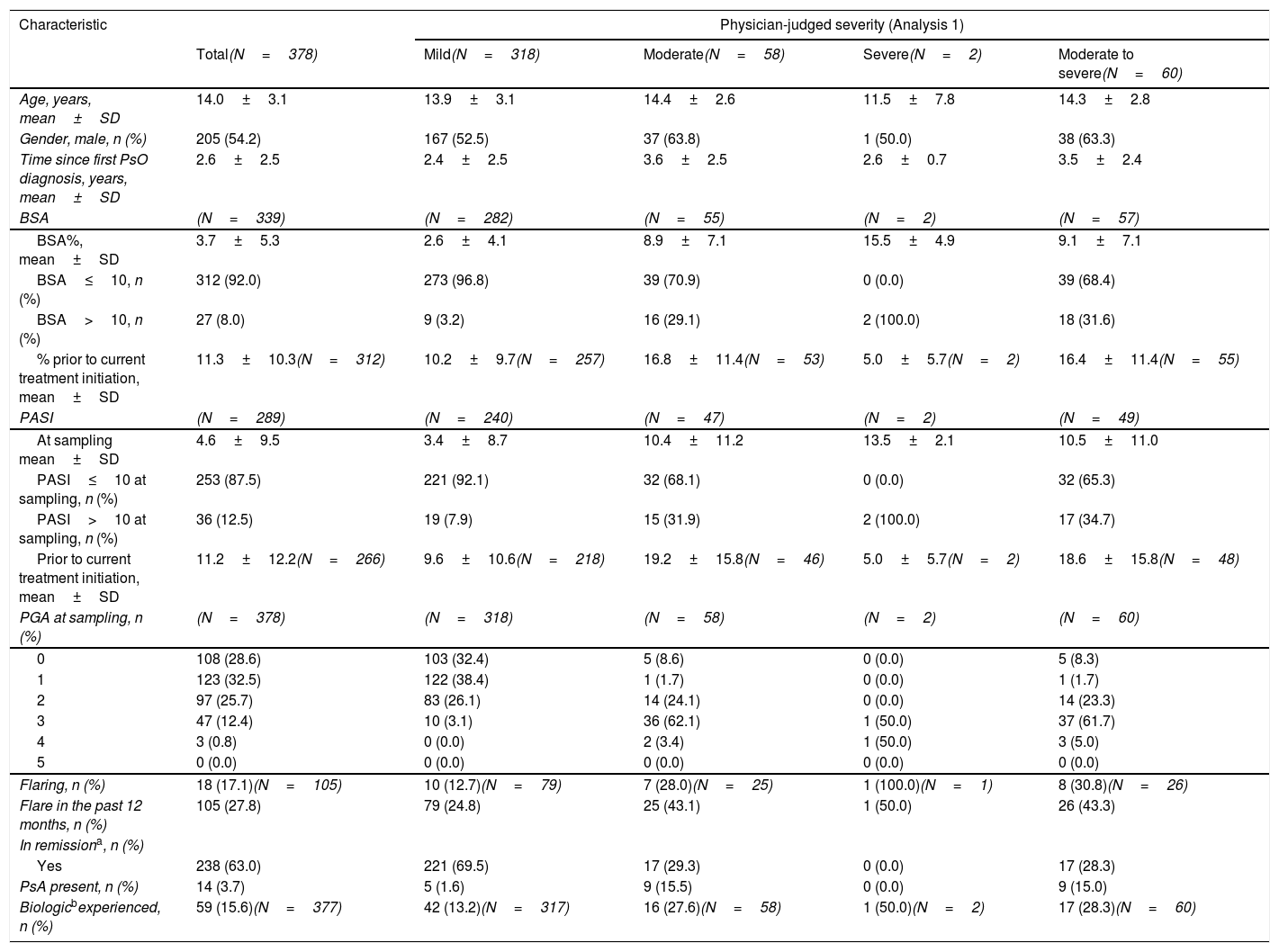
PsO disease severityTable 1 details the clinical characteristics of patients with paediatric PsO overall at the time of sampling (Analysis 1). At sampling, 84.1% (318/378) of patients had mild disease, and 15.9% (60/378) had moderate to severe disease. Retrospectively reported physician-judged severity at the time of PsO diagnosis (Analysis 2) recorded 41.8% (158/378) of patients with mild disease, and 58.2% (220/378) had moderate to severe disease (Fig. 2B).
