A 5% risk of reactivation of hepatitis B virus (HBV) infection has been reported in patients with diseases other than psoriasis treated with tumor necrosis factor inhibitors. The aim of this study was to investigate the risk of HBV reactivation in patients with a past history of HBV infection who were receiving biologic therapy for psoriasis.
Material and methodsThis was a multicenter study of 20 patients with psoriasis who were treated with at least 1 biologic agent. All the patients had serologic evidence of past HBV infection (positive total hepatitis B core antibody and negative hepatitis B surface antibody). We analyzed the clinical, serological, and liver function variables recorded before, during, and at the end of follow-up. The viral load at the end of follow-up was also analyzed for all patients.
ResultsNone of the patients fulfilled the criteria for HBV reactivation at the end of a median follow-up period of 40 months. Combining our data with data from other studies of psoriasis patients with a past history of HBV infection who were treated with a biologic, we calculated a maximum estimated risk of HBV reactivation for a mean follow-up period of 30 months of 2.7 reactivations per 100 patients.
ConclusionsBiologic therapy did not cause HBV reactivation in our series of patients. Nonetheless, because of the potentially serious complications associated with HBV reactivation, it is important to measure viral load in patients with a history of HBV infection prior to initiation of biologic therapy to rule out occult carriage. These patients should also be monitored regularly in conjunction with a hepatologist.
Se ha reportado un riesgo de reactivación de hepatitis B pasada de hasta el 5% en pacientes tratados con fármacos dirigidos contra el factor de necrosis tumoral para enfermedades distintas a la psoriasis. Nuestro objetivo es investigar el riesgo de reactivación del virus de la hepatitis B en pacientes con hepatitis B pasada y psoriasis tratada con biológicos.
Material y métodosEstudio multicéntrico en el que se incluyeron 20 pacientes con serología sugestiva de hepatitis B pasada (antiHBc+, antígeno HBs–) y diagnóstico de psoriasis tratada con al menos un biológico. Se recogieron variables clínicas, serológicas y de función hepática antes, durante y al final del seguimiento. Se obtuvo una carga viral al final del seguimiento en todos los pacientes.
ResultadosNingún paciente mostró criterios de reactivación de hepatitis B al final del estudio, con una mediana de seguimiento de 40 meses. Sumando los datos de otras series publicadas de pacientes con psoriasis y hepatitis B pasada tratados con biológicos, el riesgo máximo sería de 2,7 reactivaciones por 100 pacientes tratados con un seguimiento medio de unos 30 meses.
ConclusionesEn nuestro estudio el tratamiento con biológicos no provocó ninguna reactivación de hepatitis B. Sin embargo, debido a las graves complicaciones asociadas a la misma, se aconseja descartar portadores ocultos en pacientes con hepatitis B pasada antes de iniciar tratamiento biológico (solicitando una carga viral al inicio del mismo), así como un seguimiento conjunto con un hepatólogo.
Infection with the hepatitis B virus (HBV) is a worldwide health issue. An estimated 350 million individuals are carriers, although there are marked differences in their geographic distribution.1 Spain is a country with an intermediate prevalence of affected individuals. After establishing universal vaccination programs against HBV, the prevalence in 2007 of carriers of the HB surface antigen (HBsAg) and those with past hepatitis B infection decreased to 0.7% and 8.7%, respectively, although the prevalences are higher in elderly individuals and immigrants.2 Chronic infection with HBV is a dynamic process. Individuals with past hepatitis B infection are HBsAg- although a low viral replication rate may persist.3
Psoriasis is an inflammatory skin disease whose prevalence varies considerably according to the populations studied.4 In Spain, the most recent study reported that 2.3% of the population were affected.5 Biologic agents targeting tumor necrosis factor (TNF) or interleukin 12/23 have revolutionized the treatment of severe psoriasis. Given their immunosuppressive properties, these agents may favor reactivation of viral infections, with occasionally fulminant outcomes.6 Reactivation can occur at any time during treatment, although it is most likely on initiation or after termination due to an immune reconstitution phenomenon.3 The objective of this study was to assess the risk of reactivation of HBV in HBsAg+ patients with psoriasis who were treated with biologic agents.
