Melanoma arising in blue nevus, also known as melanoma ex blue nevus, is a specific form of melanoma whose genetic profile is different to that of other cutaneous melanomas and surprisingly similar to that of uveal melanoma. Although melanoma ex blue nevus can appear de novo, it usually arises in a pre-existing blue nevus or dermal melanocytosis. Not all nodular lesions arising in association with blue nevus or dermal melanocytosis are melanomas, however, and because clinical and histologic findings may be insufficient for a definitive diagnosis, additional studies such as comparative genomic hybridization are important. Detection of chromosomal aberrations supports a diagnosis of malignancy. Studies of the BAP1 gene are particularly useful in this setting because loss of expression is indicative of melanoma. We present 3 cases on the spectrum of blue nevus to melanoma ex blue nevus that were studied using molecular biology techniques.
El melanoma sobre nevus azul o melanoma ex-blue nevus es una variedad de melanoma peculiar que tiene un perfil genético diferente al del resto de los melanomas cutáneos y sorprendentemente superponible al perfil del melanoma uveal. Aunque puede aparecer de novo, el melanoma ex-blue nevus se suele desarrollar sobre un nevus azul previo o sobre una melanocitosis dérmica. No todas las lesiones nodulares desarrolladas sobre un nevus azul o una melanocitosis dérmica son melanomas, y los hallazgos clínicos e histológicos pueden ser insuficientes para llegar a un diagnóstico de certeza. Así, cobran relevancia estudios adicionales, como la hibridación genómica comparada, pues la presencia de aberraciones cromosómicas favorece el diagnóstico de malignidad. Es de especial utilidad el estudio del gen BAP1, cuya pérdida de expresión orienta a melanoma en este espectro de lesiones. Presentamos tres casos del espectro nevus azul a melanoma ex-blue nevus con estudios de biología molecular.
