Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematodermic neoplasm usually involving the skin. In this retrospective case series, 10 cases of BPDCN were identified, 90% of which had skin involvement and exhibited predominantly violaceous nodules and/or bruise-like plaques. Skin lesions showed diffuse or nodular dermal-based infiltrates of intermediate sized blasts with a grenz zone. Tumor immunophenotyping was CD4(+), CD56(+), CD123(+) and CD303(+). The most frequently mutated genes according to targeted next-generation sequencing were TET2 (3/7) and NRAS (2/7). Multiagent chemotherapy (CT) was administered as first-line therapy, and a total of 5 patients underwent allogenic hematopoietic stem cell transplantation (allo-HSCT). Better outcomes were observed in younger patients and those treated with acute lymphoblastic leukemia (ALL)-like CT followed by allo-HSCT. This study shows the clinical range of cutaneous lesions of BPDCN. Despite the absence of a gold standard therapy, patients treated with myeloablative intensive regimens and allo-HSCT seems to have a more favorable prognosis.
La neoplasia blástica de células dendríticas plasmocitoides (NBCDP), es una neoplasia hematodérmica poco frecuente y agresiva. En esta serie de casos retrospectiva, se identificaron 10 casos de NBCDP, con un 90% de afectación de la piel, presentándose predominantemente como nódulos violáceos y/o placas hematoma-like. Las lesiones cutáneas mostraban infiltrados dérmicos difusos o nodulares de blastos de tamaño intermedio con zona de grenz. El inmunofenotipado fue CD4+, CD56+, CD123+ y CD303+. Los genes mutados más frecuentes fueron TET2 (3/7) y NRAS (2/7). Se administró multi-quimioterapia (QT) como tratamiento de primera línea, y 5 pacientes se sometieron a trasplante alogénico de progenitores hematopoyéticas (alo-TPH). Se observaron mejores resultados en los pacientes más jóvenes y aquellos tratados con QT similar a la leucemia linfoblástica aguda (LLA) seguida de alo-TPH. Este estudio muestra el rango clínico de las lesiones cutáneas de NBCDP. A pesar de no haber un gold standard terapéutico, los regímenes de QT mieloablativos y alo-TPH parecen tener un pronóstico más favorable.
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and aggressive hematologic neoplasm with cutaneous tropism, which represents 0.7% of all cutaneous hematologic neoplasms.1 It was first described in 1995 as “acute agranular CD4+ NK-cell leukemia”2 and, since 2008, it has been recognized as a distinct entity by the WHO.3–5
BPDCN arises from clonal precursors of plasmacytoid dendritic cells (pDC), characterized by CD4+/CD56+/CD123+ cells that are negative for lineage-specific markers.6,7 Additionally, BDCA-2/CD303, BDCA-4/CD304 and TCL1 are specific markers of pDC.8 Although final diagnosis is based on immunophenotyping, the skin is the primarily affected organ (>80% of patients) and clinical recognition is key to early treatment.9
There is no standard therapy for the management of this entity. Chemotherapy regimens achieve high complete response (CR) rates, but relapses occur early. There is evidence on the benefit of both autologous10 and allogeneic stem cell transplantation (allo-HSCT).10,11 Recently, new targeted therapeutic options have appeared, which may improve patient outcome.12,13
In the present article, we’ll be characterizing the clinical characteristics, mutational profile, and outcomes of 10 patients with BPDCN from a single institution.