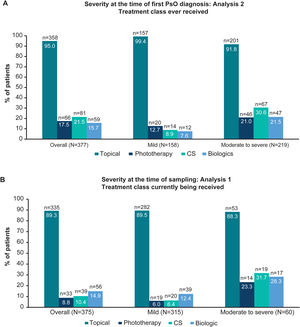
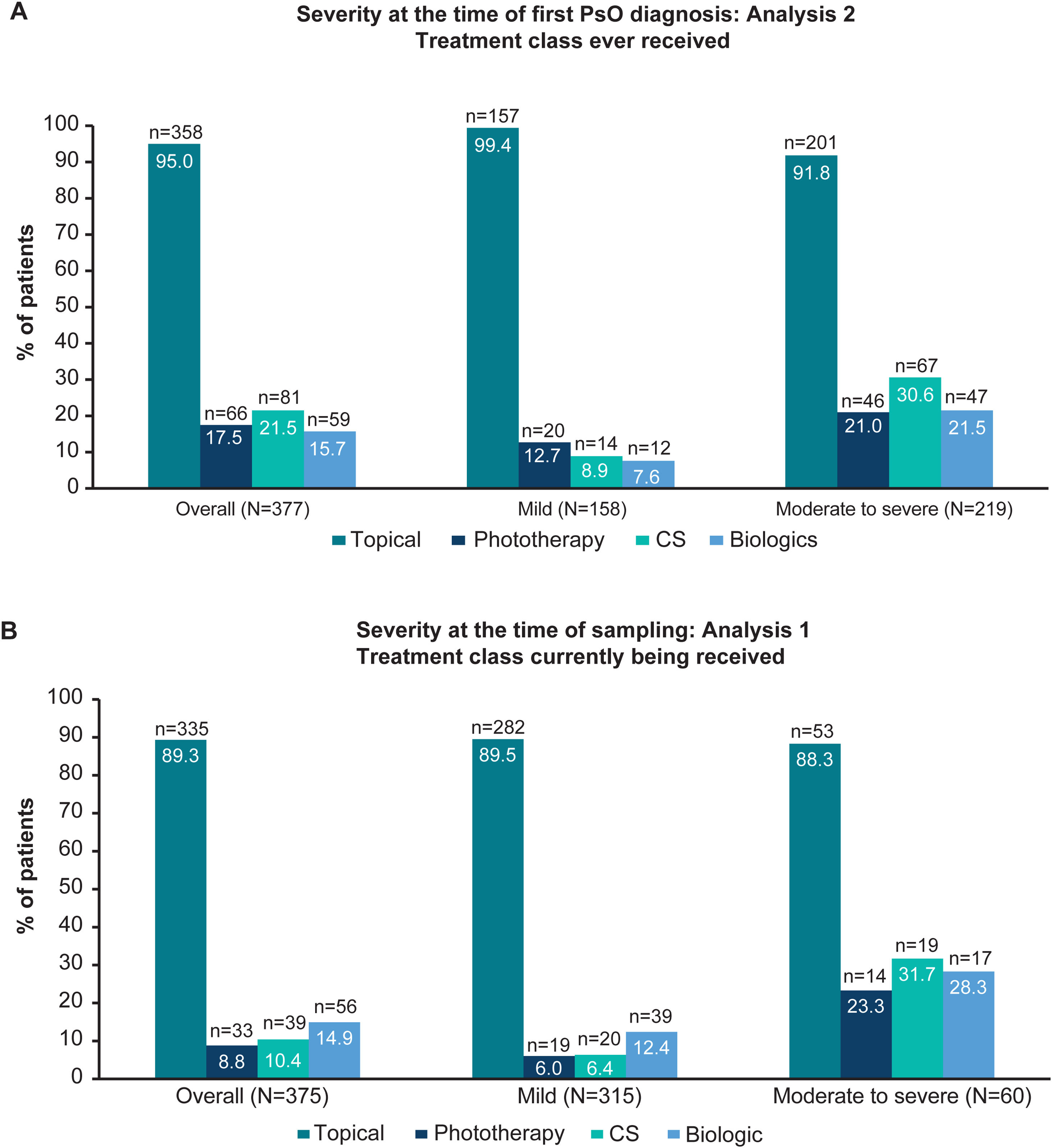
Treatment classes ever received by severity at first diagnosis (Analysis 2) and treatment classes currently being received by severity at the time of sampling (Analysis 1). Due to rounding, data may not summate to 100%. (A) Bar graphs showing the proportion of patients ever receiving topical, phototherapy, CS and biologic therapy (including biosimilars) overall and in patients with physician-judged mild and moderate to severe disease at the time of first diagnosis (Analysis 2). (B) Bar graphs showing the proportion of patients currently receiving topical, phototherapy, CS, and biologic therapy (including biosimilars) overall and in patients with physician-judged mild and moderate to severe disease at the time of sampling (Analysis 1). CS, conventional systemics; n, number of patients with outcome; N, total number of patients in the group; PsO, psoriasis.
