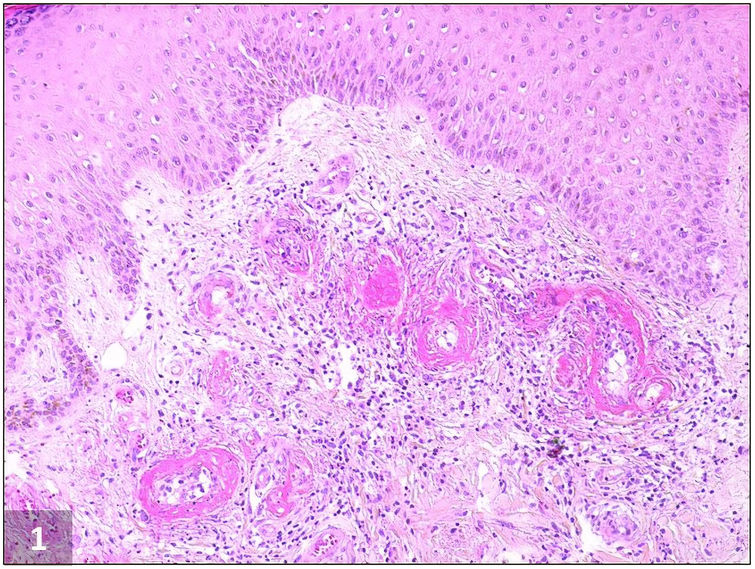
Livedoid vasculopathy (LV) is an uncommon occlusive thrombotic skin disease that primarily affects the small blood vessels of the lower extremities. It is a recurrent and painful condition characterized by persistent livedo associated with recurrent painful ulcerations, mainly around the malleoli, that heal with atrophic scars (atrophie blanche).1–3 The diagnosis is confirmed through a skin biopsy2,3 that shows characteristic vascular abnormalities, including intraluminal thrombosis, endothelial proliferation, and subintimal hyaline degeneration (Fig. 1).1,2,4 This condition is more common in middle-aged women.1–3 Its pathogenesis is unclear, but it may be associated with various coagulopathies and associated syndromes.2,4,5 Plasminogen activator inhibitor-1 (PAI-1) is an important inhibitor of the fibrinolytic system and elevated levels of PAI-1 have been described in patients with LV.4,6 Treatment for LV is challenging and not well established, aiming to improve skin lesions, relieve pain and prevent recurrence. The most widely prescribed therapies are anticoagulants (most commonly as monotherapy, achieving a favourable response in 98% of patients), anabolic steroids (to control acute symptoms due to their anti-inflammatory effect although they may also act by enhancement of fibrinolysis and inhibition of coagulation), intravenous immunoglobulins, and antiplatelets, among a variety of other therapies that include dipyridamole, pentoxifylline and nifedipine.1,7,8 Recently, the new non-vitamin K antagonist oral anticoagulants (NOACs), especially rivaroxaban, have also been used with considerable success.5,7–9
Livedoid vasculopathy, histopathological picture: Fibrin deposition within both the wall and lumen of dermal blood vessels resulting in thickened vessels walls and vascular occlusion by thrombosis. Dermal fibrosis and a perivascular lymphocytic infiltrate are also observed (H&E, ×100).
We report two cases of LV in middle-aged women with recalcitrant disease who harboured PAI-1 promoter increased risk polymorphisms and that were successfully treated with NOACs.
