Alopecia areata (AA) is an autoimmune disease characterized by non-scaring hair loss and preservation of hair follicles. The information available on disease course, and clinical features of AA is scarce worldwide, and almost nonexistent in Colombia.
ObjectiveTo determine the clinical and sociodemographic characteristics of patients diagnosed with AA who presented to a dermatology consultation in five Colombian cities.
Material and methodsThis was a retrospective and multicenter study on data from an ongoing National Registry of Alopecia Areata in Colombia (RENAAC) collected in Bogota, Cali, Cartagena, Barranquilla, and Medellin, Colombia from March 2022 through April 2023. Data was recorded in a standardized form by trained physicians. The variables were expressed as measures of central tendency and dispersion, and absolute and relative frequencies.
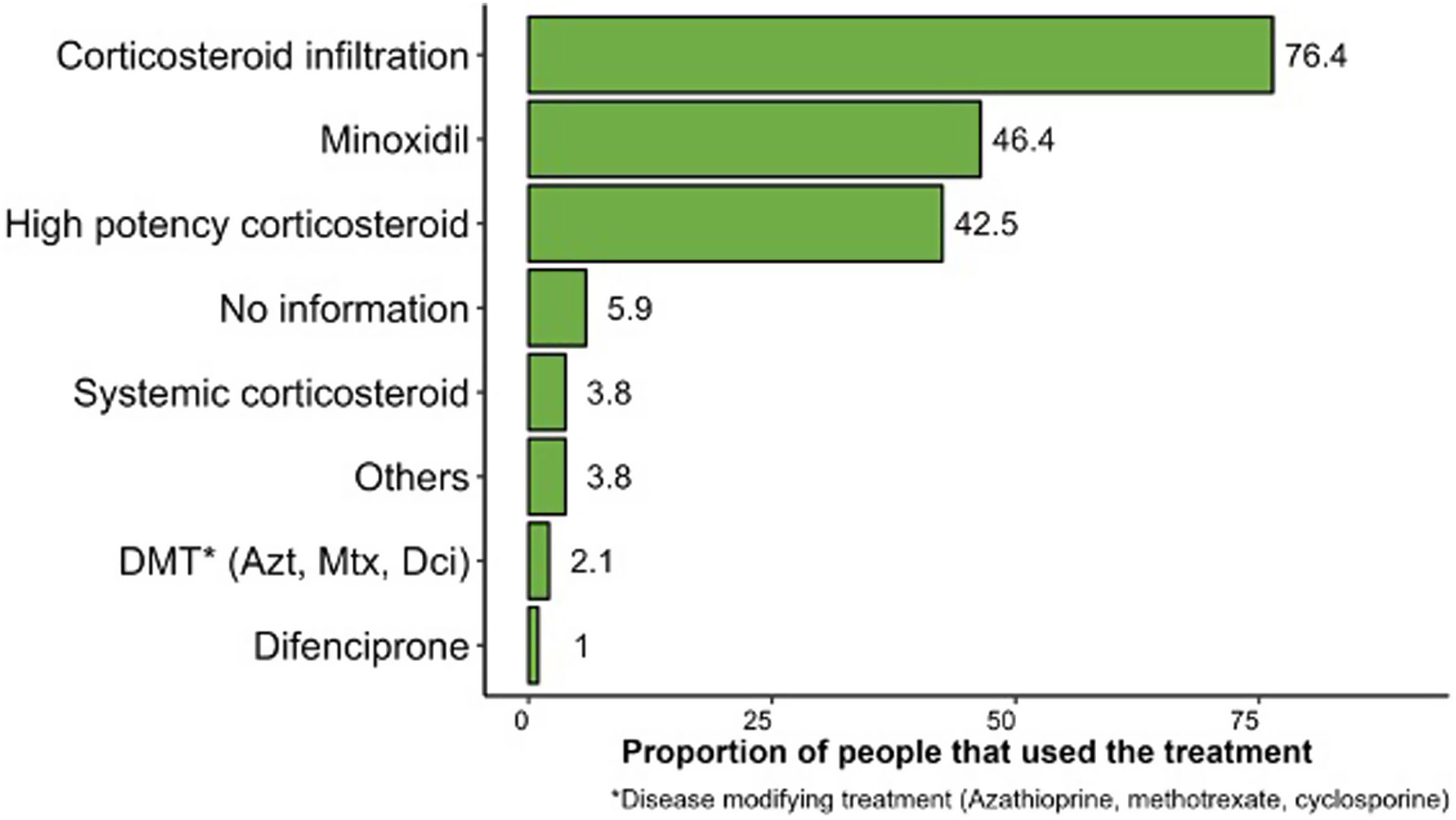
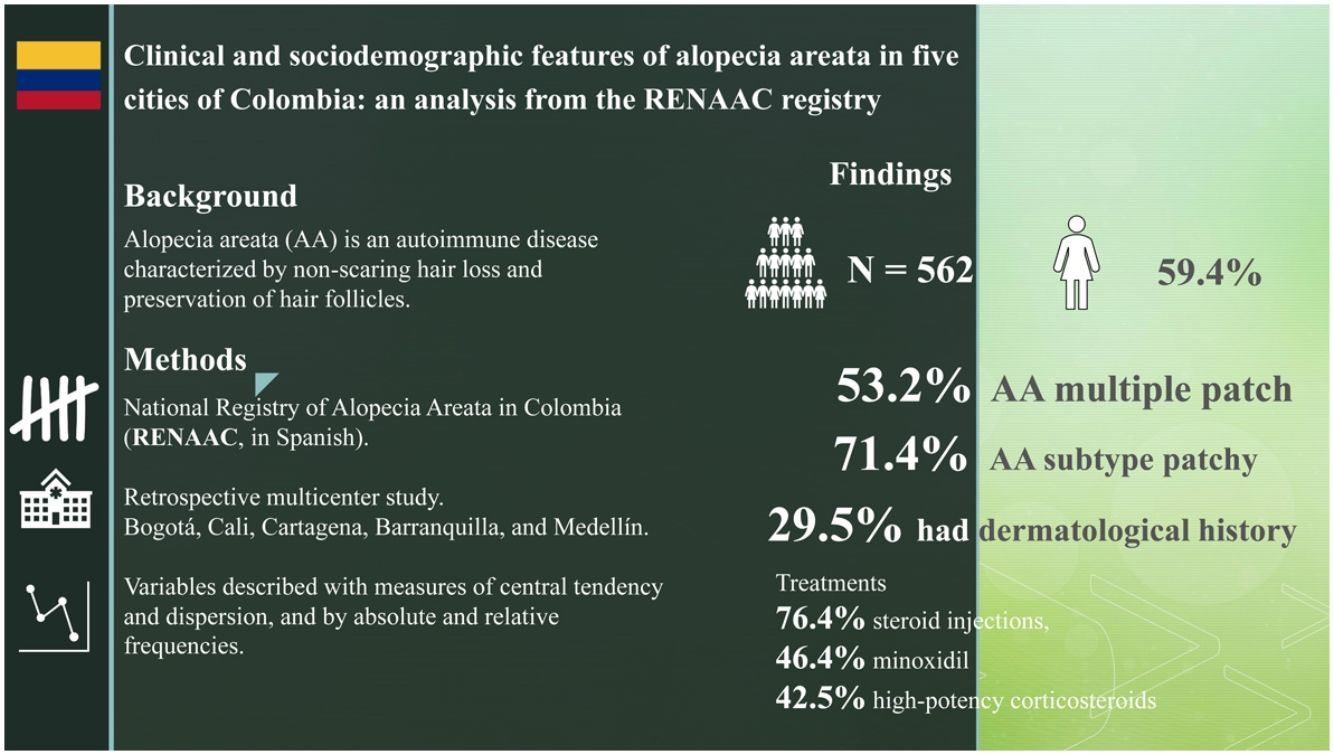
ResultsA total of 562 patients were included, 59.4% of whom were women, aged between 15 and 49 years (63.9%) with a mean disease course of 1.7 years. The most common finding was multiple plaque (53.2%), the predominant AA subtype was patchy (71.4%), and 29.5% of the patients had a past dermatological history, 18.3% had a past endocrinological history, and 8.9% had a past psychiatric history. The treatments most widely used were steroid injections (76.4%), 5% topical minoxidil (46.4%), followed by high-potency corticosteroids (42.5%).