Nodules arising on dermal melanocytoses or on blue nevi constitute a diagnostic challenge, since the classic histologic landmarks that enable us to distinguish between melanoma and melanocytic nevus are insufficient in this type of lesion. In contrast, the loss of BAP1 expression is indicative of melanoma among the lesions that are included in this spectrum, even in the absence of histologic indicators of malignancy.1 Similarly, demonstration of chromosomal abnormalities using comparative genomic hybridization (CGH) points to malignant disease.2,3
We report on 3 patients whose findings illustrate the spectrum of nodular lesions that can arise on dermal melanocytoses, ranging from benign cellular blue nevus to clinically evident melanoma and including a mixed case lying between atypical blue nevus and melanoma ex blue nevus.
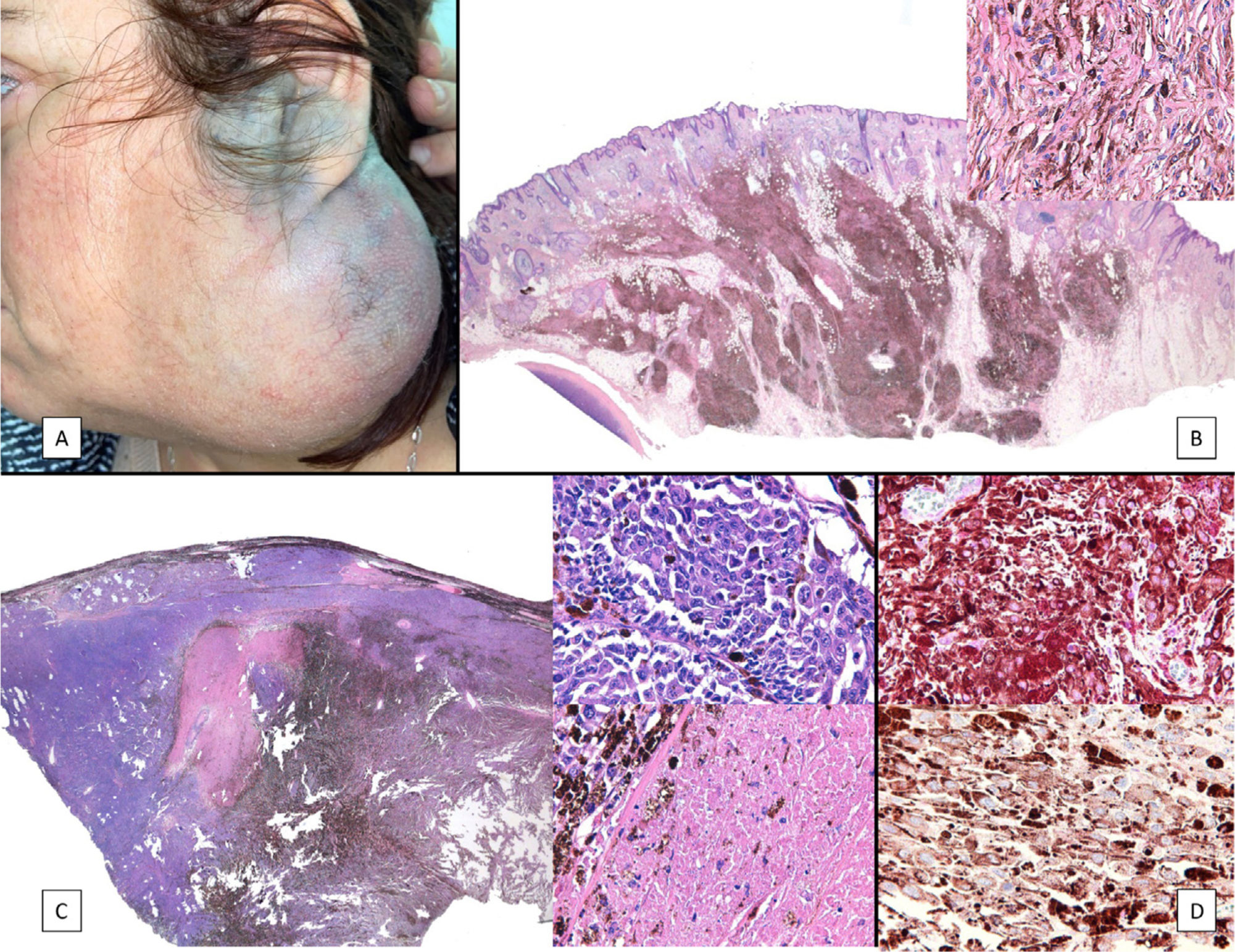
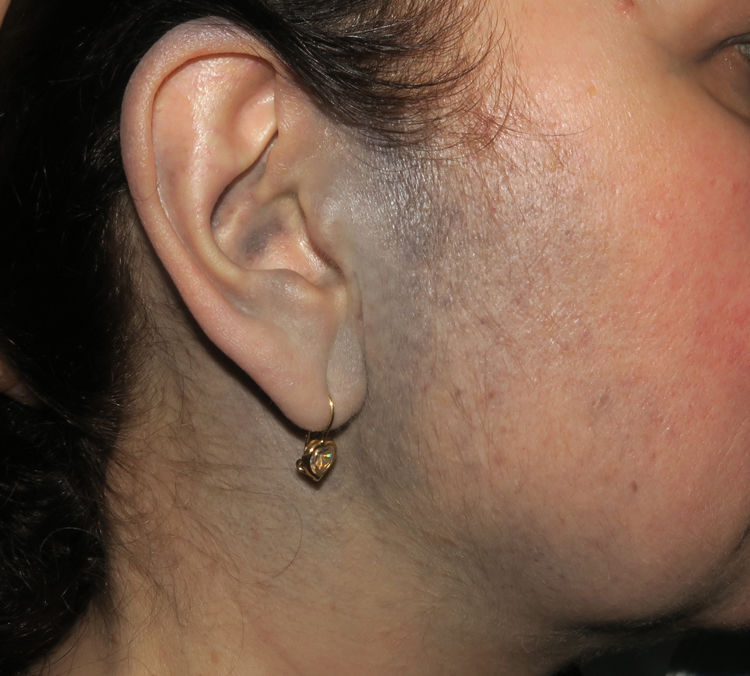
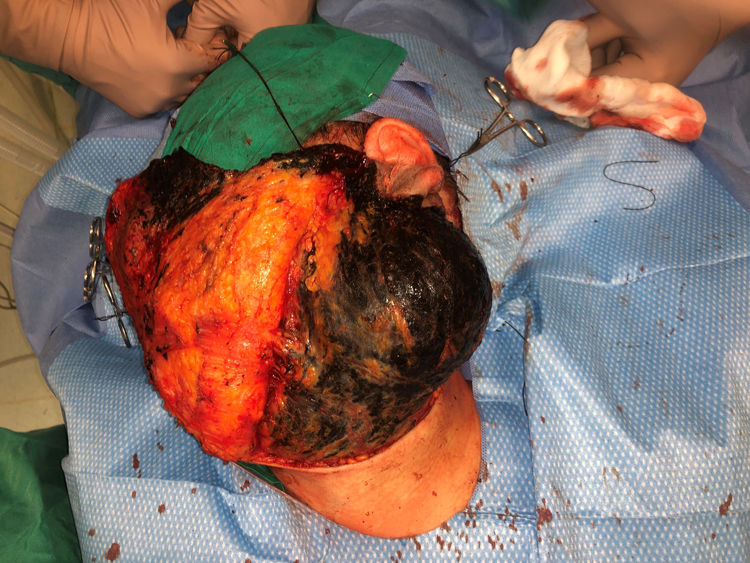
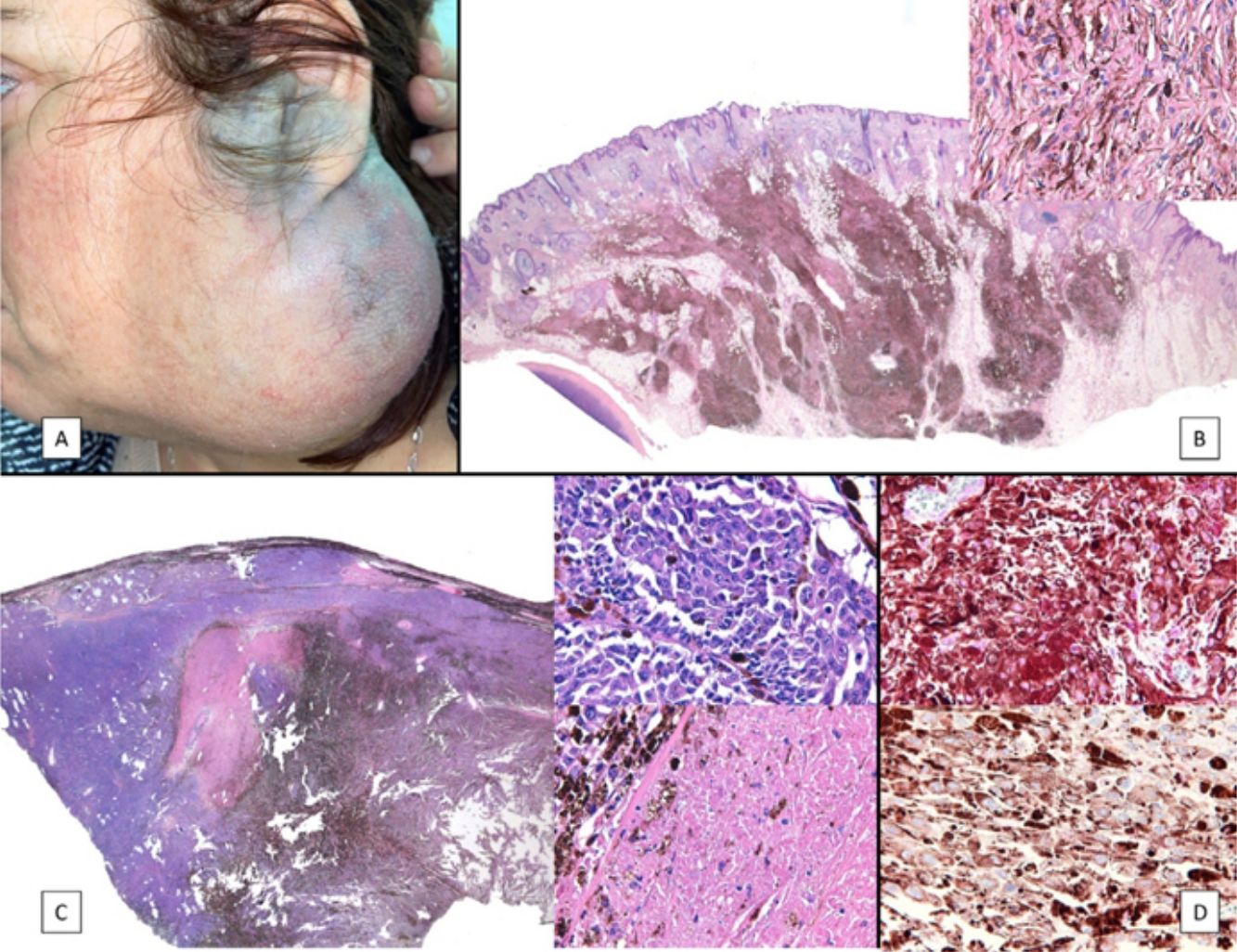
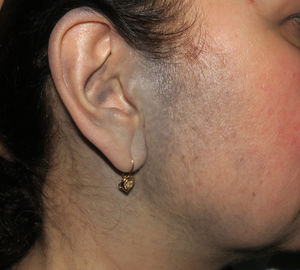
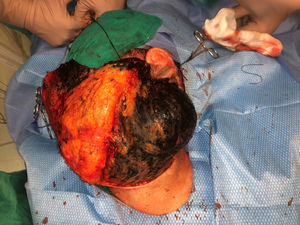
Case PresentationsPatient 1A 49-year-old woman was assessed for a large, firm subcutaneous mass measuring some 12cm in diameter in the parotid area. The lesion was located on the lower portion of a grayish-blue plaque that had been present since birth and affected the left pinna, as well as the adjacent side of the neck and preauricular skin (Fig. 1A). The plaque had never been biopsied and was clinically labeled as a “vascular malformation”.