Using severity data between the time of diagnosis and the time of sampling (Fig. 1A), the unmet needs in patients could be determined (Fig. 1B [Analysis 2 vs. Analysis 1]). Of patients with mild disease at diagnosis (41.8% [158/378]), 7.6% (12/158) progressed to moderate disease at sampling. Of patients with moderate disease at diagnosis (51.3% [194/378]), 18.6% (36/194) still had moderate disease and 0.5% (1/194) progressed to severe disease. Of patients with severe disease at diagnosis (6.9% [26/378]), 38.5% (10/26) had moderate disease at sampling, while 3.8% (1/26) still had severe disease at sampling.
PsO disease characteristics, symptoms and affected areasAt sampling, the mean±SD BSA and PASI scores overall were 3.7%±5.3% and 4.6±9.5, respectively. Overall, 38.9% (147/378) of patients had a PGA score ≥2, indicating an absence of clear or almost clear skin, 17.1% (18/105) were currently experiencing a flare, 27.8% (105/378) had reported a flare in the previous 12 months, and 63.0% (238/378) were in remission (Table 1). Of the patients with moderate to severe disease, 31.6% (18/57) had a BSA of >10%, 34.7% (17/49) had a PASI>10, and 30.8% (8/26) were experiencing a flare. Table SI (see supplementary) shows further symptoms and areas affected by PsO at sampling.
Disease characteristics, overall and by physician-judged disease severity at sampling (Analysis 1).
| Characteristic | Physician-judged severity (Analysis 1) | ||||
|---|---|---|---|---|---|
| Total(N=378) | Mild(N=318) | Moderate(N=58) | Severe(N=2) | Moderate to severe(N=60) | |
| Age, years, mean±SD | 14.0±3.1 | 13.9±3.1 | 14.4±2.6 | 11.5±7.8 | 14.3±2.8 |
| Gender, male, n (%) | 205 (54.2) | 167 (52.5) | 37 (63.8) | 1 (50.0) | 38 (63.3) |
| Time since first PsO diagnosis, years, mean±SD | 2.6±2.5 | 2.4±2.5 | 3.6±2.5 | 2.6±0.7 | 3.5±2.4 |
| BSA | (N=339) | (N=282) | (N=55) | (N=2) | (N=57) |
| BSA%, mean±SD | 3.7±5.3 | 2.6±4.1 | 8.9±7.1 | 15.5±4.9 | 9.1±7.1 |
| BSA≤10, n (%) | 312 (92.0) | 273 (96.8) | 39 (70.9) | 0 (0.0) | 39 (68.4) |
| BSA>10, n (%) | 27 (8.0) | 9 (3.2) | 16 (29.1) | 2 (100.0) | 18 (31.6) |
| % prior to current treatment initiation, mean±SD | 11.3±10.3(N=312) | 10.2±9.7(N=257) | 16.8±11.4(N=53) | 5.0±5.7(N=2) | 16.4±11.4(N=55) |
| PASI | (N=289) | (N=240) | (N=47) | (N=2) | (N=49) |
| At sampling mean±SD | 4.6±9.5 | 3.4±8.7 | 10.4±11.2 | 13.5±2.1 | 10.5±11.0 |
| PASI≤10 at sampling, n (%) | 253 (87.5) | 221 (92.1) | 32 (68.1) | 0 (0.0) | 32 (65.3) |
| PASI>10 at sampling, n (%) | 36 (12.5) | 19 (7.9) | 15 (31.9) | 2 (100.0) | 17 (34.7) |