Study limitations and conclusionsAA was slightly predominant in women. As seen in other populations, this disease had an earlier onset in men vs women. Presentation in pediatric age was uncommon. The previous history of other dermatological diseases was checked in almost one third of the patients. Analysis of the co-presentation of AA with other autoimmune diseases is biased due to excluding patients with systemic erythematous lupus from the study.
La alopecia areata (AA) es una enfermedad autoinmune caracterizada por la pérdida de cabello no cicatrizante y la preservación de los folículos pilosos. La información disponible sobre el curso de la enfermedad y sus características clínicas es escasa en todo el mundo y prácticamente ausente en Colombia.
ObjetivoDeterminar las características clínicas y sociodemográficas de los pacientes con diagnóstico de AA que acudieron a una consulta de dermatología en cinco ciudades colombianas.
MétodosEstudio multicéntrico retrospectivo con datos del Registro Nacional de Alopecia Areata en Colombia (RENAAC) en Bogotá, Cali, Cartagena, Barranquilla y Medellín entre marzo de 2022 y abril de 2023. Los datos fueron registrados en forma estandarizada por médicos entrenados. Las variables se describieron con medidas de tendencia central y dispersión, y por frecuencias absolutas y relativas.
ResultadosSe incluyeron 562 pacientes, el 59,4% mujeres, con edades comprendidas entre los 15 y los 49 años (63,9%), con un tiempo medio de evolución de 1,7 años. El tipo de placa más frecuente fue múltiple (53,2%), el subtipo de AA predominante fue parches (71,4%), el 29,5% de los pacientes tenían antecedentes dermatológicos, 18,3% antecedentes endocrinológicos y 8,9% psiquiátricos. Los tratamientos más utilizados fueron las inyecciones de esteroides (76,4%), el minoxidil tópico al 5% (46,4%) y los corticoides de alta potencia (42,5%).