Material and MethodsPatients were selected from those included in the Spanish Registry of Adverse Systemic Drug Reactions in Psoriasis (BIOBADADERM). The methodology used in this prospective registry has been described previously.7 The following study inclusion criteria were applied:
- -
Treatment with anti-TNF or ustekinumab
- -
Serological evidence of past HBV infection before treatment (presence in serum of antibodies against the hepatitis B core antigen [antiHBc] and absence of HBsAg, with presence or absence of hepatitis B surface antibody [antiHBs])3
- -
At least one assessment of HBV DNA during biologic therapy
Reactivation of HBV was defined as detection of HBV DNA in blood±conversion to HBsAg+.3
Hepatitis was defined as alanine aminotransferase (ALT) elevation to 5 times the upper limit of normal.8
Demographic variables (sex), personal history, type of psoriasis, hepatotoxic and/or concomitant immunosuppressive therapies, and type and duration of biologic therapy received (adalimumab [ADA], etanercept [ETA], efalizumab [EFA], infliximab [IFX], and ustekinumab) were extracted retrospectively from the patients’ medical records. In addition, we also extracted laboratory data (ALT, antiHBc, antiHBs, HBsAg, HVB viral load) to determine serological status at the start of treatment, during treatment at various intervals, and at the end of treatment.
The confidence intervals for the incidence of reactivations in the absence of events were calculated using the rule of three, according to the method described by Hanly et al.9
ResultsOf the 1030 patients with psoriasis treated with biologic agents included in the BIOBADADERM registry up until October 2013, 20 met the aforementioned inclusion criteria (5 women and 15 men). A further 24 patients who had psoriasis treated with a biologic agent with serology findings indicative of past HBV infection were identified but not included in this study as no information was available on viral load (viral reactivation could not be ruled out). These patients did not, however, have any clinical manifestations of hepatitis.
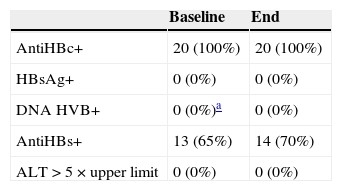
All patients were antiHBc+ and no patient was a carrier of HBsAg at the start of treatment. The median duration of follow-up was 40 months (9-84 months). Patients received a mean of 2 biologic treatments. Thirteen patients received ADA, 14 ETA, 7 IFX, 6 UST, and 1 EFA. The median duration of treatment was 13 months for ADA, 16 months for ETA, 22 months for IFX, 3 months for EFA, and 18 months for ustekinumab. At the end of treatment, no patient had converted to HBsAg+. Of the 13 patients who were antiHBs+ at the start of treatment, 2 had converted to antiHBs- at the end of treatment. Three patients who were antiHBs– at the start of treatment had positive serological titers at the end of treatment. Transaminase levels remained within normal range at the start and end of treatment in all patients. The median number of serological assessments during follow-up was 5. All patients had information on viral load available at the end of treatment and in all cases, the virus was undetectable. The initial viral load was measured in only 7 patients, and the virus was undetectable in all of them (Table 1). No patient received prophylaxis for hepatitis B during therapy. Reactivation according to the definition given above of hepatitis B was not detected in any patients. The risk of HBV reactivation during the study was 0% (95% confidence interval, 0%-14%).
Patient Serological Characteristics (n=20).