| Prior to current treatment initiation, mean±SD | 11.2±12.2(N=266) | 9.6±10.6(N=218) | 19.2±15.8(N=46) | 5.0±5.7(N=2) | 18.6±15.8(N=48) |
| PGA at sampling, n (%) | (N=378) | (N=318) | (N=58) | (N=2) | (N=60) |
| 0 | 108 (28.6) | 103 (32.4) | 5 (8.6) | 0 (0.0) | 5 (8.3) |
| 1 | 123 (32.5) | 122 (38.4) | 1 (1.7) | 0 (0.0) | 1 (1.7) |
| 2 | 97 (25.7) | 83 (26.1) | 14 (24.1) | 0 (0.0) | 14 (23.3) |
| 3 | 47 (12.4) | 10 (3.1) | 36 (62.1) | 1 (50.0) | 37 (61.7) |
| 4 | 3 (0.8) | 0 (0.0) | 2 (3.4) | 1 (50.0) | 3 (5.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Flaring, n (%) | 18 (17.1)(N=105) | 10 (12.7)(N=79) | 7 (28.0)(N=25) | 1 (100.0)(N=1) | 8 (30.8)(N=26) |
| Flare in the past 12 months, n (%) | 105 (27.8) | 79 (24.8) | 25 (43.1) | 1 (50.0) | 26 (43.3) |
| In remissiona, n (%) | |||||
| Yes | 238 (63.0) | 221 (69.5) | 17 (29.3) | 0 (0.0) | 17 (28.3) |
| PsA present, n (%) | 14 (3.7) | 5 (1.6) | 9 (15.5) | 0 (0.0) | 9 (15.0) |
| Biologicbexperienced, n (%) | 59 (15.6)(N=377) | 42 (13.2)(N=317) | 16 (27.6)(N=58) | 1 (50.0)(N=2) | 17 (28.3)(N=60) |
Also includes biosimilar therapy.
Where the full number of patients is not available, the number of patients per group is indicated within the table (N). Due to rounding, some percentages may not summate to 100%. BSA, Body Surface Area; N, total number of patients per group; n, number of patients with outcome; PASI, Psoriasis Area and Severity Index; PGA, Physician's Global Assessment; PsA, psoriatic arthritis; PsO, psoriasis; SD, standard deviation.
An analysis was carried out on the subset of patients not currently experiencing a flare (Table SII. See supplementary). At sampling, patients with mild disease reported a mean BSA of 2.6%±4.1%, a mean PASI of 3.4±8.8; 28.2% (87/308) of patients with mild disease had a PGA score 2–4. Of patients with moderate to severe disease not currently experiencing a flare, patients had a mean BSA of 7.9%±6.2%, a mean PASI score of 9.1±7.8, and 88.5% (46/52) had a PGA score 2–4.
Severity by type of treating physicianA higher proportion of patients attended dermatologists (mild, 84.0% [200/238]; moderate to severe, 16.0% [38/238]), than paediatricians (mild, 79.4% [27/34]; moderate to severe, 20.6%[7/34]) or GP/PCPs (mild, 92.0% [81/88], moderate to severe, 8.0% [7/88]) (Analysis 1, Table SIII. See supplementry). Dermatologists reported a lower average PASI score in patients with moderate to severe disease compared with GP/PCPs and paediatricians, however, GP/PCPs reported the lowest average BSA% with moderate to severe disease.