Conclusiones y limitacionesLa AA fue ligeramente predominante en mujeres. Como se observó en otras poblaciones, esta enfermedad tuvo un inicio más temprano en hombres en comparación con las mujeres. La presentación en edad pediátrica fue infrecuente. Se observaron antecedentes de otras afecciones dermatológicas en casi un tercio de los pacientes. El análisis de la comorbilidad de AA con otras enfermedades autoinmunes está sesgado por la exclusión de pacientes con lupus eritematoso sistémico del estudio.
Alopecia areata (AA) is a multifactorial autoimmune disease characterized by non-scarring hair loss and preservation of hair follicles.1 AA is usually recurrent or persistent, especially when hair loss is extensive. Hair loss can take the form of ≥1 well-demarcated round or oval patches. AA can affect the scalp, or the body. If AA compromises the entire scalp it is known as AA totalis, and if it compromises the entire body, it is called AA universalis. Conditions commonly associated with AA are asthma, allergic rhinitis, atopic dermatitis, thyroid disease, and autoimmune diseases, such as thyroiditis and vitiligo.2
AA affects nearly 2% of the world population at some point in life.3 It is estimated that the approximate prevalence of the disease ranges from 0.1% to 0.2%, with a mean age of onset of 31.5 years for men and 36.2 years for women.4 In the United States, the estimated incidence rate of AA is 20.9 per 100,000 persons-years, with a cumulative lifetime incidence rate of 2.1%.4 Epidemiology data on AA in Latin America is scarce, with prevalence rates ranging from 0.2% to 3.8% according to dermatology centers.5
There is no determined predisposition by age or race, with conflicting results regarding sex predisposition.6 The burden of disease estimated by the 2019 global burden of disease (GBD) study is 3580 (2296–5371) disability-adjusted life years (DALYs) due to AA worldwide.7
A few descriptive studies have been conducted among the Colombian population on the distribution of different types of alopecia.8–10 A single-city study indicated that AA accounts for 2.5% of all dermatologic consultations among women. Another multinational study included 256 Colombian patients with only data for Latin American countries was shown stated that AA represents nearly 13% of all types of consultations for alopecia. No data on the clinical behavior of this disease have ever been reported in Colombia. This study presents the first results of an ongoing National Registry of Alopecia Areata in Colombia (RENAAC), whose main objective is to determine the clinical and sociodemographic characteristics of people diagnosed with AA who attended a dermatology consultation in Colombia.
MethodsStudy designThis was a multicenter and retrospective study conducted with information from the health records of patients with AA who attended a dermatology consultation in five Colombian cities. The research protocol was approved by Instituto Médico de Alta Tecnología Oncomédica S.A. (Authorization Code ONC-CEI-CEI-159.2022) and Hospital Alma Mater de Antioquia Ethics and Research Committees (code: IN32-2022).
Health care providers and cities of data collectionIn Colombia, the general system of social security regarding health comprises mandatory affiliation regimes: contributory, subsidized, and special. The contributory regime is financed with contributions from employers, workers, and pensioners, the subsidized one with public resources to take care of poor people, and the special regime covers few unionized workers and members of the armed forces. At the same time, there is a private sector that is accessed by people who can afford it and use it due to a lack of health care coverage or else looking for better access conditions.11 In this registry, although all persons are affiliated to one of the mandatory regimes, the information comes from consultations from private centers.
Data was gathetered by general practitioners specifically trained for this purpose in the offices of three dermatologists who participated in the study located in Bogotá, D.C., Cali, Barranquilla, and Cartagena, all in Colombia. Additionally, we included patients from Hospital Alma Mater de Antioquia located in Medellin, Colombia, a high-complexity hospital with a dermatology unit.
Information was collected from the health records of people diagnosed with AA from March 2022 through April 2023, using a case report form (CRF) designed on the Kobotoolbox® platform. Information on the sociodemographic and clinical variables of people with AA, including treatment patterns was collected.
Inclusion and exclusion criteriaThe study included people of all ages, of both sexes (sex assigned at birth), with a diagnosis of AA (ICD-10 codes: L638 and L639) as reported in their health history, while cases with a diagnosis of systemic lupus erythematosus (ICD-10: M320, M321, M328 and M329), androgenic alopecia (ICD-10: L640, L648 and L649), scarring alopecia (ICD-10: L66), and infections such as syphilis (ICD-10: A50-A539) or tinea capitis (ICD-10: B350) were excluded.
Statistical analysisContinuous variables were expressed as measures of central tendency and dispersion, while the categorical ones were expressed as absolute and relative frequencies. When calculating the treatments received, a private center that was not allowed to access prescription information was eliminated from the overall number of patients analyzed to calculate relative frequencies (n=478). All analyses were conducted using the R project for Statistical Computing, language version 4.3.0.
