Studies on the use of systemic therapy for psoriasis in pediatric patients are scarce. The main aim of this study was to describe the systemic treatments used for moderate to severe psoriasis in pediatric clinical settings. The second aim was to describe the effectiveness and safety of these treatments.
Material and methodsDescriptive, cross-sectional, multicenter study of patients under 18 years of age with moderate to severe psoriasis who were being treated or had been treated with a systemic drug (conventional or biologic) or phototherapy. We recorded demographic and clinical information, treatments received, tolerance, adverse effects, and response to treatment.
ResultsData were collected for 40 patients (60% female; mean age, 13 years) who had received 63 treatments in total. The most common first treatment (n=40) was phototherapy (administered to 68% of patients), followed by acitretin (15%). The most common treatments overall (n=63) were phototherapy (57%) and methotrexate (16%). At week 12 (evaluation of systemic treatment and phototherapy), 66% of the patients were classified as good responders and 22% as partial responders. The respective rates for week 24 (evaluation of systemic treatment only) were 36% and 32%. The treatments were well tolerated (97%) and adverse effects were reported in just 11% of cases. There were no treatment discontinuations because of adverse effects.
ConclusionsPhototherapy, followed by methotrexate, were the most common treatment for moderate to severe psoriasis in this series of patients under 18 years. The treatments showed a favorable safety profile and were associated with a good response rate of 66% at week 12 (systemic treatment and phototherapy) and 36% at week 24 (systemic treatment only).
Los trabajos sobre el tratamiento sistémico de la psoriasis en edad pediátrica son escasos. El objetivo principal de este trabajo consistió en describir qué tratamientos sistémicos se emplean en práctica clínica en psoriasis moderada-grave en edad pediátrica. Secundariamente se describió la efectividad y perfil de seguridad de dichos tratamientos.
Materiales y métodosEstudio descriptivo transversal multicéntrico, de los pacientes con psoriasis moderada-grave, que siendo menores de 18 años estuviesen recibiendo o hubieran recibido tratamiento sistémico (clásico o biológico) o fototerapia. Se recogieron datos clínico-demográficos, tipo de tratamiento recibido, y tolerancia, efectos indeseables y respuesta al mismo.
ResultadosSe obtuvieron datos de 40 pacientes (60% sexo femenino, edad media 13 años), que realizaron 63 ciclos de tratamiento. Teniendo en cuenta el primer tratamiento (n=40), la fototerapia fue la opción más frecuente (68%), seguida de acitretino (15%). Considerando el total de ciclos de tratamiento (n=63), el tratamiento más frecuentemente empleado fue la fototerapia (57%), seguida de metotrexato (16%). En la semana 12 (incluye evaluación de fototerapia), el 66% y el 22% fueron buenos respondedores o respondedores parciales, respectivamente. En la semana 24 (datos exclusivos sobre fármacos sistémicos), el 36% y el 32% continuaron con respuestas buenas y parciales. Los tratamientos fueron bien tolerados (97%) y los efectos indeseables escasos (11%), sin que en ningún caso motivasen la suspensión del fármaco.
ConclusionesEn la población menor de 18 años con psoriasis moderada-grave evaluada la fototerapia fue el tratamiento más utilizado, seguida de metotrexato. Los tratamientos consiguieron porcentajes de buenos respondedores del 66% en la semana 12 (incluida fototerapia), y del 36% en la semana 24 (fármacos sistémicos sin fototerapia), presentando un buen perfil de seguridad.
Psoriasis is not uncommon in childhood, but only scant information is available on the epidemiology of the condition and its management with systemic therapy in the pediatric population.1,2
Studies on pediatric psoriasis report that around 8% of these patients have moderate to severe disease requiring treatment with phototherapy or systemic drugs.3 Given the chronic nature of psoriasis and the need for prolonged treatment, it is important to carefully select the best treatment option based on both its effectiveness and the safety profile, especially in very young children.4
Our principal objective was to describe the systemic treatments used in clinical practice to treat moderate to severe psoriasis in children.
Our second aim was to describe the effectiveness and safety of these treatments.