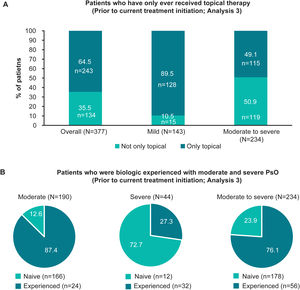
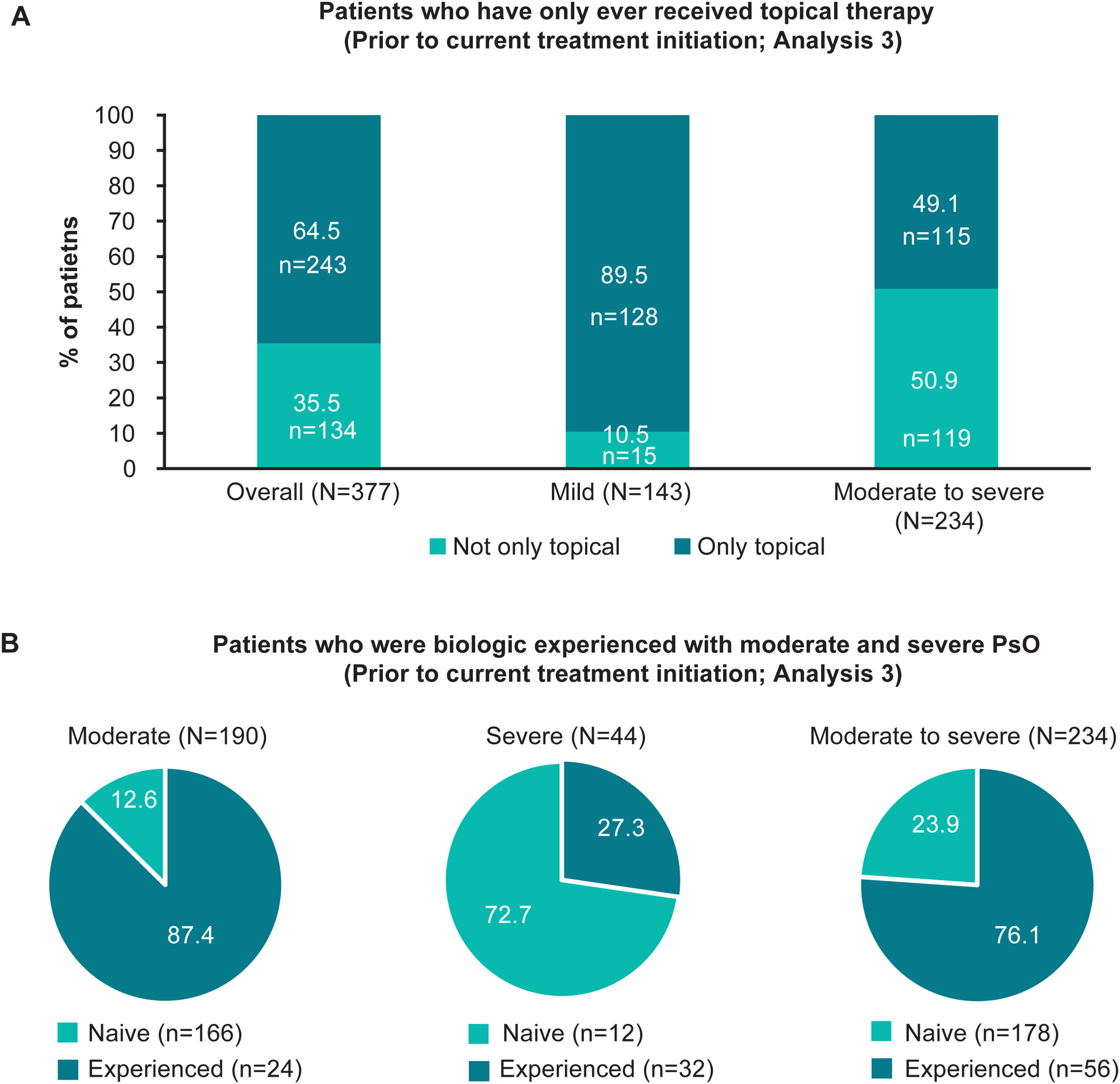
Treatment patternsTreatment historyOverall, 95.0% (358/377) of patients had ever received topical therapy, and only 17.5% (66/377), 21.5% (81/377) and 15.7% (59/377) of patients had ever received phototherapy, conventional systemics and biologic therapy, respectively (Fig. 2A; Analysis 2, at diagnosis). Of patients categorised with mild and moderate to severe disease at diagnosis, 8.9% (14/158), and 30.6% (67/219) had ever received conventional systemics, and 7.6% (12/158) and 21.5% (47/219) had ever received biologic therapy for their PsO, respectively (Analysis 2, Fig. 2A). Categorised by physician-judged severity prior to current treatment initiation, 49.1% (115/234) of patients with moderate to severe disease had ever been treated with only a topical therapy for their PsO (Analysis 3, Fig. 3A). Of note, 93.3% (56/60) of patients with moderate to severe disease were biologic-experienced (Fig. 3B).