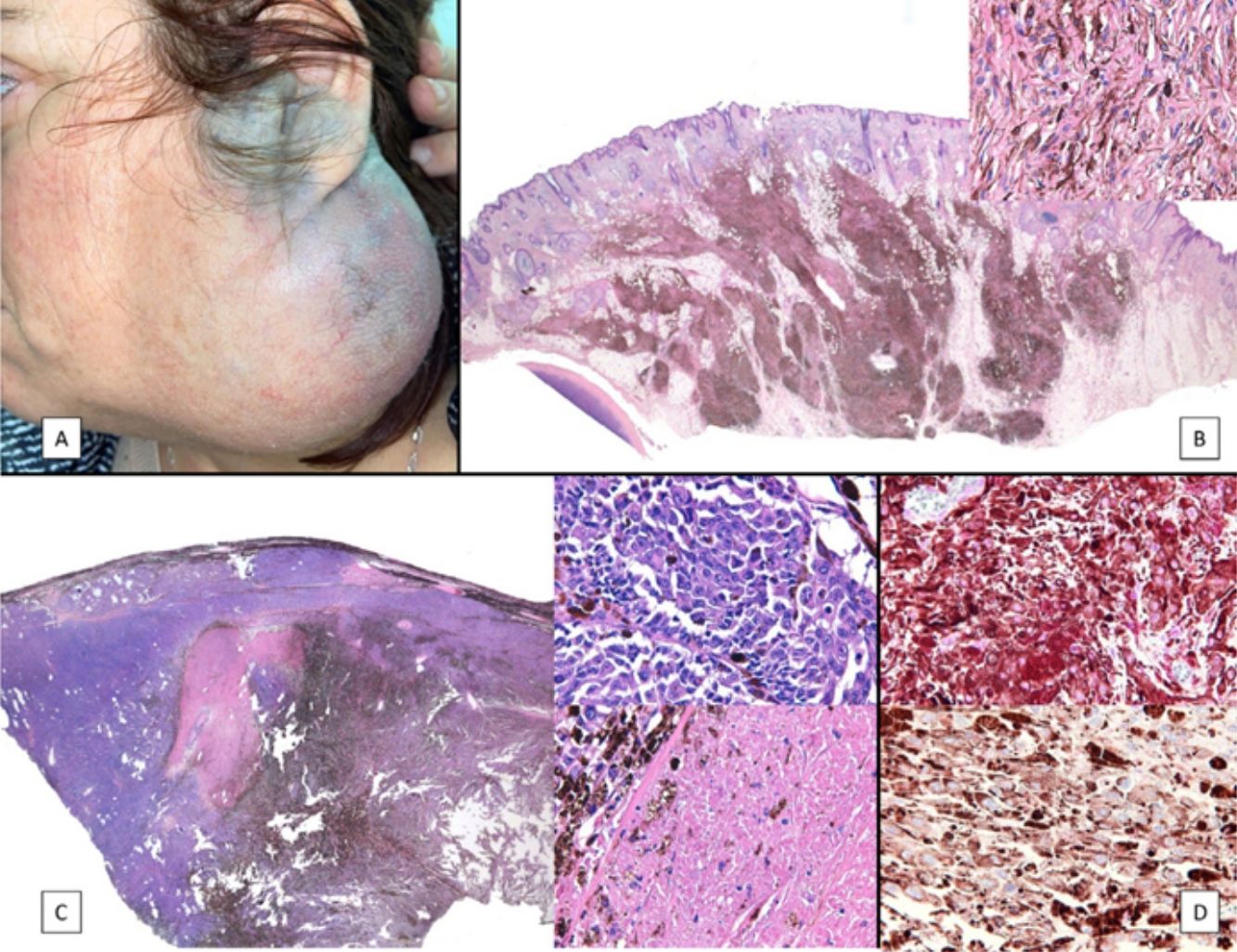
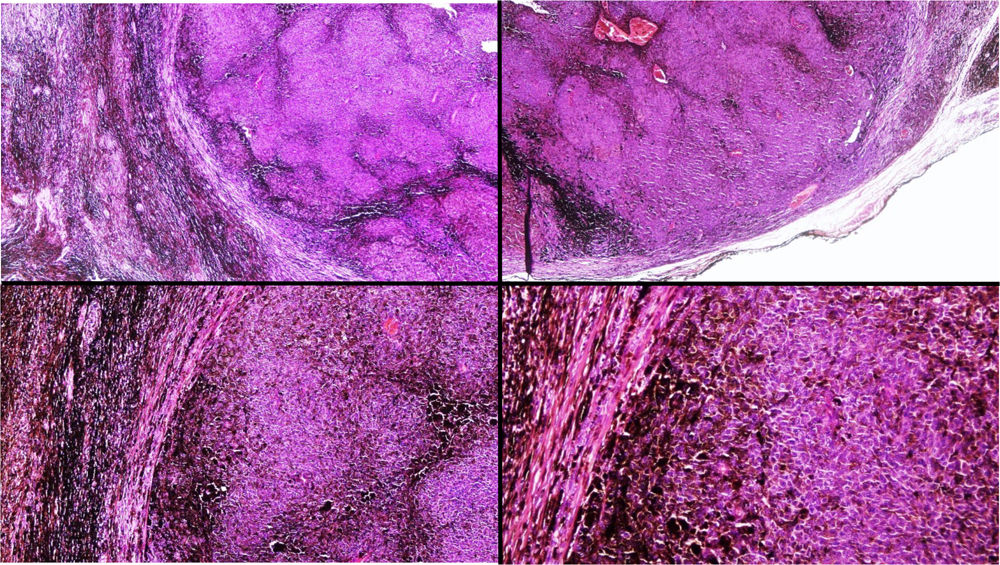
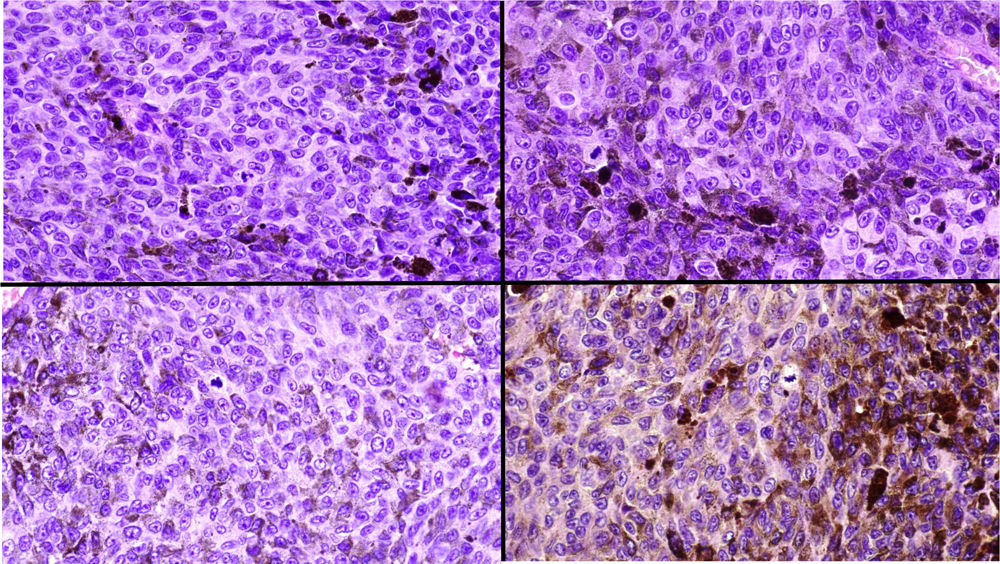
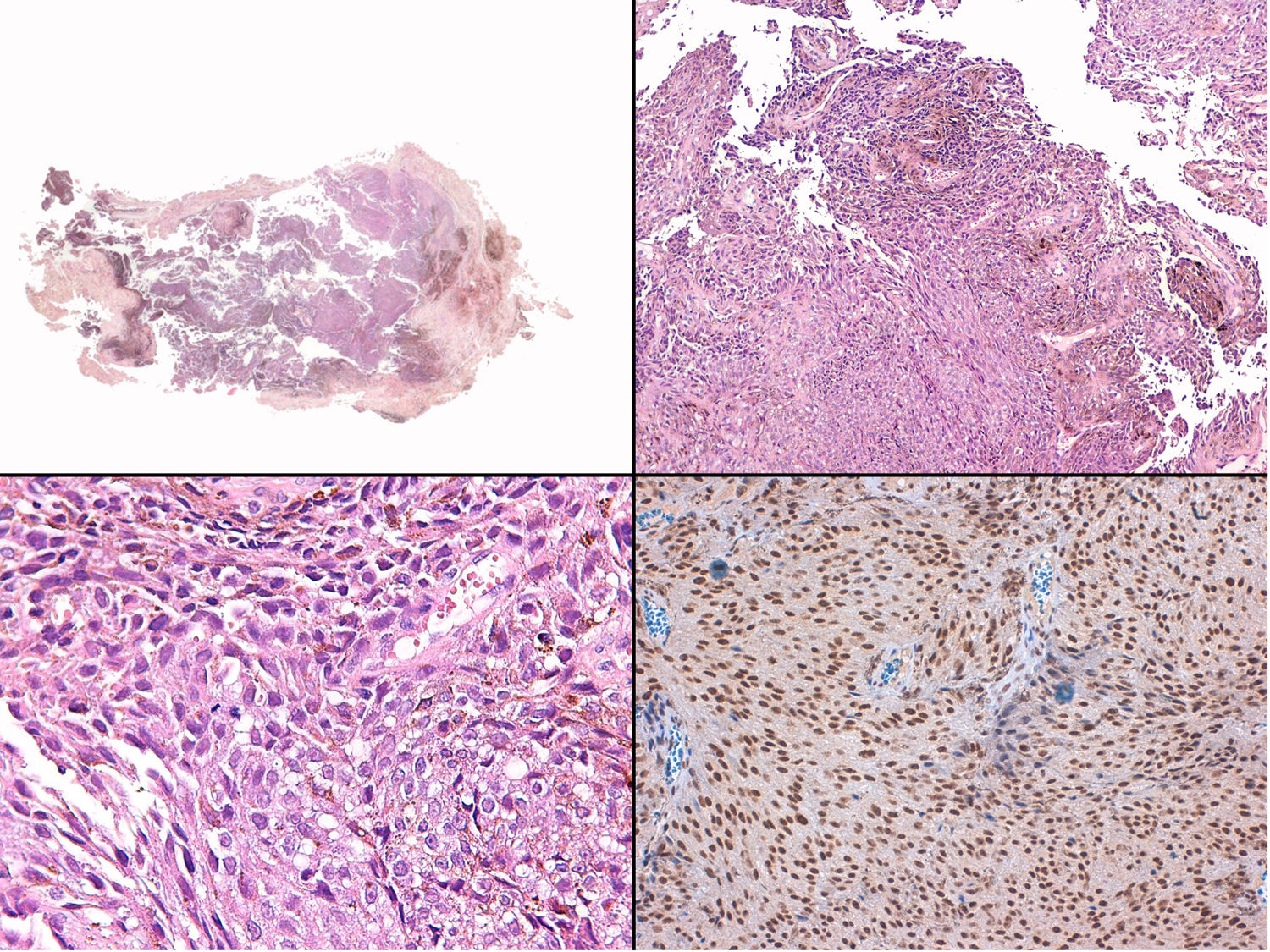
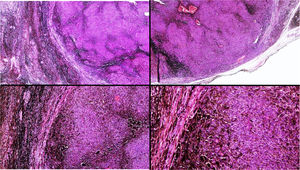
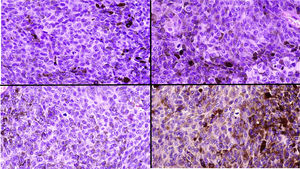
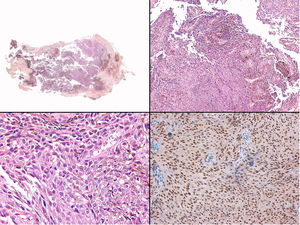
A, Large mass on the skin of the mastoid area on a blue plaque situated on the skin of the left pinna, preauricular region, and side of the neck. B, Plaque-type blue nevus. Panoramic view with inset showing detail: bundles of spindle cells, abundant melanophages, and sclerotic collagen occupying the subcutaneous fat and, to a lesser extent, the dermis, albeit without involvement of the dermal–epidermal junction. C, Central area of the tumor: note the predominance of dense sheets of epithelioid and pleomorphic cells replacing the pre-existing structures. The lower half of the inset shows areas of mass coagulative necrosis. The upper half of the inset shows densely melanocytic areas with no melanin content. D, Upper panel. Nuclear expression of BAP1 (in red) preserved in the plaque-type blue nevus portion. Lower panel. Loss of BAP1 expression in the nuclei of the melanoma portion.
