Primary cutaneous marginal zone lymphoma (PCMZL) is a low-grade B cell primary skin lymphoma,1 usually found in male adults around 50–55 years old.2,3 This disease can present as single or multiple erythematous macules, plaques, or tumors. The most common locations are the trunk and upper limbs.4 Treatment of this disease is based on only a limited number of short case series, and well-designed clinical trials are lacking; therefore, strong evidence is scarce. Currently, solitary lesions are treated via surgery or local radiotherapy, whereas multiple lesions are treated with radiotherapy, intravenous administration of rituximab, or watchful follow-up.5 The prognosis of this type of lymphoma is excellent, with a five-year survival rate of 95–100%.4 Nevertheless, PCMZL skin relapses are common (44–50%).6 This study aims to describe the epidemiology and outcome of patients with PCMZL in a hospital with rare skin lymphoma management expertise.
This observational, longitudinal, retrospective study included all patients histologically diagnosed as PCMZL at our institution from January 2007 to December 2020. Clinical data were collected, processed, and analyzed using SPSS v.25 statistical software.
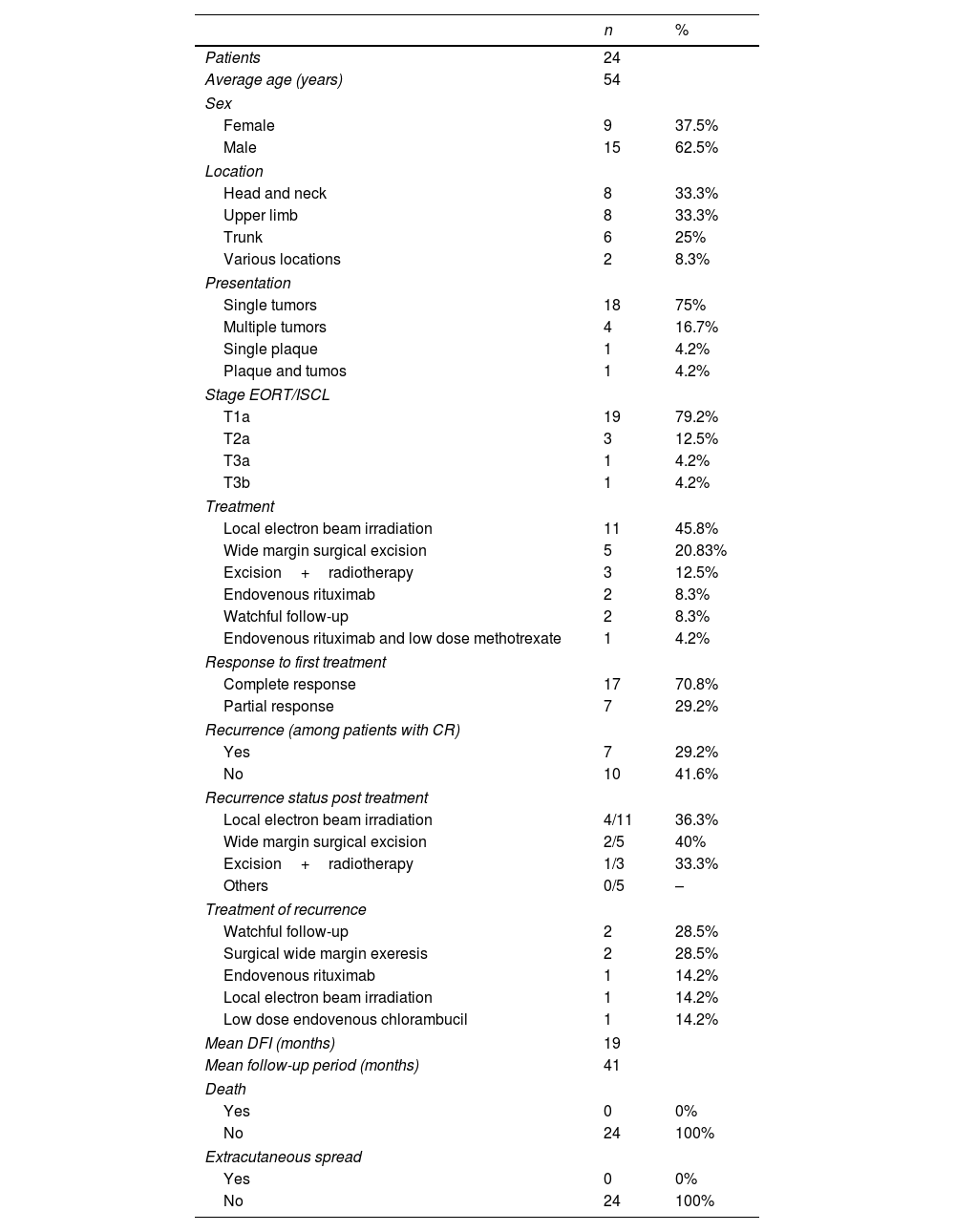
Ultimately, 24 patients were evaluated during the study period, of which 62.5% (n=15) were men, and the median age was 58. A solitary nodule (75%, n=18) was the most frequent presentation of PCMZL, followed by multiple nodules (16.2%, n=4). These lesions were localized more frequently in the head and neck area (33.3%, n=8), upper limbs (33.3%, n=8), and trunk (25%, n=6). In all patients, the results of whole-body computed tomography (CT) showed no extracutaneous involvement. T1A was the most common EORT/ISCL stage (79.2%, n=19). Local radiotherapy of the lesion and the surrounding skin (1cm) using electron beam irradiation (30Gy) was the most common treatment applied (n=11, 45.8%), followed by a wide margin (1–1.5cm) surgical excision (n=5, 20.8%). A complete response was achieved in 17 patients (70.8%), while seven patients (29.2%) showed a partial response with disease stabilization. Among patients with a complete response to treatment, seven (29.2%) experienced a disease relapse. For these seven patients, the primary treatment was radiotherapy (n=4), surgical excision (n=2), and surgical excision followed by radiotherapy (n=1). In five of these patients, the recurrence was found at the same site as the primary lesion (see Table 1 for a short resume of clinical characteristics, response to treatment, and outcomes).