Material and MethodsThis was a descriptive, cross-sectional, multicenter study carried out in the dermatology units managed by the 7 health districts (Estructuras de Gestión Integrada) that make up the Galician Public Health network (SERGAS). We included patients with moderate to severe psoriasis aged under 18 years who were currently receiving or had been treated with systemic drugs (classic or biologic) or phototherapy between January 2005 and August 2017. The decision to include patients aged up to 17 years (and not just pediatric patients up to 12 or 14 years of age) was taken because 18 is the age at which a patient is considered to be an adult. It is also the lowest age at which most systemic treatments (with the exception of etanercept5 and more recently adalimumab6 and ustekinumab2) are approved for use in this setting (the prescription of other systemic treatments to children aged under 18 years is deemed to be “off-label” use).
The following data was collected for each patient: clinical and demographic information, the results of a basic laboratory workup, the characteristics and severity of psoriasis (assessed using the Psoriasis Area and Severity Index [PASI] or the Body Surface Area [BSA]), and the presence of comorbidities. Psoriasis was considered to be moderate to severe when the PASI was at least 10 or when the PASI was under 10 but the condition failed to respond to appropriate treatment. With the BSA, the cut-off point for moderate to severe psoriasis was 10%. We also collected data on the duration and dosage of and response to systemic treatments, as well as on adverse events, tolerance, and the reasons for withdrawal of treatment.
Treatment response was assessed at weeks 12 and/or 24 for all the treatments except phototherapy, which was only assessed at week 12. The reason for this difference is that opposed to systemic therapies, in routine clinical practice in our units, we do not perform a standardized assessment at week 24 after finishing phototherapy.
Taking into account the PASI, the BSA, and the descriptive information recorded by the dermatologist in the patients’ clinical record, definitions were established for classifying patients as good responders, partial responders, and non-responders. A good response was defined as an improvement in the PASI score of at least 75% over baseline (PASI 75) at week 12 or week 24. Also included in this group were patients whose clinical history indicated: “Very good or excellent outcome”, “almost complete clearing” of psoriasis, “total or almost total remission”, or “withdrawal of treatment owing to a good response”. When no PASI value was available, a BSA of 0% or 1% was also considered as a good response. Partial response was defined as an improvement of more than 50% but less than 75% in the PASI score or an indication in the clinical record describing the outcome as “partial improvement”, “partial clearance”, or “partial remission”. Patients who did not achieve a PASI 50 response or whose medical record included the terms “little improvement”, “minimal effect”, “no improvement”, “lesions unchanged with respect to start of treatment”, or “withdrawal of treatment due to lack of response” were classified as non-responders.
We distinguished between “adverse events” (treatment-related clinical signs or abnormalities in test results observed by the physician and recorded in the medical record, whether or not they resulted in withdrawal of treatment) and “intolerance” (subjective symptoms or discomfort reported by the patient or their parents thought to be related to the treatment).
The motives for withdrawal of treatment were as follows: good response, poor response, lack of response, loss of response (secondary treatment failure), use of an intermittent treatment regimen, express wish of the patient or the parents, poor adherence to therapy, therapy limited by a clinical trial, interruption of therapy for medical reasons (surgery, active infection, or the need for other treatments that might interact or were contraindicated with the treatment in question), intolerance, and adverse effects.
The protocol of this study was reviewed and approved by the Clinical Research Ethics Committee of Galicia. It was also classified by the Spanish Agency for Medicines and Health Products (AEMPS) as a post-authorization study with a design other than prospective follow-up (EPA-OD).
Statistic AnalysisAll the data collected were recorded on an Excel spreadsheet and analyzed with the R Statistics program (version R i386 3.4.2). We calculated the frequency distribution for qualitative variables and the mean and standard deviation for quantitative variables. The Chi square test (or Fisher's exact test if appropriate depending on the number of observations) was used to determine the relationships between qualitative variables. Student's T test was use to compare quantitative variables by treatment group. Statistical significance was set at P less than .05.
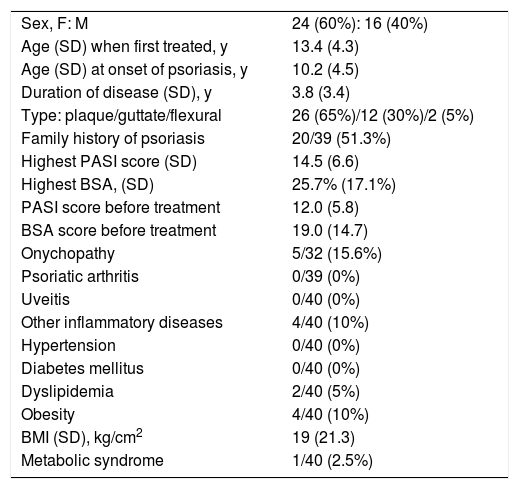
ResultsThe final analysis included 40 patients (60% female, mean age at the start of the first treatment 13 years, 65% with plaque psoriasis) who underwent a total of 63 treatment cycles. The characteristics of the study population are shown in Table 1.