The nodule was diagnosed as spindle-cell melanoma based on a cytology study of a sample obtained via fine-needle aspiration. The results were positive for Melan-A, human melanoma black (HMB) 45, and S-100. No distant lesions were revealed in the extension study, which included full-body positron emission tomography/computed tomography and fundoscopy. The patient underwent surgery in the ear, nose, and throat department. During the procedure, in addition to the tumor described above, she was observed to have very extensive and vague jet-black pigmentation affecting both the wide lateral margins and the deep margins of the resection (Fig. 1). The thyroid gland, facial nerve, adjacent cervical muscle fascia, and several lymph nodes were totally pigmented.
Histopathology of the surgical specimen revealed 2 very different areas. The portion of the pre-existing peripheral plaque comprised bundles of spindle cells accompanied by abundant melanophages and sclerotic collagen arranged in thick fascicles occupying mainly the subcutaneous fat and, to a lesser extent, the dermis, albeit sparing the dermal–epidermal junction (Fig. 1B). The nuclei of these cells were small, with no atypia, pleomorphism, or mitosis (Fig. 1B, inset). The abundant melanin followed a uniform pattern throughout the tumor. The whole lesion was considered to be plaque-type blue nevus. The central portion of the large tumor contained more mixed areas, with mostly dense sheets of epithelioid and pleomorphic cells replacing all the pre-existing structures (Fig. 1C). Large areas of coagulative necrosis in mass were observed (Fig. 1C, lower inset), as was a very irregularly distributed melanin pattern, with areas comprising a high density of melanophages heavily laden with melanin that contrasted with the densely melanocytic areas that did not contain melanin (Fig. 1C, upper inset). The results of immunohistochemistry with Melan-A, HMB45, and S-100 were positive. The findings were interpreted as melanoma arising in plaque-type nevus. Immunohistochemistry also revealed expression of BAP1 in the nucleus of cells in the plaque-type blue nevus portion (Fig. 1D, upper) and loss of BAP1 expression in the melanoma portion (Fig. 1D, lower).
The molecular study included somatic analysis of 42 genes based on next-generation sequencing with the Somatic Tumor Genetics kit (Sophia Genetics) in a NextSeq sequencer (Illumina) (analysis of the coding and splicing regions of the genes AKT1, ALK, BRAF,CDK4, CDKN2A, CTNNB1, DDR2, DICER1, EGFR, ERBB2, ERBB4, FBXW7, FGFR1, FGFR2, FGFR3, FOXL2,GNA11, GNAQ, GNAS, H3F3A, H3F3B, HIST1H3B, HRAS, IDH1, IDH2, KIT, KRAS, MAP2K1, MET, MYOD1,NRAS, PDGFRA, PIK3CA, PTPN11, RAC1, RAF1, RET, ROS1, SF3B1, SMAD4, TERT, and its promoter, TP53), both of the blue nevus portion and of the melanoma portion, with the only finding being that of the pathogenic mutation p.Q209L in GNAQ in both portions. Moreover, CGH revealed the complete absence of chromosomal abnormalities in the plaque-type blue nevus portion but detected several abnormalities in the melanoma portion, including gains of 1q, Cr 2, 5p, 6p, Cr 7 and 8 (4n), and Cr 13, 14, 17, 19, 20, 21, and 22, as well as a loss of heterozygosity for 1p, Cr 3, and 5q and a loss of 6q and CrX.
Six months after surgery, the patient experienced a locoregional recurrence, with multiple reddish, ulcerated nodules in the left cervical and auricular areas, as well as metastasis to the lymph nodes in the neck, mediastinum, lungs, and liver. She went on to receive systemic treatment with ipilimumab and nivolumab. After a 4-month follow-up, the patient was still alive, with progressive disease.
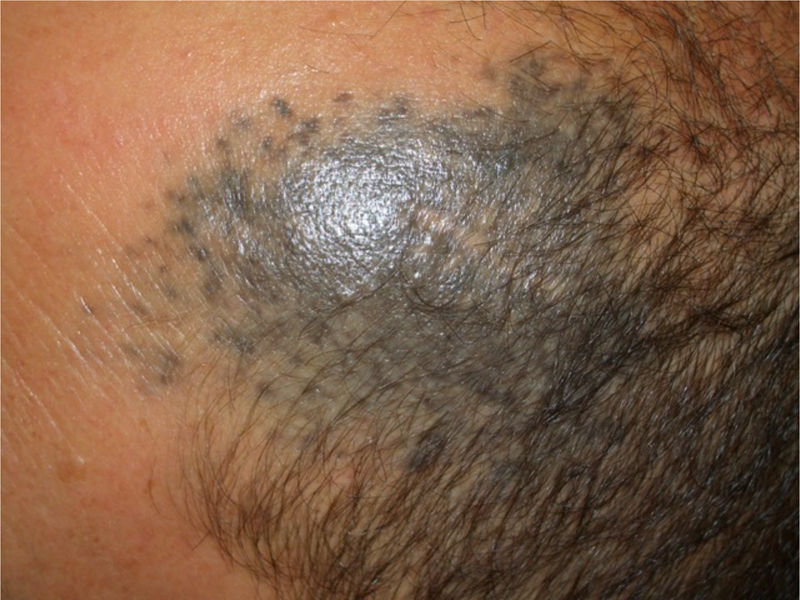
Patient 2A 53-year-old man was being followed up because of a bluish-gray pigmented plaque measuring 8cm×5cm in diameter on the left frontotemporal region that had first appeared many years previously (Fig. 2, Supplementary Material). The lesion had been biopsied on 2 occasions (2002 and 2004), leading to a diagnosis of blue nevus. At the most recent visit, a nodule could be felt inside the plaque. Ultrasound showed the nodule to be in the subcutaneous cellular tissue.