Resume of clinical characteristics, response to treatment and outcomes.
| n | % | |
|---|---|---|
| Patients | 24 | |
| Average age (years) | 54 | |
| Sex | ||
| Female | 9 | 37.5% |
| Male | 15 | 62.5% |
| Location | ||
| Head and neck | 8 | 33.3% |
| Upper limb | 8 | 33.3% |
| Trunk | 6 | 25% |
| Various locations | 2 | 8.3% |
| Presentation | ||
| Single tumors | 18 | 75% |
| Multiple tumors | 4 | 16.7% |
| Single plaque | 1 | 4.2% |
| Plaque and tumos | 1 | 4.2% |
| Stage EORT/ISCL | ||
| T1a | 19 | 79.2% |
| T2a | 3 | 12.5% |
| T3a | 1 | 4.2% |
| T3b | 1 | 4.2% |
| Treatment | ||
| Local electron beam irradiation | 11 | 45.8% |
| Wide margin surgical excision | 5 | 20.83% |
| Excision+radiotherapy | 3 | 12.5% |
| Endovenous rituximab | 2 | 8.3% |
| Watchful follow-up | 2 | 8.3% |
| Endovenous rituximab and low dose methotrexate | 1 | 4.2% |
| Response to first treatment | ||
| Complete response | 17 | 70.8% |
| Partial response | 7 | 29.2% |
| Recurrence (among patients with CR) | ||
| Yes | 7 | 29.2% |
| No | 10 | 41.6% |
| Recurrence status post treatment | ||
| Local electron beam irradiation | 4/11 | 36.3% |
| Wide margin surgical excision | 2/5 | 40% |
| Excision+radiotherapy | 1/3 | 33.3% |
| Others | 0/5 | – |
| Treatment of recurrence | ||
| Watchful follow-up | 2 | 28.5% |
| Surgical wide margin exeresis | 2 | 28.5% |
| Endovenous rituximab | 1 | 14.2% |
| Local electron beam irradiation | 1 | 14.2% |
| Low dose endovenous chlorambucil | 1 | 14.2% |
| Mean DFI (months) | 19 | |
| Mean follow-up period (months) | 41 | |
| Death | ||
| Yes | 0 | 0% |
| No | 24 | 100% |
| Extracutaneous spread | ||
| Yes | 0 | 0% |
| No | 24 | 100% |
CR: complete response; DFI: disease free interval.
The median disease-free interval of patients who suffered a relapse was 19 months. All recurrences occurred within the first three years of follow-up. The mean follow-up period in our series was 42 months (median 31 months). None of the patients experienced nodal relapse or visceral or bone marrow involvement during the follow-up period. No deaths caused by PCMZL were reported. At the end of the study, the percentage of disease-free patients was 54.1% (n=13).