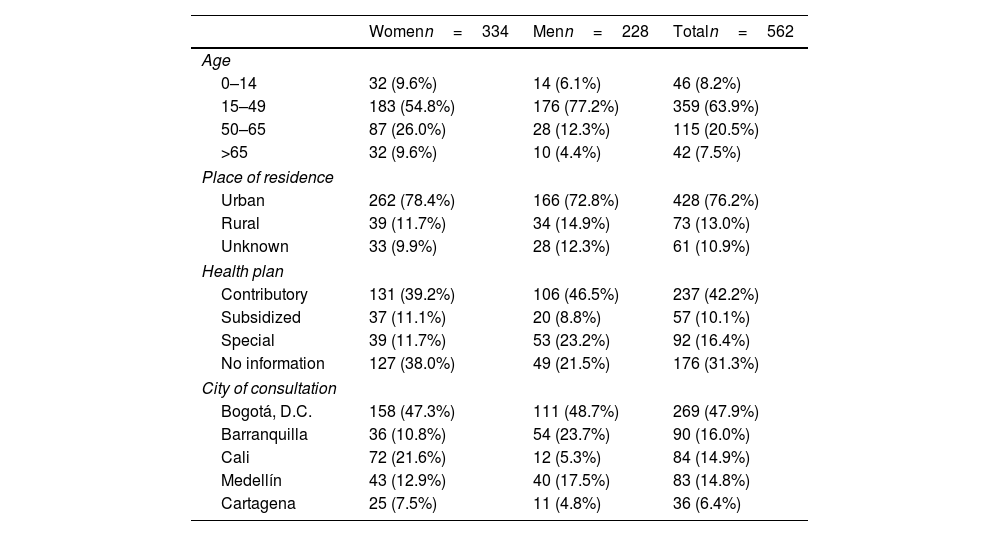
ResultsA total of 562 people with AA were included, with a woman to man ratio of 1.5:1 and a mean age of 39.2 years (standard deviation [SD], 17.1) (Table 1). Most people lived in urban areas (76.2%) and belonged to a contributory regime (42.2%). However, some patients consulted privately, making it difficult to distinguish the type of health plan in 31.3% of the cases and fell into the “no information” category. Data was collected from five Colombian cities: Bogotá (47.9%), Barranquilla (16.0%), Cali (14.9%), Medellín (14.8%), and Cartagena (6.4%).
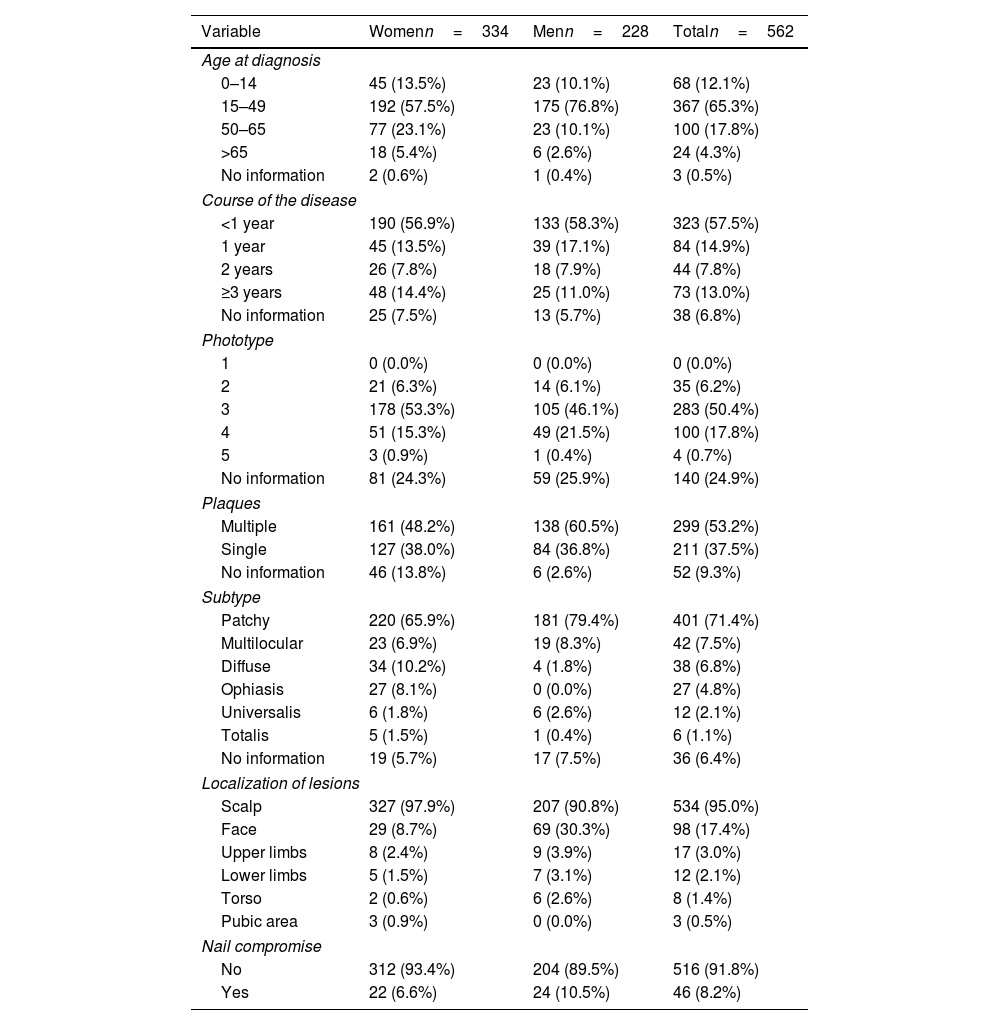
Sociodemographic characteristics of cases of AA.