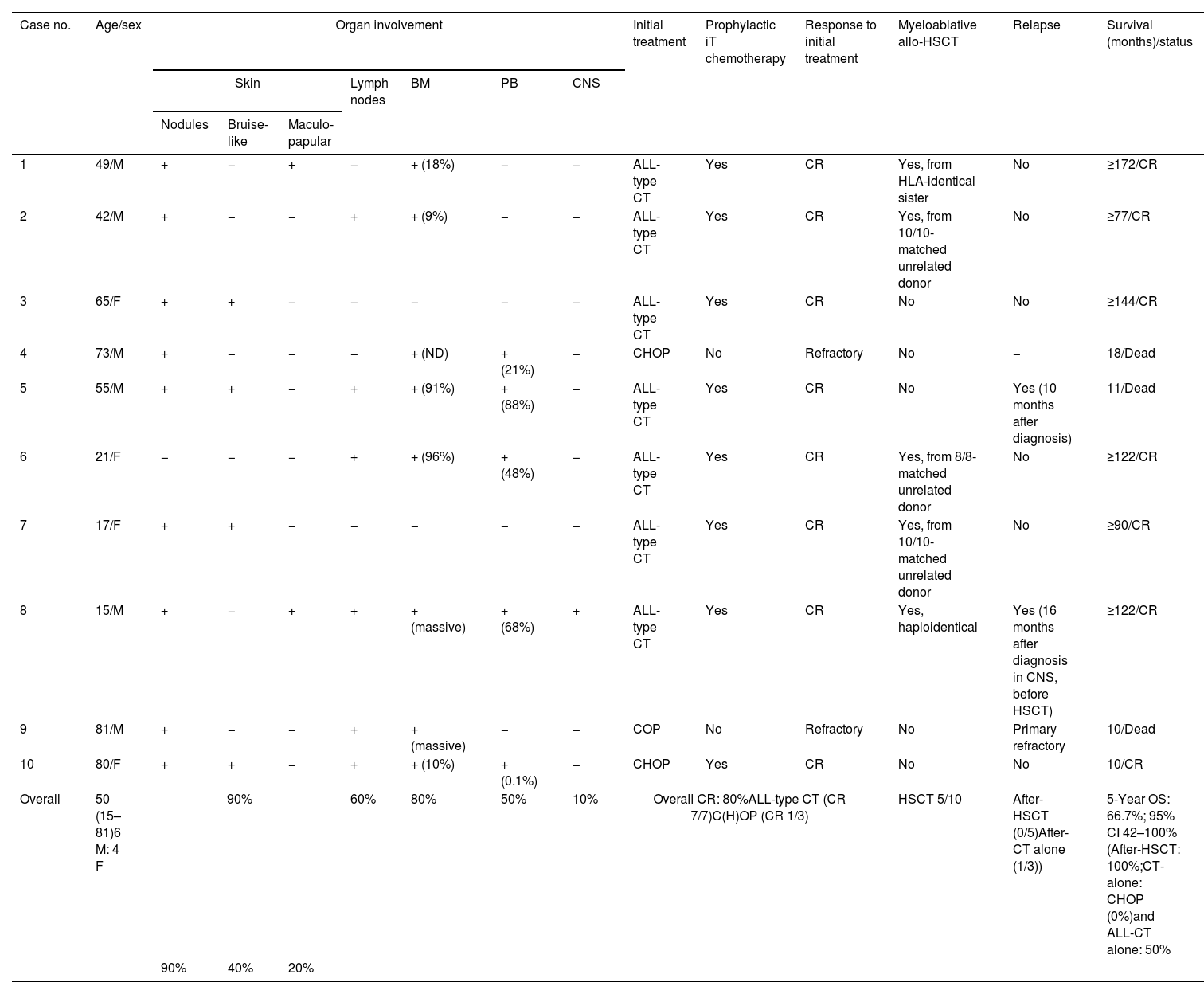
ReportClinical featuresThe main characteristics of the patients are shown in Table 1. Ten patients with BPDCN were included, 6 men, with a median age of 50 years (range, 15–81). Three patients had a previous hematologic neoplasm (myelodysplastic syndrome, n=2; primary myelofibrosis, n=1). The skin was the most frequently affected organ (90%), with 2 patients having only cutaneous signs, followed by the bone marrow (80%), the lymph nodes (60%), peripheral blood (50%) and the central nervous system (10%).
Clinical description, treatment regimens and therapeutic response in patients with blastic plasmacytoid dendritic cell neoplasm.