| Baseline | End | |
|---|---|---|
| AntiHBc+ | 20 (100%) | 20 (100%) |
| HBsAg+ | 0 (0%) | 0 (0%) |
| DNA HVB+ | 0 (0%)a | 0 (0%) |
| AntiHBs+ | 13 (65%) | 14 (70%) |
| ALT>5×upper limit | 0 (0%) | 0 (0%) |
Currently guidelines for the management of psoriasis list hepatitis B infection as a relative contraindication for the use of anti-TNF agents.10 They recommend that seropositive patients for HBsAg are treated with antiviral therapy before starting treatment. In addition, close monitoring of hepatic function and viral load is recommended in patients with serology suggestive of HBV infection, given that increased viral load is usually the first event in the reactivation process, before increased transaminases and seroconversion.3,11
Anti-TNF drugs are a risk factor for reactivation of hepatitis B, as TNF appears to inhibit viral replication and stimulate T lymphocte cytotoxic response.12 Most cases of HBV reactivation with use of anti-TNF agents have been reported in patients affected by diseases other than psoriasis (inflammatory bowel disease and rheumatic diseases). In the review published by Pérez-Álvarez et al.13 in 2011 (168 patients with past hepatitis B in treatment with anti-TNF agents), the risk of reactivation is reported as 5%, with a mortality rate in these cases of 11%. Lee et al.14 published a meta-analysis of 468 antiHBc+and HBsAg+patients from 9 different studies. The authors found 8 cases of HBV reactivation (1.7%) and in 7 of these HBV DNA was detected.
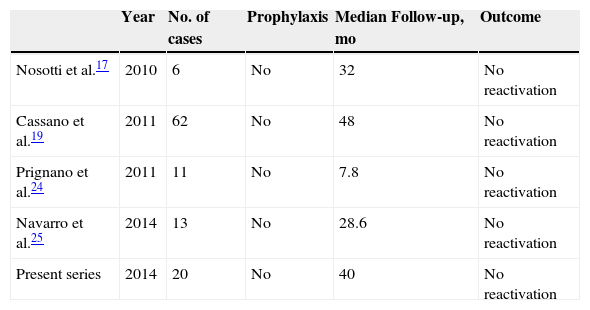
However, the experience in psoriasis is much less extensive, with reports only of sporadic cases or small series, and most reactivations occurred in chronic HBV carriers (HBsAg+).15–22 These patients constitute the group with highest risk of reactivation (20%-50%), and HBV prophylaxis is mandatory.23 In past infection (antiHBc+with HBsAg–), reactivation is rare, although the risk can increase by up to 10% when antiHBs status is lost over time.23 In our study, 2 patients lost antiHBs status, but HBV reactivation did not occur. We have only found 4 studies of patients with psoriasis treated with anti-TNF agents and past hepatitis B (Table 2).17,19,24,25 As in our series of cases, no hepatitis reactivation was observed with the use of anti-TNF agents.
The relationship between HBV and ustekinumab is less well known. It has been suggested that IL12 could be necessary to maintain control over viral replication, promoting Th-1 response, and stimulating interferon gamma production.26 Recently, an isolated case of hepatitis B reactivation was reported in an antiHBc+ and HBsAg– patient in treatment with ustekinumab for psoriasis.27 We have only found 2 studies in patients with hepatitis B and psoriasis treated with ustekinumab. Navarro et al.22 described 5 chronic HBsAg carriers in whom HBV reactivation did not occur with associated antiviral treatment. Chui et al.28 reported 14 patients treated with ustekinumab, and of these, 3 had past hepatitis B. None of these patients received antiviral prophylaxis and there were no reactivations. The 11 remaining patients were chronic carriers of the virus. Seven had not received any antiviral prophylaxis and 2 experienced hepatitis B reactivation.
From the above, 2 main conclusions can be drawn: the first is that the risk of reactivation depends on serological status, as this reflects the immune control of infection and therefore the risk of reactivation. In chronic HBV carriers (HBsAg+), antiviral prophylaxis is recommended due to the high risk of reactivation. In contrast, this risk is lower in patients with past hepatitis B (HBsAg– with antiHBc+). Thus, in clinical practice, the treating physician does not routinely request viral load assessments during follow-up (in our study, such assessments had been requested in 45% of the patients). However, it would be advisable in these patients with HBV+ serology to have an assessment of viral load before starting biologic therapy to rule out occult carriers, which would be an exceptional though possible occurrence. A study of patients with rheumatoid arthritis treated with anti-TNF agents found 4 patients positive for viral DNA among 12 patients with serology consistent with past hepatitis B (antiHBc+, antiHBs–, and HBsAg–).29 One of these had HBV reactivation. The authors also found that the risk of reactivation depended on the disease for which the biologic therapy had been indicated. The reason could potentially be related to the concomitant treatment associated with the biologic agent. Concomitant use of immunosuppressants such as methotrexate, azathioprine, and oral corticosteroids is more frequent in rheumatic diseases and inflammatory bowel disease than in psoriasis. In fact, Loras et al.30 found that the only risk factor for reactivation of hepatitis B in a multivariate analysis was association of 2 or more immunosuppressive agents. Of note, however, is a prospective study published recently with 42 patients with rheumatoid arthritis and past hepatitis B.31 No cases of viral reactivation were detected despite therapy with anti-TNFα agents and disease-modifying agents.