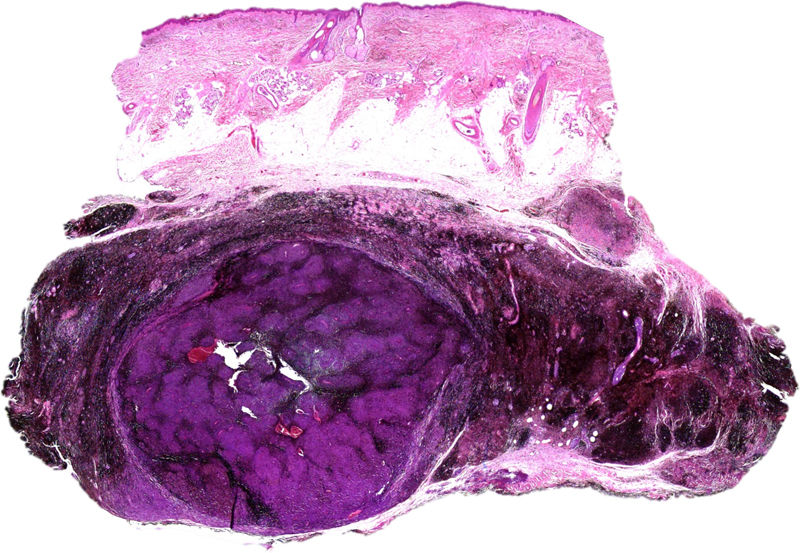
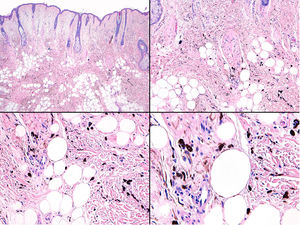
Histology revealed the lesion to be part typical discrete dendritic blue nevus and part highly pigmented plaque-type blue nevus below the hypodermis (Fig. 3, Supplementary Material). A much more cellular expansive nodule with a lower amount of melanin than the adjacent plaque was embedded in the subcutaneous portion of the plaque-type blue nevus (Fig. 4, Supplementary Material). The cells of the nodular portion had nuclei with loose chromatin and evident nucleoli. Despite the scarce atypia and pleomorphism in most fields, focal areas with moderate atypia and some mitotic figures (2/mm2) were observed (Fig. 5, Supplementary Material).
Fluorescence in situ hybridization (FISH) of the regions coding for C-MYC, CDKN2A, and CCDN1 revealed no abnormalities in the superficial portion of the dendritic blue nevus or the in portion of the plaque-type blue nevus. Amplification of the 8q region (C-MYC) was observed in the deep nodular portion. In addition, CGH of the lesion revealed chromosomal abnormalities (loss of 6q and 8p and gain of 6p and 8q). Nuclear expression of BAP1 in immunohistochemistry was preserved both in the nodule and in the blue nevus portion. The patient was diagnosed with melanoma in plaque-type blue nevus based on the presence of chromosomal abnormalities in the nodular portion.
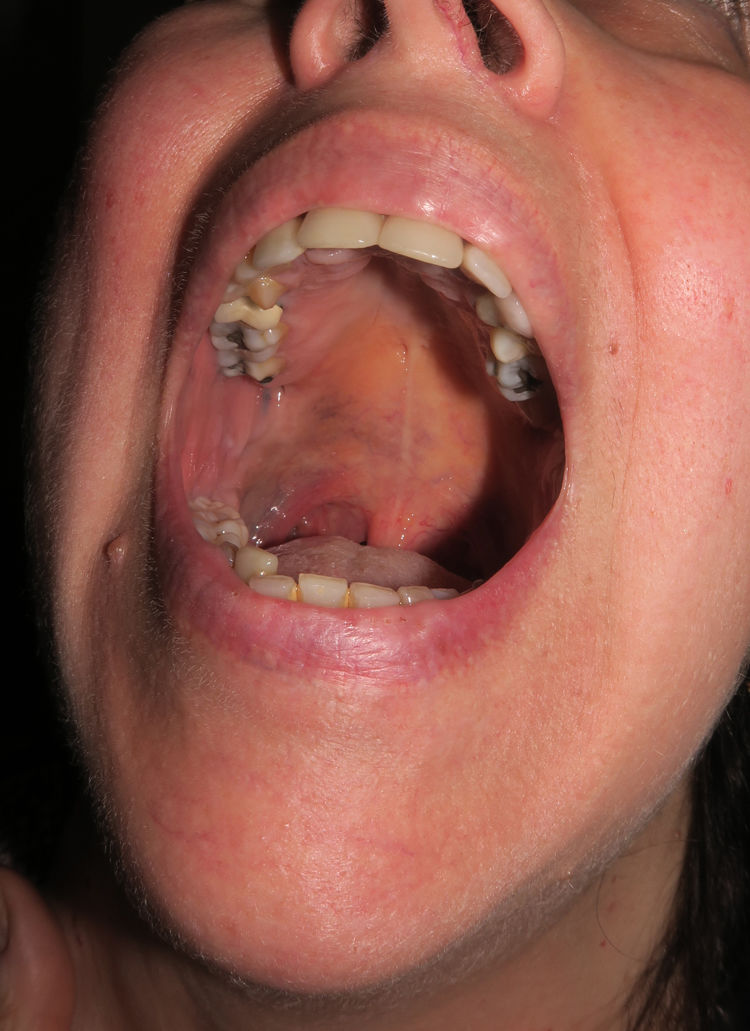
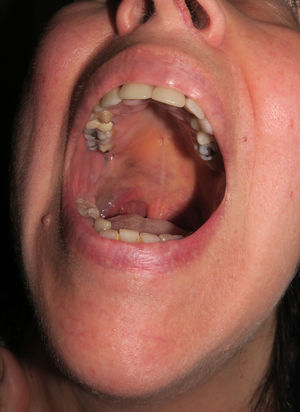
Patient 3The third patient was a 58-year-old woman with grayish-blue pigmentation (onset at puberty) that initially affected the right auricular and adjacent regions (Fig. 6, Supplementary Material) before extending progressively to the skin of the submandibular area and the ipsilateral buccal and palatal mucosa (Fig. 7, Supplementary Material). The ipsilateral sclera was completely white. Over the course of a month, the patient developed a preauricular nodule, which was clinically considered an abscess, although once removed, it was diagnosed as melanoma in situ.