| Case no. | Age/sex | Organ involvement | Initial treatment | Prophylactic iT chemotherapy | Response to initial treatment | Myeloablative allo-HSCT | Relapse | Survival (months)/status | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Lymph nodes | BM | PB | CNS | ||||||||||
| Nodules | Bruise-like | Maculo-papular | ||||||||||||
| 1 | 49/M | + | − | + | − | + (18%) | − | − | ALL-type CT | Yes | CR | Yes, from HLA-identical sister | No | ≥172/CR |
| 2 | 42/M | + | − | − | + | + (9%) | − | − | ALL-type CT | Yes | CR | Yes, from 10/10-matched unrelated donor | No | ≥77/CR |
| 3 | 65/F | + | + | − | − | − | − | − | ALL-type CT | Yes | CR | No | No | ≥144/CR |
| 4 | 73/M | + | − | − | − | + (ND) | + (21%) | − | CHOP | No | Refractory | No | − | 18/Dead |
| 5 | 55/M | + | + | − | + | + (91%) | + (88%) | − | ALL-type CT | Yes | CR | No | Yes (10 months after diagnosis) | 11/Dead |
| 6 | 21/F | − | − | − | + | + (96%) | + (48%) | − | ALL-type CT | Yes | CR | Yes, from 8/8-matched unrelated donor | No | ≥122/CR |
| 7 | 17/F | + | + | − | − | − | − | − | ALL-type CT | Yes | CR | Yes, from 10/10-matched unrelated donor | No | ≥90/CR |
| 8 | 15/M | + | − | + | + | + (massive) | + (68%) | + | ALL-type CT | Yes | CR | Yes, haploidentical | Yes (16 months after diagnosis in CNS, before HSCT) | ≥122/CR |
| 9 | 81/M | + | − | − | + | + (massive) | − | − | COP | No | Refractory | No | Primary refractory | 10/Dead |
| 10 | 80/F | + | + | − | + | + (10%) | + (0.1%) | − | CHOP | Yes | CR | No | No | 10/CR |