Our experience with patients with PCLZM in our hospital is similar to other centers; lesions are more common in 50–60-year-old men with a relatively high recurrence rate but an excellent survival rate. Primary staging in patients with PCMZL should include a physical examination, laboratory tests with a complete blood count and lactate dehydrogenase levels, skin biopsy, and complete body CT or FDG-PET scan. A bone marrow biopsy is not indicated for PCMZL.5
Concerning treatment for localized lesions, local electron radiotherapy (20–35Gy and 1–1.5cm margin)7 or wide margin excision are indicated as the first-choice treatments.5 Some authors found no difference in recurrence rates between the two treatment groups, while others showed a higher relapse rate with excised lesions, although this difference was not statistically significant.6 In other studies, recurrence was found in non-irradiated areas.8 For multifocal disease, low-dose local radiotherapy, intralesional corticosteroids, intravenous or intralesional3 rituximab, or a watchful follow-up period are the most commonly applied treatments.5 Intravenous and intralesional rituximab achieved a complete response in most cases, but relapses after suspension may occur.9 Due to the scarcity of patients included in our case series, it was not possible to make statistical inferences by comparing the different treatments applied, which is a limitation of our study. The relapse rate ranged from 36%10 to 60%.8 Relapses seem more common in multifocal disease (T3 EORTC stage) but have also been described in the T1–T2 stages.6
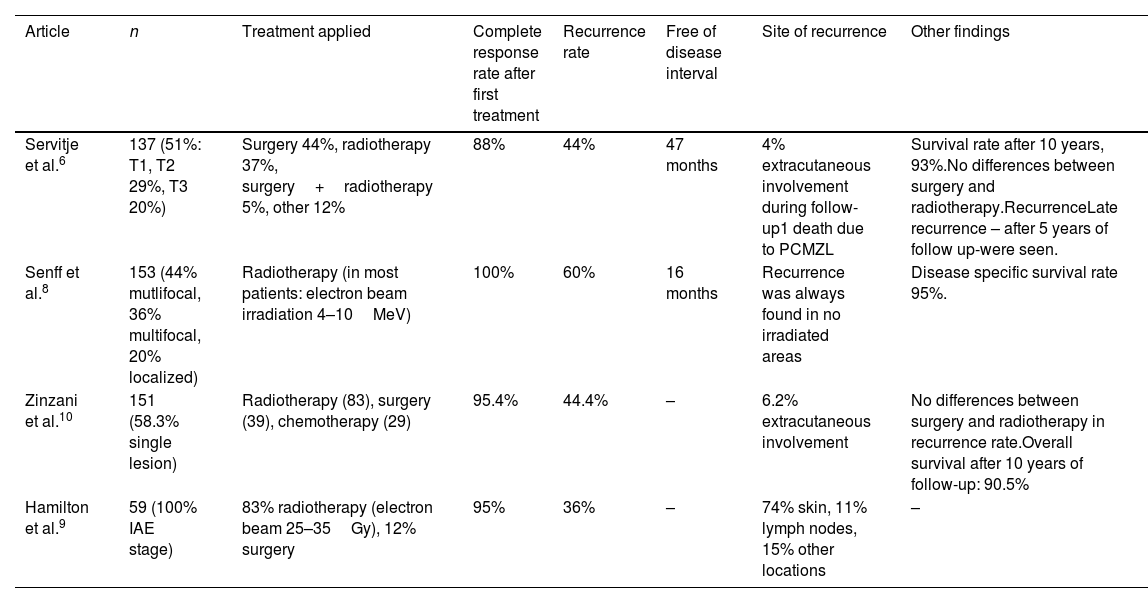
The spread of PCMZL in other organs was uncommon during follow-up (4–6.2%).6,11 The prognosis of patients with PCMZL is excellent, with a 5-year survival rate of >90% (93% and 95%).6,8 An extended follow-up period for patients with PCLZM, sometimes as long as five years after a complete response to a primary treatment, is recommended due to possible late relapse6 (see Table 2 for a relation of the results provided by other PCMZL case series). The optimal management of these patients requires a multidisciplinary approach. Multicentric studies and clinical trials are needed to assess the best therapeutic approach and management of patients with PCMZL.
Results provided by other PCMZL case series.
| Article | n | Treatment applied | Complete response rate after first treatment | Recurrence rate | Free of disease interval | Site of recurrence | Other findings |
|---|---|---|---|---|---|---|---|
| Servitje et al.6 | 137 (51%: T1, T2 29%, T3 20%) | Surgery 44%, radiotherapy 37%, surgery+radiotherapy 5%, other 12% | 88% | 44% | 47 months | 4% extracutaneous involvement during follow-up1 death due to PCMZL | Survival rate after 10 years, 93%.No differences between surgery and radiotherapy.RecurrenceLate recurrence – after 5 years of follow up-were seen. |
| Senff et al.8 | 153 (44% mutlifocal, 36% multifocal, 20% localized) | Radiotherapy (in most patients: electron beam irradiation 4–10MeV) | 100% | 60% | 16 months | Recurrence was always found in no irradiated areas | Disease specific survival rate 95%. |
| Zinzani et al.10 | 151 (58.3% single lesion) | Radiotherapy (83), surgery (39), chemotherapy (29) | 95.4% | 44.4% | – | 6.2% extracutaneous involvement | No differences between surgery and radiotherapy in recurrence rate.Overall survival after 10 years of follow-up: 90.5% |
| Hamilton et al.9 | 59 (100% IAE stage) | 83% radiotherapy (electron beam 25–35Gy), 12% surgery | 95% | 36% | – | 74% skin, 11% lymph nodes, 15% other locations | – |
The authors declare that they have no conflict of interest.