Characteristics of Patients Under 18 Years of Age With Moderate to Severe Psoriasis on Systemic Treatment (Including Phototherapy) (n=40).
| Sex, F: M | 24 (60%): 16 (40%) |
| Age (SD) when first treated, y | 13.4 (4.3) |
| Age (SD) at onset of psoriasis, y | 10.2 (4.5) |
| Duration of disease (SD), y | 3.8 (3.4) |
| Type: plaque/guttate/flexural | 26 (65%)/12 (30%)/2 (5%) |
| Family history of psoriasis | 20/39 (51.3%) |
| Highest PASI score (SD) | 14.5 (6.6) |
| Highest BSA, (SD) | 25.7% (17.1%) |
| PASI score before treatment | 12.0 (5.8) |
| BSA score before treatment | 19.0 (14.7) |
| Onychopathy | 5/32 (15.6%) |
| Psoriatic arthritis | 0/39 (0%) |
| Uveitis | 0/40 (0%) |
| Other inflammatory diseases | 4/40 (10%) |
| Hypertension | 0/40 (0%) |
| Diabetes mellitus | 0/40 (0%) |
| Dyslipidemia | 2/40 (5%) |
| Obesity | 4/40 (10%) |
| BMI (SD), kg/cm2 | 19 (21.3) |
| Metabolic syndrome | 1/40 (2.5%) |
Abbreviations: BMI, Body Mass Index; BSA, Body Surface Area; F, female; M, male; PASIP, soriasis Area and Severity Index.
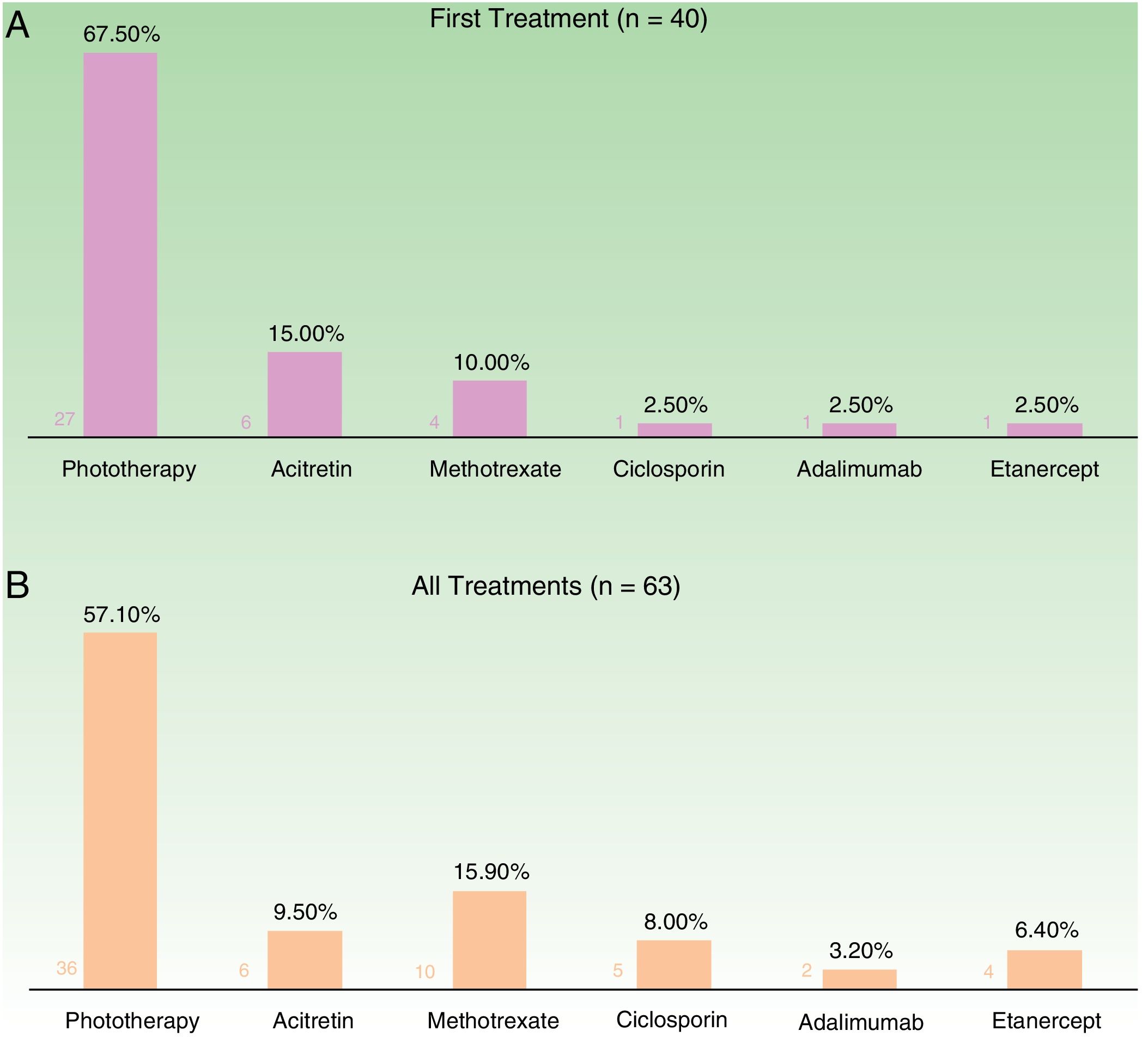
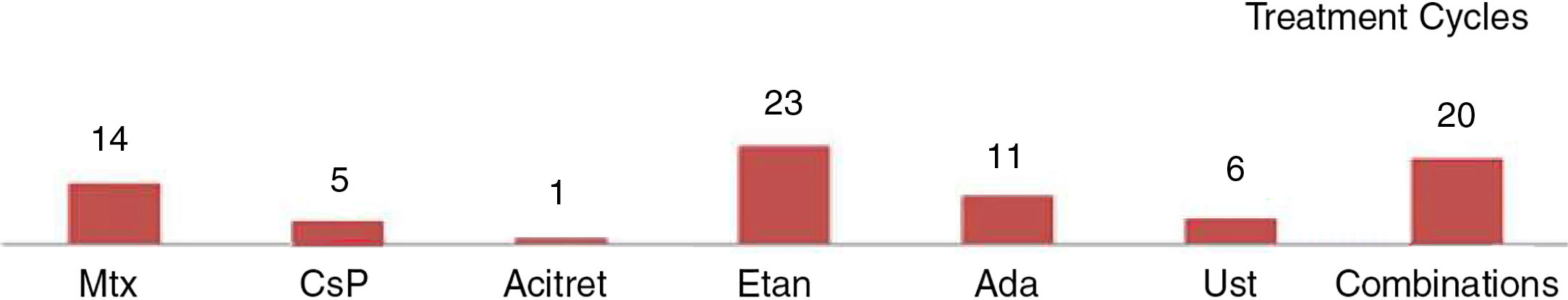