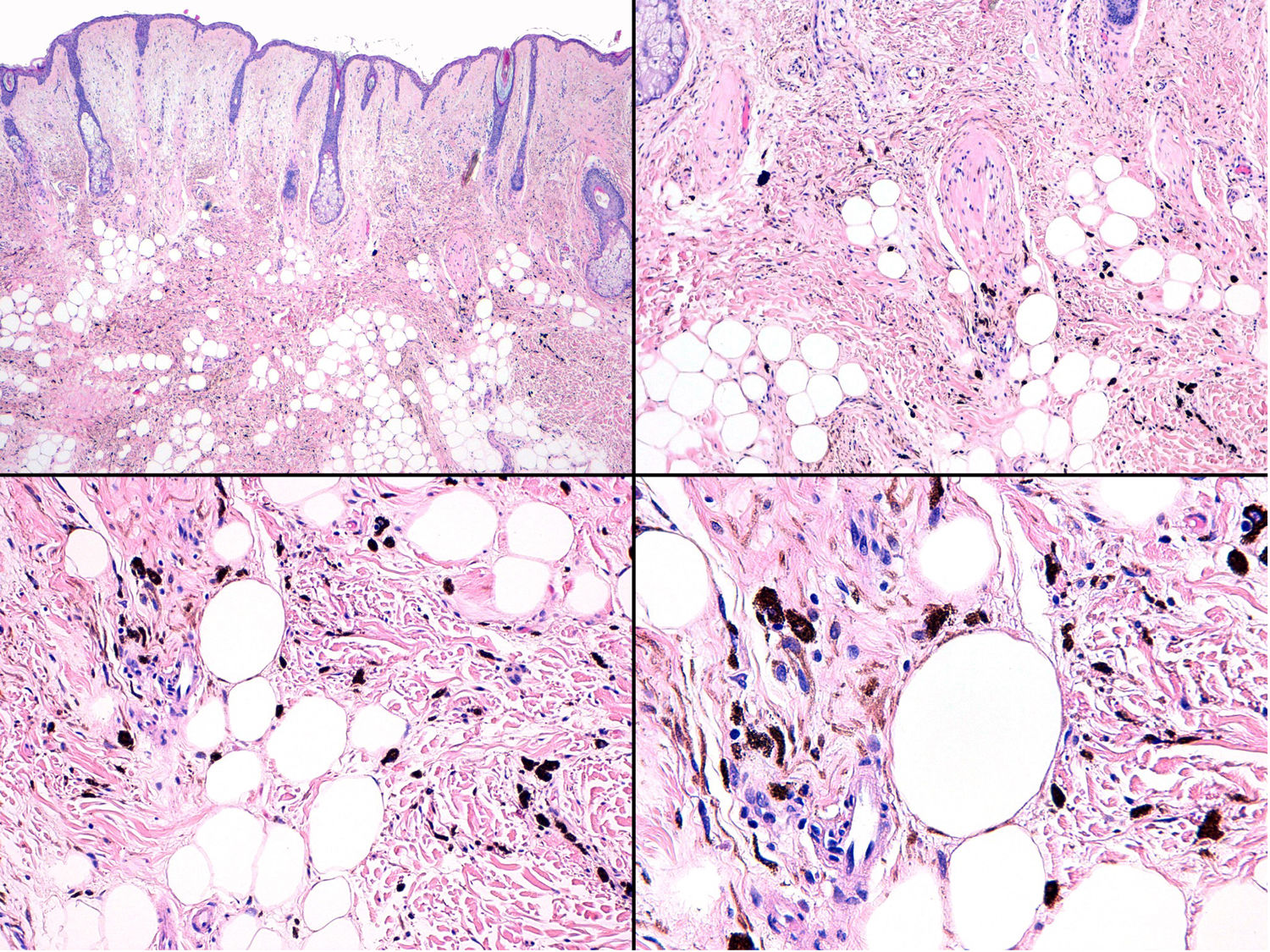
Histopathology of the fast-growing nodule revealed a fragmented and damaged specimen comprising epithelioid cell aggregates with no clear atypia or pleomorphism accompanied at the periphery by abundant melanophages (Fig. 8, Supplementary Material). Only a single mitotic figure was observed in the nodule. Histology of the pre-existing grayish-blue patch showed scant spindle-cell melanocytes and melanophages scattered through the dermis (Fig. 9, Supplementary Material). Given the clinical context, this finding was diagnosed as dermal melanocytosis in the form of nevus of Ota.
Somatic molecular testing using next-generation sequencing (see Patient 1) revealed the pathogenic mutation p.Q209P in GNAQ, both in the nodule and in the dermal melanocytosis.
Immunohistochemistry for BAP1 revealed preserved nuclear expression in the nodule that arose in the nevus of Ota (Fig. 8, Supplementary Material. Immunohistochemistry).
The extension study did not reveal distant lesions; therefore, treatment was limited to surgery. The result of the sentinel node biopsy was negative, and the patient has remained recurrence-free during 9 months of follow-up.
The patient was eventually diagnosed with cellular blue nevus in dermal melanocytosis.
DiscussionPlaque-type blue nevus is a generally cellular form of blue nevus that usually develops during early childhood. It is large, measuring 2–3cm and sometimes reaching 10cm or more.4,5 It manifests as a speckled plaque, as in patient 2, formed by a confluent cluster of blue nevi, or as a uniformly bluish plaque. The histologic diagnosis must correlate with the clinical diagnosis, since the lesion has no specific histologic characteristics. The proliferation is usually deep and may even reach the fascia.6
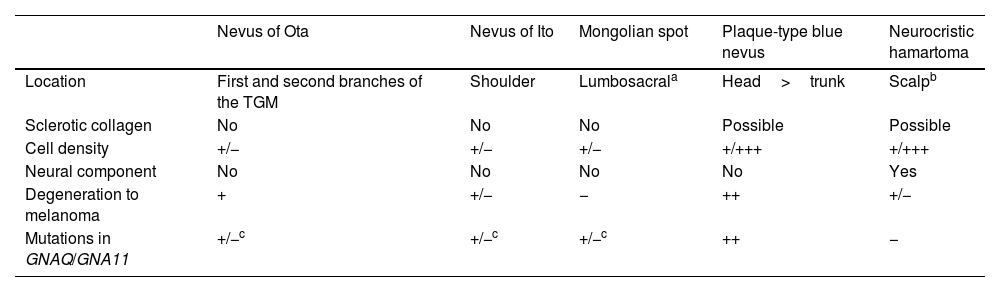
Plaque-type blue nevus, other dermal melanocytoses, and neurocristic hamartoma share various characteristics (Table 1). Neurocristic hamartoma results from an abnormal migration of melanocytes from the neural crest to the epidermis, as in blue nevus, although unlike this condition, it is accompanied by a mesenchymal component and, more particularly, a neural component (Schwann cells). However, the distinction is not clear, and there have been reports of cases described as neurocristic hamartoma that many dermatologists would diagnose as blue nevus.7,8 Plaque-type blue nevus could probably be considered simply as a neurocristic hamartoma with only a melanocytic component.