Patients who received only topical therapy and who were biologic-experienced, overall and by severity prior to current treatment initiation (Analysis 3). (A) Bar graphs showing the proportion of patients who had only ever received topical therapy, overall and in patients with physician-judged mild and moderate to severe disease prior to current treatment initiation (Analysis 3). (B) Pie charts illustrating the frequency of patients who were biologic naïve and experienced with physician-judged moderate, severe and moderate to severe disease at the time of current treatment initiation (Analysis 3). n, number of patients with outcome; N, total number of patients in the group; PsO, psoriasis.
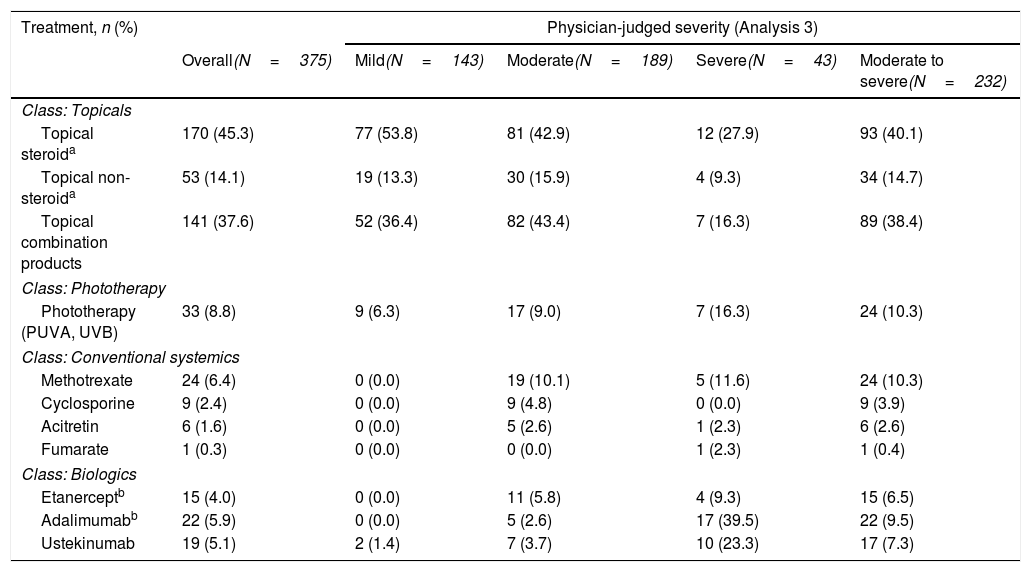
Overall, 89.3% (335/375) of patients were currently receiving topical PsO therapy at the time of sampling compared with 8.8% (33/375), 10.4% (39/375) and 14.9% (56/375) of patients who were currently receiving phototherapy, conventional systemics and biologic therapy, respectively (Fig. 2B). For patients with moderate to severe disease at sampling, 31.7% (19/60) received conventional systemics and 28.3% (17/60) biologic therapy (Fig. 2B). A detailed description of current treatment is presented in Table 2.