| Overall | 50 (15–81)6 M: 4 F | 90% | 60% | 80% | 50% | 10% | Overall CR: 80%ALL-type CT (CR 7/7)C(H)OP (CR 1/3) | HSCT 5/10 | After-HSCT (0/5)After-CT alone (1/3)) | 5-Year OS: 66.7%; 95% CI 42–100%(After-HSCT: 100%;CT-alone: CHOP (0%)and ALL-CT alone: 50% | ||||
| 90% | 40% | 20% | ||||||||||||
Abbreviations: ALL, acute lymphoblastic leukemia; allo-HSCT, allogeneic–hematopoietic stem cell transplantation; BM, bone marrow; C(H)OP, cyclophosphamide, (doxorubicin,) vincristine and prednisone; CNS, central nervous system; CR1, first complete response; CR2, second complete response; CT, chemotherapy; F, female; iT, intratecal; M, male; MRD, minimal residual disease; OS, overall survival; PB, peripheral blood.
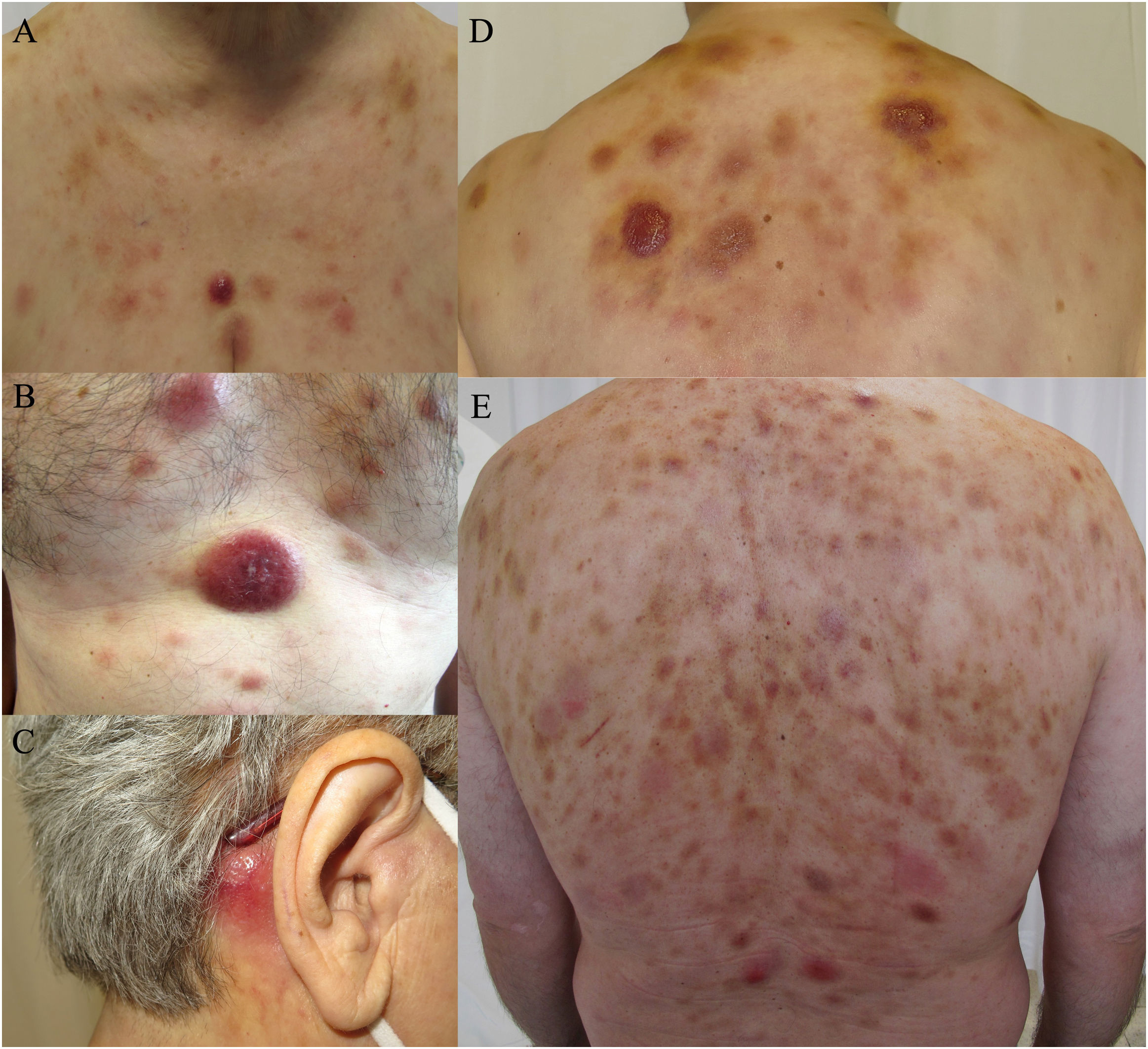
The skin lesions displayed 3 main morphologies: erythematous-violaceous indurated nodules (9/10), bruise-like plaques (4/10) and maculopapular exanthemas (2/10) (Fig. 1). Different types of lesions frequently coexisted (60% of cases). The lesions did not present ulceration, nor did the patients show any signs of pain or pruritus. The trunk was the most frequently affected area.
Clinical spectrum of skin lesions of blastic plasmacytoid dendritic cell neoplasm. (a, b, d) Multiple erythematous nodules with bruise-like plaques on the chest and upper back. (c) Isolated erythematous nodular lesion on the retroauricular area. (e) Diffuse bruise-like plaques on the back with presence of scattered nodular violaceous lesions.
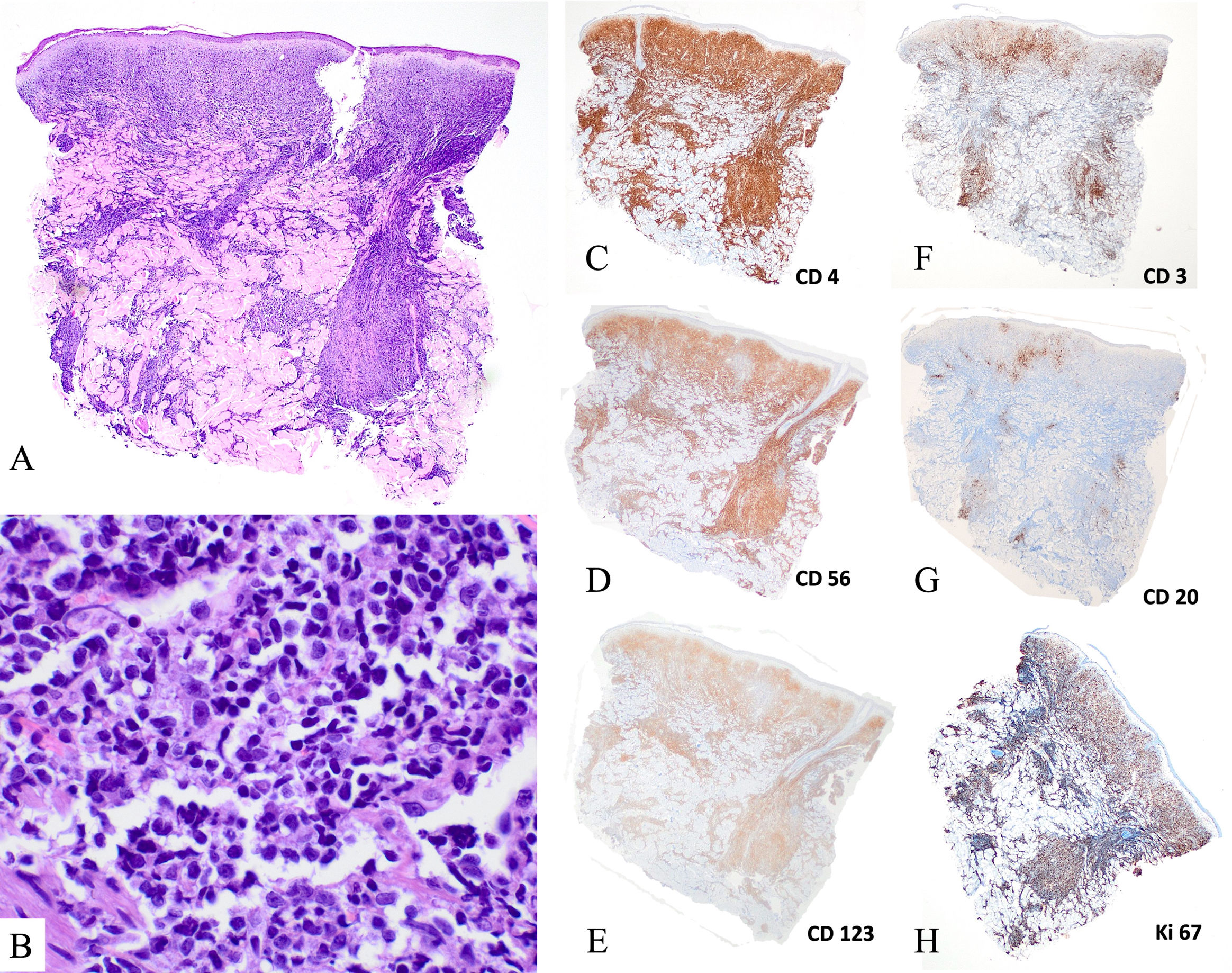