In our series (n=20), in which no reactivation was reported, the maximum risk of hepatitis B reactivation was 14%, according to the rule of three. According to the authors who reported the method, and on this point we fully agree, the absence of events in a study does not imply that it is impossible for an event to occur. They therefore propose a change of approach in the interpretation of these types of findings, and suggest considering the maximum risk of the event according to the sample size of the study instead focusing on the lack of an event. The maximum risk in our study (14%) is probably an overestimate because the number of patients in our cohort is small. In addition, the observational nature of the study excludes patients who have not undergone any assessment of viral load and these patients probably are at lower risk of reactivation. If we add our cases to those published (Table 2), in which there were also no cases of reactivation, the sample size is 112. With this number, the maximum estimated risk of hepatitis B reactivation is 2.7 cases per 100 patients with psoriasis and past hepatitis B who receive treatment with biologic agents, without antiviral prophylaxis, and with a mean follow-up of approximately 30 months.
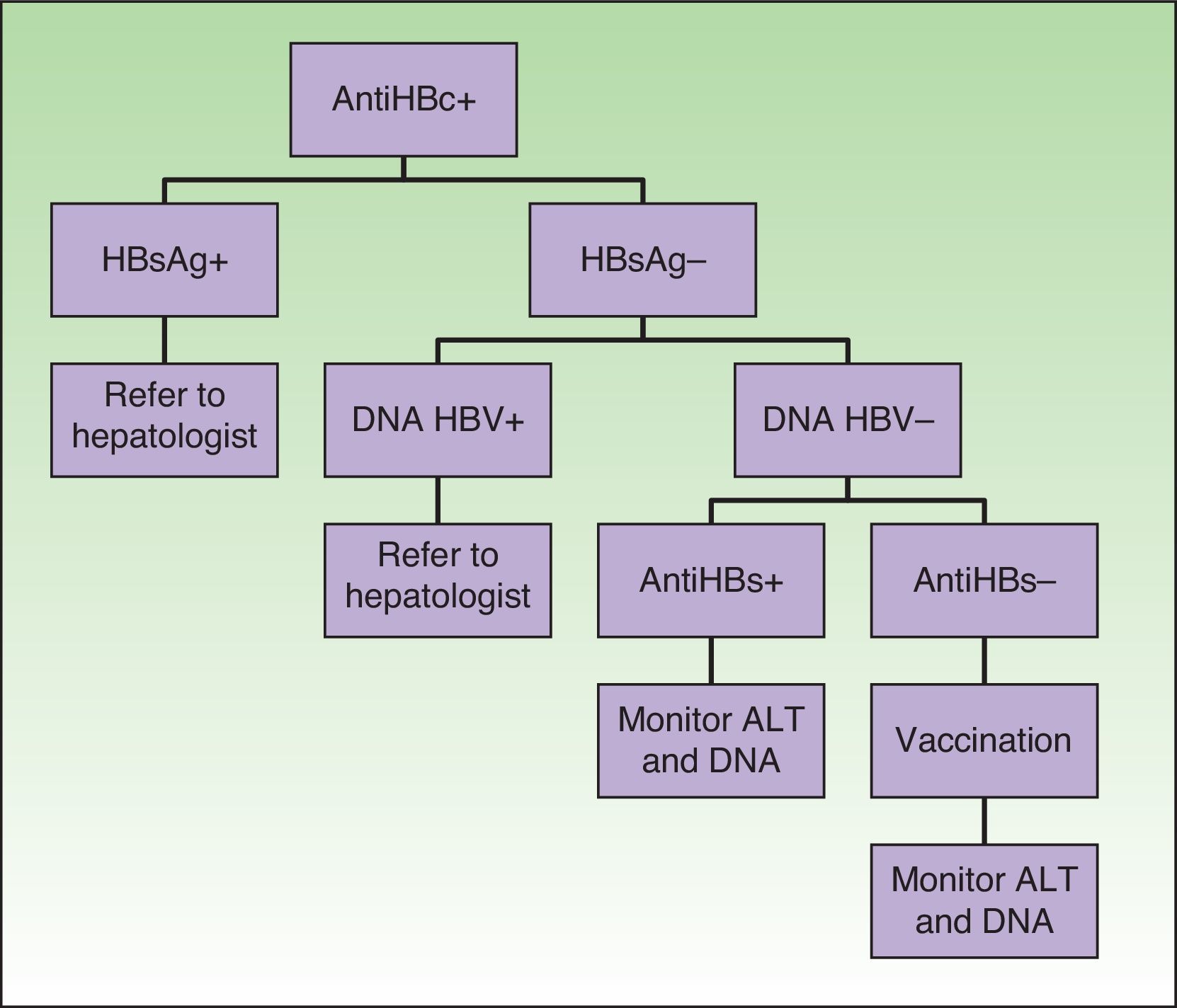
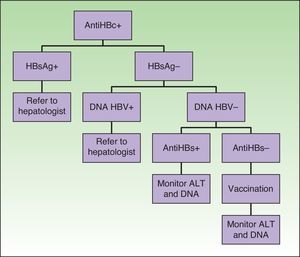
Special mention should be made of patients with serology consistent with past hepatitis B and antiHBs–, in whom it would be advisable to rule out occult carriers (with a higher risk of reactivation and in whom antiviral prophylaxis is recommended, as in chronic carriers) by requesting viral DNA before starting biologic therapy (Fig. 1).14,28,29
The importance of HBV reactivation lies in the fact that it is a preventable event and so the risk of developing a potentially serious or even fatal case of acute hepatitis can be avoided. Close follow-up is recommended in patients with HBV+ serology, and a hepatologist should assess the risk of viral reactivation in these patients before initiating biologic therapy.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors obtained the informed consent of patients and/or subjects mentioned in this article. The informed consent form is located in the archives of the corresponding author.