| Womenn=334 | Menn=228 | Totaln=562 | |
|---|---|---|---|
| Age | |||
| 0–14 | 32 (9.6%) | 14 (6.1%) | 46 (8.2%) |
| 15–49 | 183 (54.8%) | 176 (77.2%) | 359 (63.9%) |
| 50–65 | 87 (26.0%) | 28 (12.3%) | 115 (20.5%) |
| >65 | 32 (9.6%) | 10 (4.4%) | 42 (7.5%) |
| Place of residence | |||
| Urban | 262 (78.4%) | 166 (72.8%) | 428 (76.2%) |
| Rural | 39 (11.7%) | 34 (14.9%) | 73 (13.0%) |
| Unknown | 33 (9.9%) | 28 (12.3%) | 61 (10.9%) |
| Health plan | |||
| Contributory | 131 (39.2%) | 106 (46.5%) | 237 (42.2%) |
| Subsidized | 37 (11.1%) | 20 (8.8%) | 57 (10.1%) |
| Special | 39 (11.7%) | 53 (23.2%) | 92 (16.4%) |
| No information | 127 (38.0%) | 49 (21.5%) | 176 (31.3%) |
| City of consultation | |||
| Bogotá, D.C. | 158 (47.3%) | 111 (48.7%) | 269 (47.9%) |
| Barranquilla | 36 (10.8%) | 54 (23.7%) | 90 (16.0%) |
| Cali | 72 (21.6%) | 12 (5.3%) | 84 (14.9%) |
| Medellín | 43 (12.9%) | 40 (17.5%) | 83 (14.8%) |
| Cartagena | 25 (7.5%) | 11 (4.8%) | 36 (6.4%) |
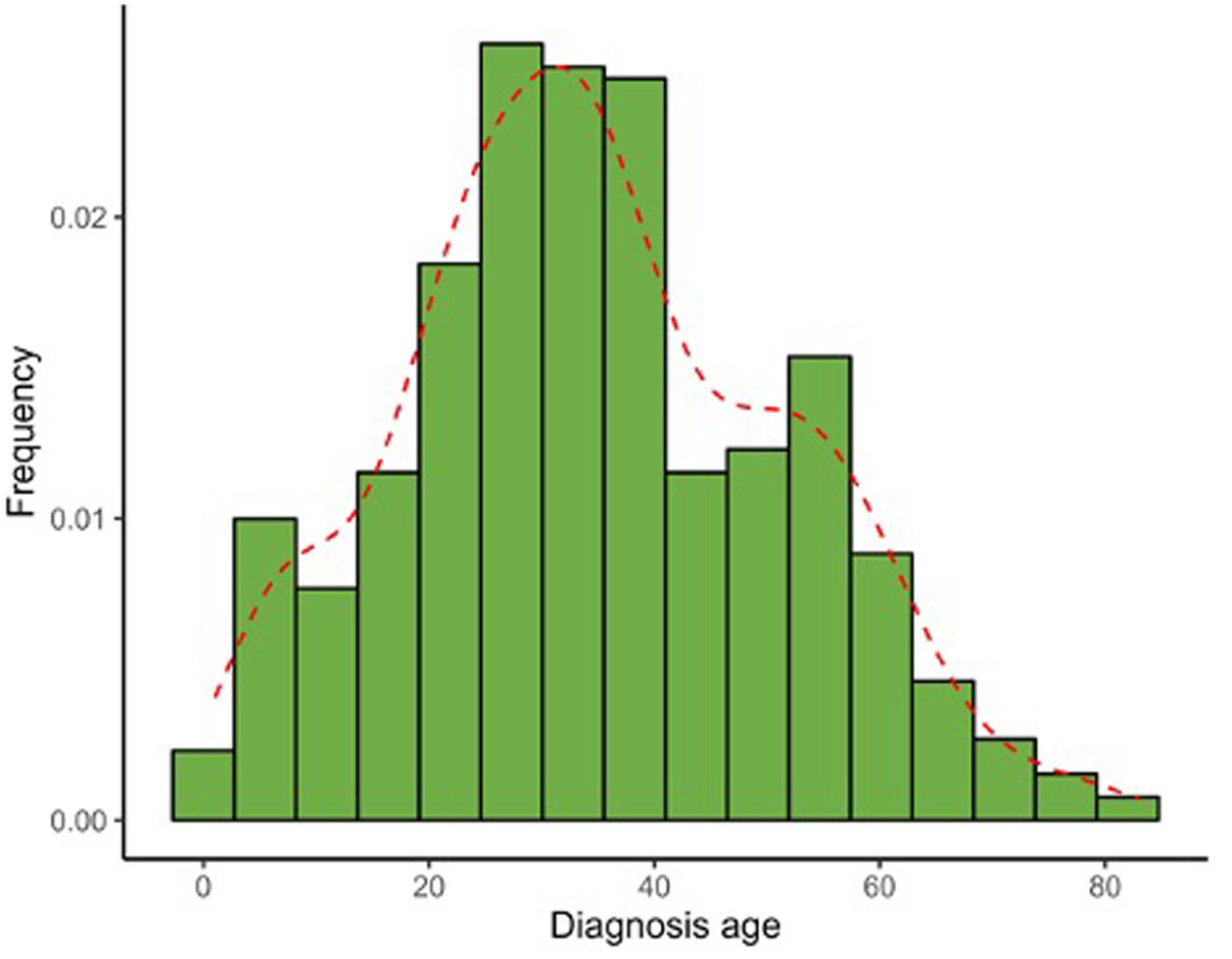
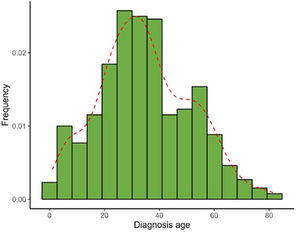
Table 2 illustrates the clinical characteristics of AA seen in the health records studied. The mean age at diagnosis was 35.0 years (SD, 17.0) and was notably lower in men (31.7 years; SD, 15.1) vs women (37.3 years, SD, 17.9). Most cases were diagnosed between the ages of 25 and 30 years old, evidencing that the diagnosis of AA usually occurs before the age of 50 (77.4%), although a 2nd peak was also reported between the ages of 50 and 56 years old (Fig. 1). The course of the disease in the 1st consultation ranged from 1 month to 40 years and most people (57.5%) consult <1 year after the onset of the lesions.