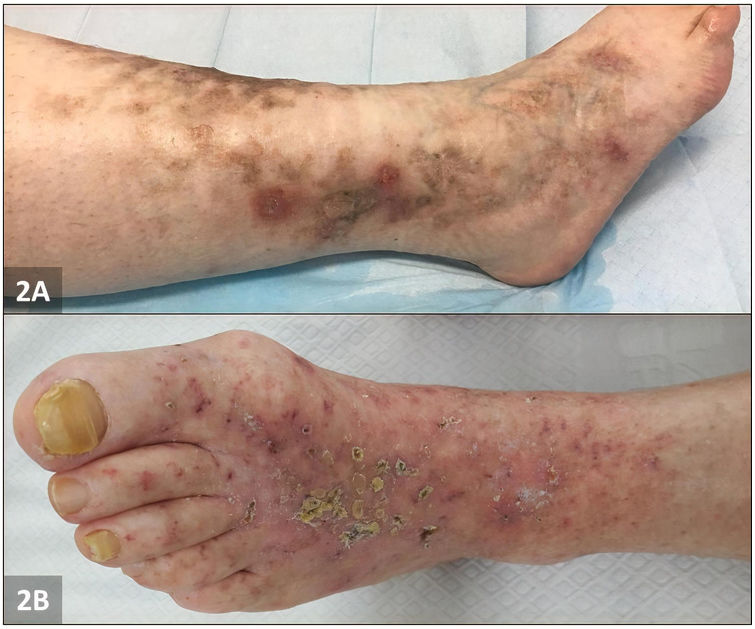
Patient number 1 was a 42-year old woman who presented to our outpatient clinic with a 14-month history of tender, coalescent, purpuric papules on her lower legs which had evolved into deep and painful ulcerations. She had no relevant past medical history. Physical examination revealed mottled skin and punched-out ulcers surrounded by purpuric erythema and hyperpigmentation on both malleolar regions with reticular and livid erythema on the legs. Several ulcers had healed with white atrophic stellate scars (Fig. 2A). Skin biopsy was compatible with LV (Fig. 1). PAI-1 promoter 4G/4G homozygosity was detected at DNA sequencing. Aspirin and oral prednisolone were prescribed with mild improvement. Prednisolone dose was gradually tapered (until 5mg/daily), and aspirin was kept at the standard dose as maintenance therapy. However, in 4 months, a new recurrence was documented. Low dose edoxaban (15mg/d) was then introduced with complete clinical resolution in 5 months. She remains well at 7-month follow-up.
Patient number 2 was a 72-year old woman with a 6-year history of pruritic and painful papules involving both legs, mottled skin, and painful ulcerations. At presentation multiple pinpoint ulcerations and crusts on a livedoid base on both ankles and feet were seen (Fig. 2B). The pain severity and ulcers worsened during the summer and remitted during the winter. A skin biopsy confirmed the diagnosis of LV. An insertion (5G)/deletion (4G) polymorphism at position −675 of the PAI-1 gene was detected by PCR-RFLP analysis. Mono- and combination therapy with aspirin, oral prednisolone and pentoxifylline was tried for 4 months with no clinical improvement. Monotherapy with rivaroxaban was then initiated (20mg/daily for 12 weeks and then 10mg/daily) with complete clinical resolution in 6 months and no documented disease recurrence at 6-month follow-up.
In both patients a complete workup, including full blood count, urinalysis, coagulation studies, rheumatoid factor, serum complement levels, tests for cyclic citrullinated peptide antibodies, antinuclear antibody and antineutrophil cytoplasmic antibodies, serum cryoglobulins, serum protein electrophoresis, immunofixation and doppler ultrasound of the legs, was carried out. There was no evidence of other coagulopathy nor systemic diseases associated with LV besides the PAI-1 promoter polymorphisms.
The 4G allele is slightly more transcriptionally active than the 5G so, the 4G allele homozygosity and some 4G/5G polymorphisms in the PAI-1 promoter are associated with increased PAI-1 protein levels, impaired fibrinolysis, and increased risk of thrombosis, being increasingly recognized as a risk factor for diseases such as juvenile myocardial infarction and stroke.4,6 In the past years, a few studies have proposed a link between PAI-1 promoter mutations and LV.2,6 This discovery has prompted the use of thrombolytic agents, such as tissue plasminogen activator, as a therapeutic option, with some promising outcomes.2,6,10 However, its safety is a matter of concern and it is not recommended as first-line treatment.4,7
We should keep in mind that although many affected patients have a prothrombotic condition no single abnormality characterizes LV, which can be regarded as a pattern of cutaneous expression of increased coagulation involving dermal vessels or of abnormal fibrinolysis.3 As such, anticoagulants are the most used therapy for LV7 and NOACs, like rivaroxaban and edoxaban, are easier to handle than warfarin. These are factor Xa direct inhibitors, thus acting at the end factor of both the intrinsic and extrinsic coagulation pathways, which in turn leads to the production of thrombin.5,8,9 As such, factor Xa is a desirable and safe target for anticoagulation therapies. Our patients had refractory LV and both harboured PAI-1 promoter polymorphisms related to an increased prothrombotic risk. These patients experienced a good clinical outcome with NOACs. The presented cases highlight that the screening for prothrombotic risk factors should include genetic polymorphisms in PAI-1 promoter in LV patients and how NOACs may be a valid option as first-line therapy in these patients.