Current treatment types, overall and by severity at time of current treatment initiation (Analysis 3).
| Treatment, n (%) | Physician-judged severity (Analysis 3) | ||||
|---|---|---|---|---|---|
| Overall(N=375) | Mild(N=143) | Moderate(N=189) | Severe(N=43) | Moderate to severe(N=232) | |
| Class: Topicals | |||||
| Topical steroida | 170 (45.3) | 77 (53.8) | 81 (42.9) | 12 (27.9) | 93 (40.1) |
| Topical non-steroida | 53 (14.1) | 19 (13.3) | 30 (15.9) | 4 (9.3) | 34 (14.7) |
| Topical combination products | 141 (37.6) | 52 (36.4) | 82 (43.4) | 7 (16.3) | 89 (38.4) |
| Class: Phototherapy | |||||
| Phototherapy (PUVA, UVB) | 33 (8.8) | 9 (6.3) | 17 (9.0) | 7 (16.3) | 24 (10.3) |
| Class: Conventional systemics | |||||
| Methotrexate | 24 (6.4) | 0 (0.0) | 19 (10.1) | 5 (11.6) | 24 (10.3) |
| Cyclosporine | 9 (2.4) | 0 (0.0) | 9 (4.8) | 0 (0.0) | 9 (3.9) |
| Acitretin | 6 (1.6) | 0 (0.0) | 5 (2.6) | 1 (2.3) | 6 (2.6) |
| Fumarate | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 1 (0.4) |
| Class: Biologics | |||||
| Etanerceptb | 15 (4.0) | 0 (0.0) | 11 (5.8) | 4 (9.3) | 15 (6.5) |
| Adalimumabb | 22 (5.9) | 0 (0.0) | 5 (2.6) | 17 (39.5) | 22 (9.5) |
| Ustekinumab | 19 (5.1) | 2 (1.4) | 7 (3.7) | 10 (23.3) | 17 (7.3) |
Of patients with moderate to severe disease not receiving a biologic at sampling, physicians were asked if these patients would benefit from one; physicians warranted their use in 34.9% (15/43) of patients; the most frequent reasons for not receiving one were: ‘patient in remission’ (23.8%, 10/42), ‘formulary/insurance restrictions’ (23.8%, 10/42), ‘concerns regarding safety/side effects’ (21.4%, 9/42) and ‘parent/guardian/carer does not want to administer (21.4%, 9/42).
Treatments by type of physicianAt the time of treatment initiation, patients consulting with a paediatrician or dermatologist were currently receiving conventional systems (paediatrician, 20.6%, 7/34; dermatologist, 11.9%, 28/235) and biologic therapy (paediatrician, 26.5%, 9/34; dermatologist, 18.7%, 44/235) compared with those consulting with a GP/PCP (conventional systemics, 0.0%, 0/88; biologics, 1.1%, 1/88).
DiscussionSeyger et al. reported that despite receiving treatment for PsO, paediatric patients still exhibited a persistent disease burden and that a small proportion of patients with moderate or severe PsO are undertreated.16 The current study was a follow-up of Seyger et al. which primarily aimed to identify the physician-reported burden of disease and current treatment patterns in a real-world paediatric PsO patient cohort in Spain. Patients with a treatment duration <4 weeks for topical therapy and/or <12 weeks for conventional systemic and/or biologic therapy were excluded from the analysis.
In total, 58.2% of patients at diagnosis and 15.9% at sampling presented with moderate to severe disease and had previously received/were receiving treatment for their PsO. Patients not currently experiencing a flare exhibited high BSA and PASI scores, suggesting that while many patients with paediatric PsO are currently well managed, a proportion of patients with moderate to severe disease exhibit a high burden of disease, whether or not they were experiencing a flare, indicating that these patients may have uncontrolled PsO and may be undertreated. Of note, adult patients with PsO who have PASI or BSA higher than 10 are considered candidates for systemic treatments. Full patients with paediatric PsO, identical treatment goals should be considered to those for adults.18,19
This study showed that 93.3% of patients with moderate to severe disease at the time of sampling were biologic-experienced. Any delay for patients receiving biologics, could be explained by two factors. Firstly, there could be a lack of experienced physicians prescribing biologic therapies to paediatric patients. A survey study in adult patients, which included responses from 300 dermatologists in Spain, reported that 85% of dermatologists prescribe two or more traditional therapies before prescribing a biologic, indicating that biologics are reserved for use after other treatments have failed. The study reported that 73% of patients with PsO in Spain have an average delay of 2 years before they are switched to a biologic.20 Secondly, approvals for biologic therapies in Spain could be restricted by the national health care system, with different levels of access to biologic therapies depending on the territory.