Characteristics of Dermal Melanocytoses, Plaque-Type Blue Nevus, and Neurocristic Hamartoma.
| Nevus of Ota | Nevus of Ito | Mongolian spot | Plaque-type blue nevus | Neurocristic hamartoma | |
|---|---|---|---|---|---|
| Location | First and second branches of the TGM | Shoulder | Lumbosacrala | Head>trunk | Scalpb |
| Sclerotic collagen | No | No | No | Possible | Possible |
| Cell density | +/− | +/− | +/− | +/+++ | +/+++ |
| Neural component | No | No | No | No | Yes |
| Degeneration to melanoma | + | +/− | − | ++ | +/− |
| Mutations in GNAQ/GNA11 | +/−c | +/−c | +/−c | ++ | − |
Abbreviation: TGM, trigeminal nerve.
In clinical practice, the appearance of a cutaneous melanoma on these lesions has mainly been reported in blue nevus and, albeit much less frequently, in nevus of Ota, nevus of Ito, and neurocristic hamartoma.9–11
The term melanoma arising on blue nevus, melanoma ex blue nevus, or blue nevus-like melanoma includes both melanomas that develop on a pre-existing blue nevus (or a dermal melanocytosis) and melanomas that develop on the scar of a previously resected blue nevus, melanomas with histologic characteristics similar to those of a blue nevus, and melanomas with a blue nevus component.6 Ex blue nevus melanoma is a rare tumor, with fewer than 200 cases reported to date,12,13 although some reports of neurocristic hamartoma with metastasis could be considered melanoma ex blue nevus,14 and fatal melanomas arising on plaque-type blue nevus have been published as “nodules in plaque-type blue nevus”.15 The most common locations for melanoma ex blue nevus are the head and neck,12 although the lesion has also been reported on the buttocks and trunk. The most common age at onset is 45–55 years, and the most frequent clinical manifestation is as a large, rapidly growing nodule on a previous blue nevus, most frequently a cellular blue nevus.
The classic histopathology criteria for malignancy in melanocytic tumors cannot always be applied in melanoma arising on blue nevus.16 The asymmetrical or mainly deep silhouette, the absence of maturation, and the expansive growth pattern, as well as the uniform expression of human melanoma black 45 are common features of blue nevus. Criteria that do prove useful for the diagnosis of melanoma include the presence of necrosis, marked pleomorphism, atypia, and a high mitotic index.13 In doubtful cases, the loss of BAP1 expression and the demonstration of chromosomal abnormalities using FISH or CGH (gains and losses in chromosomes 6 and 8 and losses in chromosomes 1 and 3) are indicative of malignancy.1–3 Furthermore, unlike other cutaneous melanomas, they have a low number of mutations, and the most common driver mutations in melanoma arising on blue nevus are those found in GNAQ or GNA11 (which are common in blue nevi) and, albeit much less frequently, mutations in CYSLTR2.13,16–18 All these features lead us to consider the tumor more as uveal melanoma than as cutaneous melanoma. Furthermore, driver mutations appear at onset of the lesion, similar to mutations in BRAF or NRAS in conventional cutaneous melanoma. These are mutually exclusive mutations that are usually acquired somatically, although in early phases of embryonic development. Mutations in GNAQ/GNA11 serve somehow as “type markers” on the blue nevus/melanoma ex blue nevus spectrum. In fact, the presence of mutations in BRAF or NRAS makes it difficult to maintain a diagnosis of blue nevus or melanoma ex blue nevus.16 Pathogenic mutations in GNAQ or GNA11 lead to constitutive activation of the RAS signaling pathway.19 Their presence is essential for the development of blue nevus, although it is insufficient for causing malignancy. Abnormalities have been described in other genes. These are acquired later in the natural history of melanoma arising on blue nevus and are in fact associated with malignancy and even with a poorer prognosis, for example, loss of BAP1 expression, either by mutation or by partial or total loss of the region that encodes it (3p21). As mentioned above, this loss of BAP1 expression within the spectrum of blue nevus-like lesions has both diagnostic and prognostic implications.1,16–18 The absence of expression in the nuclei of a blue nevus-like tumor almost enables us to make a diagnosis of melanoma, even in cases with scarce atypia and no mitosis.1 Furthermore, blue nevus-like melanoma with loss of BAP1 expression metastasizes more than those that do not lose expression of BAP1.1,16 This has also been demonstrated in uveal melanoma.19 Other, later-onset mutations that are characteristic of melanoma arising on blue nevus (and of uveal melanoma) but that are not present in blue nevus include those in EIF1AX and SF3B1. The prognostic significance of these mutations is less well established. Data from a recent series show that 3 of the 5 patients with melanoma ex blue nevus and the SF3B1 mutation died from metastasis of the melanoma. The authors found this surprising, because this mutation was associated with a better prognosis in uveal melanoma.16
In this study, we report 3 new cases illustrating the spectrum of blue nevus/melanoma ex blue nevus. Case number 1 clearly involves melanoma. It was characterized clinically by early recurrence and visceral metastasis, histology revealed the presence of large areas of necrosis and marked atypia and pleomorphism, and molecular testing showed loss of BAP1 expression and the presence of multiple chromosomal abnormalities. Furthermore, the poor response to immunotherapy parallels findings for uveal melanoma, possibly owing to the low mutational burden, which considerably limits treatment options. Patient 2 was diagnosed with melanoma arising on plaque-type blue nevus owing to the presence of chromosomal abnormalities, although the BAP1 expression and much less clear histology findings in Patient 1 could be interpreted as atypical blue nevus. The progress of this case to date has not involved recurrence or metastasis. Lastly, the third case was interpreted as cellular blue nevus in dermal melanocytosis owing to the absence of pleomorphism and necrosis, the finding of a single mitotic figure in all the samples studied, and maintained expression of BAP1.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We are grateful to Dr. José Luis Rodríguez Peralto for kindly performing the comparative genomic hybridization studies.