Histologically, skin lesions displayed dermal infiltrates of medium-sized blasts with irregular nuclei. Mitoses were a common finding and there was no vascular invasion or ulceration (Fig. 2A, B). Lymph nodes showed a leukemic infiltration pattern, occasionally causing diffuse effacement of the lymph node architecture. Bone marrow involvement patterns were heterogeneous, from focal involvement detected by immunohistochemistry to massive infiltration. Blastic cells were variable in size and had a lymphoblast-like appearance.
Histological findings of the skin biopsy of blastic plasmacytoid dendritic cell neoplasm. (A, B) Hematoxylin–eosin staining (A, ×20; B, ×400). Presence of a dense dermal infiltrate of intermediate size cells with atypical nuclei, with a grenz zone and no epidermotropism or vascular tropism. (C) Immunohistochemistry staining (×2) positive for CD4, CD56 and CD123, and negative for CD3 and CD20. Presence of a high number of mitoses (ki67>50%).
Immunophenotypic and molecular biology details are provided in Table S1. The samples from all patients expressed CD4, CD56, CD123 and HLA-DR (Fig. 2C–E). In 6 patients, the expression of CD303 and CD304 were assessed, and all were positive. Some cases expressed isolated, non-lineage-defining B-cell, T-cell, myeloid or monocytic antigens (CD22, CD38, CD2, CD7, CD33 and/or CD68).
Mutational statusTargeted NGS was performed in 7 cases with a panel including genes recurrently mutated in AML (Supplementary Table S2). Overall, mutations were detected in 4 of the 7 cases. Interestingly, 2 of the 3 cases without any identifiable mutation had an exclusively cutaneous presentation. Mutations were found in TET2 (3/7), NRAS (2/7), TP53 (1/6), RUNX1 (1/6), and SRSF2 (1/6) (Table S2).