FundingBIOBADADERM receives funding from the Spanish Academy of Dermatology and Venereology, the Spanish Medicines and Health Products Agency, and the pharmaceutical industry (Abbott, Merck-Schering Plough, Pfizer-Wyeth). The collaborating companies provide similar amounts of funding and do not participate in the analysis or interpretation of the results.
Conflicts of InterestFrancisco Vanaclocha has given talks paid for by Abbott, Pfizer, MSD, and Janssen.
Ignacio García-Doval has been paid travel expenses by Merck/Schering-Plough, Pfizer, and Janssen to attend congresses
Gregorio Carretero has acted as a consultant and researcher for Abbott, Janssen-Cilag, MSD, and Pfizer, and has received fees from Abbott, Jannsen and Pfizer and equipment from MSD and Pfizer.
Esteban Daudén has carried out the following activities: advisory board member, consultant, grant recipient, research support, participation in clinical trials, and paid talks with Abbvie/Abbott, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD-Schering-Plough, Celgene, and Lilly.
Diana Patricia Ruiz-Genao has been a speaker for by Abbott, Pfizer, MSD, and Janssen. M. Mercè Alsina-Gibert has participated as a consultant for Pfizer, Abbvie, Jannsen, and MSD.
Beatriz Pérez-Zafrilla has given talks paid for by Pfizer and Wyeth.
Raquel Rivera has participated as a consultant and researcher for Abbvie, Janssen, MSD, Pfizer-Wyeth, Celgene, Leo Pharma, and Novartis.
The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Sanz-Bueno J, Vanaclocha F, García-Doval I, Torrado R, Carretero G, Daudén E, et al. Riesgo de reactivación de hepatitis B pasada en pacientes con psoriasis tratados con biológicos. Análisis retrospectivo de 20 casos. Registro de BIOBADADERM. Actas Dermosifiliogr. 2015;106:477–482.