Clinical characteristics of cases of AA included in the study.
| Variable | Womenn=334 | Menn=228 | Totaln=562 |
|---|---|---|---|
| Age at diagnosis | |||
| 0–14 | 45 (13.5%) | 23 (10.1%) | 68 (12.1%) |
| 15–49 | 192 (57.5%) | 175 (76.8%) | 367 (65.3%) |
| 50–65 | 77 (23.1%) | 23 (10.1%) | 100 (17.8%) |
| >65 | 18 (5.4%) | 6 (2.6%) | 24 (4.3%) |
| No information | 2 (0.6%) | 1 (0.4%) | 3 (0.5%) |
| Course of the disease | |||
| <1 year | 190 (56.9%) | 133 (58.3%) | 323 (57.5%) |
| 1 year | 45 (13.5%) | 39 (17.1%) | 84 (14.9%) |
| 2 years | 26 (7.8%) | 18 (7.9%) | 44 (7.8%) |
| ≥3 years | 48 (14.4%) | 25 (11.0%) | 73 (13.0%) |
| No information | 25 (7.5%) | 13 (5.7%) | 38 (6.8%) |
| Phototype | |||
| 1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 2 | 21 (6.3%) | 14 (6.1%) | 35 (6.2%) |
| 3 | 178 (53.3%) | 105 (46.1%) | 283 (50.4%) |
| 4 | 51 (15.3%) | 49 (21.5%) | 100 (17.8%) |
| 5 | 3 (0.9%) | 1 (0.4%) | 4 (0.7%) |
| No information | 81 (24.3%) | 59 (25.9%) | 140 (24.9%) |
| Plaques | |||
| Multiple | 161 (48.2%) | 138 (60.5%) | 299 (53.2%) |
| Single | 127 (38.0%) | 84 (36.8%) | 211 (37.5%) |
| No information | 46 (13.8%) | 6 (2.6%) | 52 (9.3%) |
| Subtype | |||
| Patchy | 220 (65.9%) | 181 (79.4%) | 401 (71.4%) |
| Multilocular | 23 (6.9%) | 19 (8.3%) | 42 (7.5%) |
| Diffuse | 34 (10.2%) | 4 (1.8%) | 38 (6.8%) |
| Ophiasis | 27 (8.1%) | 0 (0.0%) | 27 (4.8%) |
| Universalis | 6 (1.8%) | 6 (2.6%) | 12 (2.1%) |
| Totalis | 5 (1.5%) | 1 (0.4%) | 6 (1.1%) |
| No information | 19 (5.7%) | 17 (7.5%) | 36 (6.4%) |
| Localization of lesions | |||
| Scalp | 327 (97.9%) | 207 (90.8%) | 534 (95.0%) |
| Face | 29 (8.7%) | 69 (30.3%) | 98 (17.4%) |
| Upper limbs | 8 (2.4%) | 9 (3.9%) | 17 (3.0%) |
| Lower limbs | 5 (1.5%) | 7 (3.1%) | 12 (2.1%) |
| Torso | 2 (0.6%) | 6 (2.6%) | 8 (1.4%) |
| Pubic area | 3 (0.9%) | 0 (0.0%) | 3 (0.5%) |
| Nail compromise | |||
| No | 312 (93.4%) | 204 (89.5%) | 516 (91.8%) |
| Yes | 22 (6.6%) | 24 (10.5%) | 46 (8.2%) |
The patients’ predominant phototype was type III. Multiple plaques (53.2%) were more prevalent than single plaques (37.5%). The most frequent subtype of AA was patchy (71.4%), and the most widely recorded locations of AA were scalp (95.0%) and face (17.4%). Nail compromise was uncommon (only 8.2%).