As in adult patients, children and adolescents with BSA greater than 10%, DLQI>10, those who have not responded to standard therapies, or have contraindications, should be considered for biologic therapy.21 Some studies suggest using systemic therapies earlier in mild psoriasis and in patients with lower DLQI scores.21 Allowing patients to switch to systemic treatment may improve QoL and reduce disease progression.
Of those patients with moderate to severe disease not receiving a biologic at sampling, their physicians were asked if the current condition of each patient may warrant the use of one; 34.9% of physicians answered “yes”. The most frequent reasons reported by physicians for not prescribing a biologic were ‘patient in remission’, and ‘formulary/insurancerestrictions’ which may indicate that the cost of biologic therapies was an economic burden.
Patients with moderate and severe disease were more likely to visit a dermatologist rather than a GP/PCP or paediatrician; GPs/PCPs and paediatricians may often refer patients with moderate and severe disease to a specialist, since phototherapy and systemic/biologic treatments for PsO either require specialised unit facilities, or because drugs are restricted to hospital prescription by dermatology specialists. Similar to the findings by Seyger et al., GPs/PCPs in Spain are less likely to prescribe systemic treatment than dermatologists.16 Regional guidelines for referral to specialists in Spain may ensure that patients are treated earlier in their treatment journey. Prompt intervention is vital, particularly in severe cases, to modify the inflammatory course of the disease and prevent complications and sequelae in later life.15
LimitationsDermatologists were likely over-represented in this study of patients with paediatric PsO as there is likely a small percentage of patients who were treated by paediatricians and general practitioners. Therefore, the proportion of paediatric patients in Spain attending each type of physician may not be accurately reflected in these data. Further study limitations were previously described by Seyger et al.16
ConclusionsThese real-world data reflect the current disease burden and treatment landscape of paediatric PsO in Spain. The management of patients with paediatric PsO could be improved by further educating healthcare professionals and providing regional treatment guidelines for paediatric PsO. This may reduce the delay in the referral of patients with PsO to hospitals with experience in managing PsO with biologic therapies, hence potentially overcoming access difficulties to biologic therapies across Spain.
FundingThis research was funded by Novartis Farmacéutica S.A, Barcelona, Spain. The survey was carried out by Adelphi Real World independently of Novartis.
Conflict of interestRaúl de Lucas has nothing to disclose.
Asunción Vicente has participated in Clinical Trials as principal investigator, sub-investigator, provided scientific advice or payments for presentations at medical meetings and/or advice from: Abbvie, Amgen, Amryt, Boehringer Ingelheim, Bristol-Myers Squibb, Ferrer, Galderma, Janssen, Leti, Eli Lilly and Company, Novartis, Pierre Fabre, Pfizer, Sanofi.
Antonio Torrelo lectures for Novartis.
James Lucas and Liane Gillespie-Akar are employed by Adelphi Real World.
Craig Richardson and Lara Gómez Labrador are employed by Novartis.
The authors thank Ellen McKenna MSc, and Philip O’Gorman PhD (Novartis Ireland Ltd, Dublin, Ireland) for providing medical writing support and assistance, which was funded by Novartis Pharmaceuticals Corporation in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).


![Change in patient disease severity and data collection. (A) Schematic illustrating the collection of severity data both retrospectively (at the time of diagnosis, at the time of current treatment initiation and at the time of sampling [currently]). (B) Schematic showing the frequency of patients with mild, moderate, and severe PsO based on categorisation at the time of first PsO diagnosis (retrospective) versus at the time of sampling (currently). n, number of patients with outcome; N, total number of patients in the group; PsO, psoriasis. Change in patient disease severity and data collection. (A) Schematic illustrating the collection of severity data both retrospectively (at the time of diagnosis, at the time of current treatment initiation and at the time of sampling [currently]). (B) Schematic showing the frequency of patients with mild, moderate, and severe PsO based on categorisation at the time of first PsO diagnosis (retrospective) versus at the time of sampling (currently). n, number of patients with outcome; N, total number of patients in the group; PsO, psoriasis.](https://static.elsevier.es/multimedia/00017310/0000011400000005/v2_202306061222/S0001731023001734/v2_202306061222/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)