Treatment and outcomesTherapy and outcomes are detailed in Table 1. Seven of 10 patients were treated with high-risk acute lymphoblastic leukemia (HR-ALL)-type regimens, including prophylactic intrathecal chemotherapy (PIC), based on vincristine, daunorubicin, steroids, mitoxantrone, cytarabine, methotrexate, cyclophosphamide and l-asparaginase. Three patients underwent CHOP-like regimens (cyclophosphamide, doxorubicin, vincristine, and prednisone), based on performance status and age, with 1 patient receiving PIC. The overall response rate was 80%. All 7 patients receiving ALL-type chemotherapy and 1 of the 3 patients on CHOP achieved CRs (Fig. S1. Supplementary material). Neither one of the 2 non-responding patients treated with CHOP received PIC, and 1 died due to central nervous system involvement. Five patients underwent an allo-HSCT, all with a myeloablative conditioning regimen with cyclophosphamide and total body irradiation (TBI). None of the allografted patients died of tumor progression or transplant-related complications (Fig. S1. Supplementary material). After a median follow-up of 77 months (8–172 months), all 5 allo-HSCT patients remain in CR. The 5-year overall survival (OS) rate was 66.7% for all patients, which was higher (83%) for ALL-like treated patients (only 1 patient died, without having undergone allo-HSCT). A younger age (<50 vs >50 years; p=0.022) and ALL-like schemes (p=0.018) were favorable prognostic factors regarding OS. Although patients undergoing an allo-HSCT showed a trend toward a better survival, statistical significance was not reached. Conversely, the fact of being limited to the skin was not a prognostic factor (p=0.31) (Fig. S1. Supplementary material).
DiscussionBPDCN is a very rare, aggressive hematologic neoplasm manifesting primarily in the skin. In this retrospective case series, we provide a detailed description of the skin lesions and treatment outcomes of 10 patients with an emphasis in ALL-type CT and allo-HSCT.
Our series displays the typical features of BPDCN: male predominance and notable tropism for skin and bone marrow involvement. Age at diagnosis was lower vs other series.1,7,8 Skin lesions had 3 main morphologies (nodular violaceous lesions, bruise-like plaques and maculopapular lesions).1,7 Since the skin is the most frequently affected organ (>90% of patients), initial diagnosis is usually achieved by dermatologists.9,14 Interestingly, patients with skin-limited disease at diagnosis do not have a better prognosis.1,7 At presentation, 80% of patients had systemic involvement and 30% of patients had a history of a previous hematologic neoplasm, which highlights the risk of second or concurrent hematologic neoplasms.14
All cases expressed CD4, CD56 and CD123 and, if assessed, pDC specific antigens BDCA-2/CD303 and BDCA-3/CD304. Other lineage-specific markers were negative.6,7 We observed a mutational landscape similar to previous reports of BPDCN, with 43% of cases harboring mutations in TET2.15–17 This finding may provide a rationale to incorporate epigenetic therapies (i.e. hypomethylating agents such as azacytidine and decitabine) in patients ineligible for intensive regimens.15,18NRAS mutations—which we found in 2 out of 7 studied patients—happen to be recurrent (27.3% of cases) and mutually exclusive with KRAS and ATM mutations.19 Overall, the mutational landscape of BPDCN is reminiscent of myeloid malignancies, which may explain their sequential or concurrent presentation.
Regarding treatment, in the absence of a standard strategy, therapies go from skin-directed to systemic chemotherapies.1,9,12,13 In 2018, tagraxofusp—an antiCD123 treatment—was the first agent for BPDCN to be approved by the FDA.12,13,20 Therefore, tagraxofusp will likely be incorporated into frontline schemes, along with CNS prophylaxis and allo-HSCT.20 Historically, multiagent chemotherapy regimens13 provided 40% up to 80% CR rates, but disease tends to recur, with a median overall survival of 12 to 14 months.13 Despite harboring mutations observed in myeloid malignancies,3,14,18 ALL-type regimens have been particularly effective8,20 and have highlighted the importance of CNS prophylaxis.20,21 In recent years, the importance of allo-HSCT to consolidate the response has become increasingly clear,1,10,11,17,21 due to a lower mortality rate and longer OS.9,13,14 When feasible, patients in our study were treated in a homogeneous fashion with intensive ALL-based therapy followed by early allo-HSCT with myeloablative conditioning.17 Disease control was excellent, with no relapses after a median of 10 years of follow-up in allo-HSCT patients. However, patients ineligible for allo-HSCT, as seen in this study, frequently receive less-intensive therapies without PIC and develop early recurrences, which results in a grim prognosis. New targeted treatments, such as antiCD123, BCL-2 inhibitors (venetoclax),13,14 or CAR-T cells, may improve overall survival and allow the treatment of more fragile patients.
In conclusion, our series showed characteristic clinical, morphological, and phenotypical features of BPDCN. An ALL-based regimen followed by early allo-HSCT resulted in a high remission rate and long response duration in patients considered eligible for intensive therapy.
Conflict of interestsThe authors declare that they have no conflict of interest.