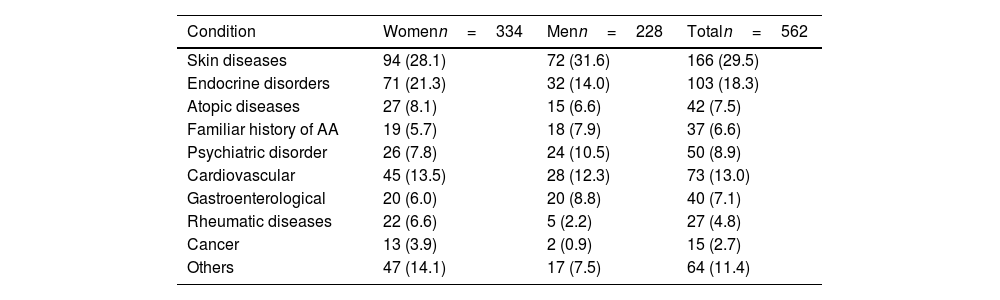
Regarding health history, 37 people (6.6%) had a family history of AA. In addition, 7.5% had a personal history of atopy with, at least, 1 allergic co-morbidity (rhinitis [4.8%], asthma [0.7%], allergic conjunctivitis [0.2%], or atopic dermatitis [2.6%]). Notably, almost a third of the patients (29.5%) had a history of skin diseases (n=166) (Table 3).
Other relevant past medical history in the study population.
| Condition | Womenn=334 | Menn=228 | Totaln=562 |
|---|---|---|---|
| Skin diseases | 94 (28.1) | 72 (31.6) | 166 (29.5) |
| Endocrine disorders | 71 (21.3) | 32 (14.0) | 103 (18.3) |
| Atopic diseases | 27 (8.1) | 15 (6.6) | 42 (7.5) |
| Familiar history of AA | 19 (5.7) | 18 (7.9) | 37 (6.6) |
| Psychiatric disorder | 26 (7.8) | 24 (10.5) | 50 (8.9) |
| Cardiovascular | 45 (13.5) | 28 (12.3) | 73 (13.0) |
| Gastroenterological | 20 (6.0) | 20 (8.8) | 40 (7.1) |
| Rheumatic diseases | 22 (6.6) | 5 (2.2) | 27 (4.8) |
| Cancer | 13 (3.9) | 2 (0.9) | 15 (2.7) |
| Others | 47 (14.1) | 17 (7.5) | 64 (11.4) |
Absolute no. of patients and relative frequency in parenthesis (%) are shown.
Additionally, an endocrinological disorder was diagnosed in 18.3% of the cases, being hypothyroidism the most frequent condition (12.3%), especially in women, with a 3.3:1 ratio vs men. This history was mainly observed in patients older than 15 years and was distributed by age group as follows: 41.7% from 15 to 49 years, 38.8% from 50 to 65 years, and 17.5% >65 years.
Psychiatric history was frequent (n=50, 8.9%), being anxiety and depression the most common diseases (n=46), with similar data reported both sexes, and mainly focused in the 15–49 (60.9%) and 50–65 age groups (28.3%).
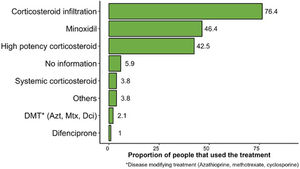
Regarding the type of treatment, steroid injections (76.4%), and more specifically triamcinolone, 5% topical minoxidil (46.4%), and high-potency corticosteroids (42.5%) were the most widely used therapeutic regimens to treat AA (Fig. 2).
Recurrence of AA was observed in 13.9% of the patients, with a woman to man ratio of 1.1:1. These recurrences range from 1 to 5 throughout the course of the disease, being 1 and 2 recurrences the most frequent pattern. Based on the years of disease progression, the maximum number of recurrences is three per year.
DiscussionThis study describes the main clinical and sociodemographic characteristics of people with AA who attended a dermatology consultation in five main cities of Colombia. A higher frequency of women was observed, with a concentration in terms of age and diagnosis within the 3rd decade of life and with two important peaks of diagnosis, as well as a family history of AA, and a personal history of other comorbidities, such as hypothyroidism or mental illness. The most common therapies of AA were identified and general relapse patterns were described. Given the scarce literature available on the characteristics of AA in Latin America, this pioneering study contributes to the clinical understanding of how the disease behaves in our region.
As mentioned earlier, during this first phase of data collection, a higher frequency of cases was found in women vs men. These results are similar to those reported by other authors12,13; nevertheless, a predominance of men has also been found,14 making the relationship between AA and sex inconclusive in terms of evidence.6 Our findings can also be explained by the difference in the frequency of attending a health consultation, which, according to the scientific medical literature available, tends to be higher in women.15
In this cohort, on average, women were older than men, and men were diagnosed at an earlier age, which is consistent with what was reported by Lundin et al. in 2014.13 Pediatric AA was not common as it has been found in studies from other regions. Late onset was recorded in 22.1% of the cases, with a higher prevalence in women, which is also consistent with other studies.16
Regarding the frequency of diagnosis of AA, along with other autoimmune diseases, we must clarify that the presence of most autoimmune diseases was among the exclusion criteria, which is a limitation for this part of the analysis. Also, the etiology of AA has not been fully described but is known to be multifactorial, with chronic inflammation and infections as triggers, alongside genetic predisposition and environmental factors.1
AA may coexist with other autoimmune diseases such as celiac disease, rheumatoid arthritis, systemic lupus erythematosus, and thyroid disease; in fact, suffering from AA increases the risk of suffering from these types of diseases.17 Genes associated with AA have been found to be involved in the development of other autoimmune diseases such as type 1 diabetes, multiple sclerosis, psoriasis, inflammatory bowel disease, or autoimmune thyroid diseases.1,17
Regarding medical history, the family history of AA is consistent with other studies conducted in Mexico, Singapore, and China, in which it was found in 0–8.6% of the cases.12,18,19 In addition to indicating genetic influence, positive family history may be a relevant prognostic factor during the anamnesis of patients.
Regarding the history of atopy, rhinitis presented the highest frequency. However, this rate is lower than the one observed in the overall population in studies conducted in Colombia (32%).20 Although former studies have reported that AA increases the risk of atopic dermatitis and is linked to atopy, based on observations of higher presentation of this atopic skin disease in cases of AA vs matched controls,21 we did not seen a frequency rate higher than the rates reported in Colombia (14%).20 Additionally, growing evidence linking intestinal microbiota with AA suggests that intestinal bacteria may play an important role in the development of autoimmune diseases, although results are inconclusive and need further research.1,22
Regarding endocrinological history, hypothyroidism was the most frequently reported disease. There is evidence of an existing relationship between abnormal values of thyroid-stimulating hormone (TSH) and AA,23 and an increased risk of developing AA when presenting thyroid diseases such as thyrotoxicosis, Graves’ disease and thyroiditis, which suggests shared pathophysiological mechanisms between AA and thyroid disease.24 In a 2019 review, Kinoshita et al. suggested that AA is more frequently associated with thyroid peroxidase antibodies (TPO-Ab) and thyroglobulin antibodies (TG-Ab) than with hypo and hyperthyroidism. Nonetheless, due to certain limitations of the review, this statement is not entirely conclusive.25
Stress may also be a trigger factor for AA, although this is controversial, as some studies report that this association has not been found,26 despite suggestive evidence that there are biological factors that link the central nervous system (CNS) to acute stress in AA.1 What is undeniable is that AA changes physical appearance, which can have a profound psychological impact on the patients. Some studies report a higher prevalence of psychiatric comorbidities in people with AA, particularly anxiety, depression, attention deficit hyperactivity disorder, and psychotic disorders, leading to the deterioration of the quality of life of both patients and their family, and differential psychosocial consequences mainly in women and children.23,27 Due to the substantial psychosocial burden associated to AA, successful comprehensive treatment is critical, beyond clinical results.
Current therapeutic options are beneficial in resolving acute episodes. However, there is no treatment that resolves AA completely. Treatments vary according to the training of the dermatologist and factors such as the patient's age, the extent and duration of the disease, or the condition of the scalp, among others.28 Most treatments, including steroids, topical immunotherapy, topical minoxidil, anthralin, and immunosuppressants, focus on inhibiting autoimmunity and reversing AA, although results vary from one patient to the other and depending on the therapy being used.28 The treatment regimens mentioned above are consistent with the results of the RENAAC. In this registry, there were no reports on the use of Janus Kinase (JAK) inhibitors for AA treatment, which could be interesting in terms of later comparisons of effectiveness on these promising therapies for the most severe cases.29 Currently, in Colombia, while baricitinib and tofacitinib are endorsed for treating various chronic inflammatory diseases, they are not yet authorized to be used vs alopecia areata (AA). However, adding these innovative treatments to Colombia's list of authorized drugs vs AA, coupled with the ongoing maintenance of this registry, will enable the generation of real-world evidence on their efficacy in the years to come. This approach will facilitate comparative analyses of recurrence rates under different treatment scenarios.
Finally, the nail involvement reported in this study was infrequent; however, it may be due to the non-recording of this information, as former works report rates of lamina involvement in 9–64.1% of the cases,30 which reinforces the importance of carefully recording basic as well as detailed information in the patient's health history.
This study is limited by its retrospective nature. Additionally, the registry is derived from health records, which restricts the exhaustivity of the information and does not allow delving into other aspects of interest. The current version of this registry did not collect detailed information on prescriptions (i.e., dosages or specific molecule names for some drug categories such as immunosuppressants). However, as this is an ongoing project, further versions of the forms will be improved with more detailed questions.
ConclusionThe creation of this first registry of people with AA contributes to the knowledge of the clinical behavior of the disease in Colombia and the Latin American region. AA was slightly predominant in women with a ratio of 1.5:1 vs men, with an earlier onset being described in men. The presence of dermatological, endocrine, and psychiatric comorbidities was frequent, which should be included in patient care protocols.
FundingThis work was supported through a grant by Instituto Científico Pfizer Colombia (ICPC).
Conflict of interestsThe authors state that they have no conflict of interests.
We wish to thank the dermatologists who gave access to their patient's health records, the administrative teams of ALZAK Foundation (Cartagena) and Funinderma (Bogotá), and the Research and Innovation area of Hospital Alma Mater de Antioquia, Antioquía, Colombia.