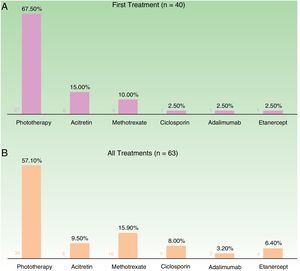
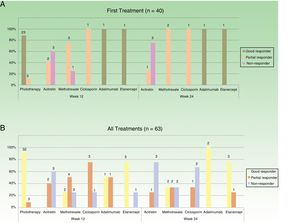
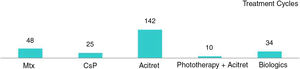
In the case of the first treatment administered (n = 40), phototherapy was the option most frequently chosen (68%), followed by acitretin (15%) (Fig. 1). In the analysis of the outcomes recorded at week 12 (which includes phototherapy), 66% (25/38) of the patients were classified as good responders and 24% (9/38) as partial responders. At week 24 (assessment of classic systemic drugs and biologic agents only), 25% of the patients (3/12) were classified as good responders and 50% as partial responders.
In the analysis of all of the treatment cycles (n=63), the most frequent treatment was phototherapy, which accounted for 57% of cycles, followed by methotrexate, accounting for 16% (Fig. 1). At week 12, 66% (38/58) were classified as good responders and 22% (13/58) as partial responders. At week 24 (analysis of classic systemic and biologic therapies only), 36% of patients (8/22) continued to have a good response and 32% (7/22) had a partial response.
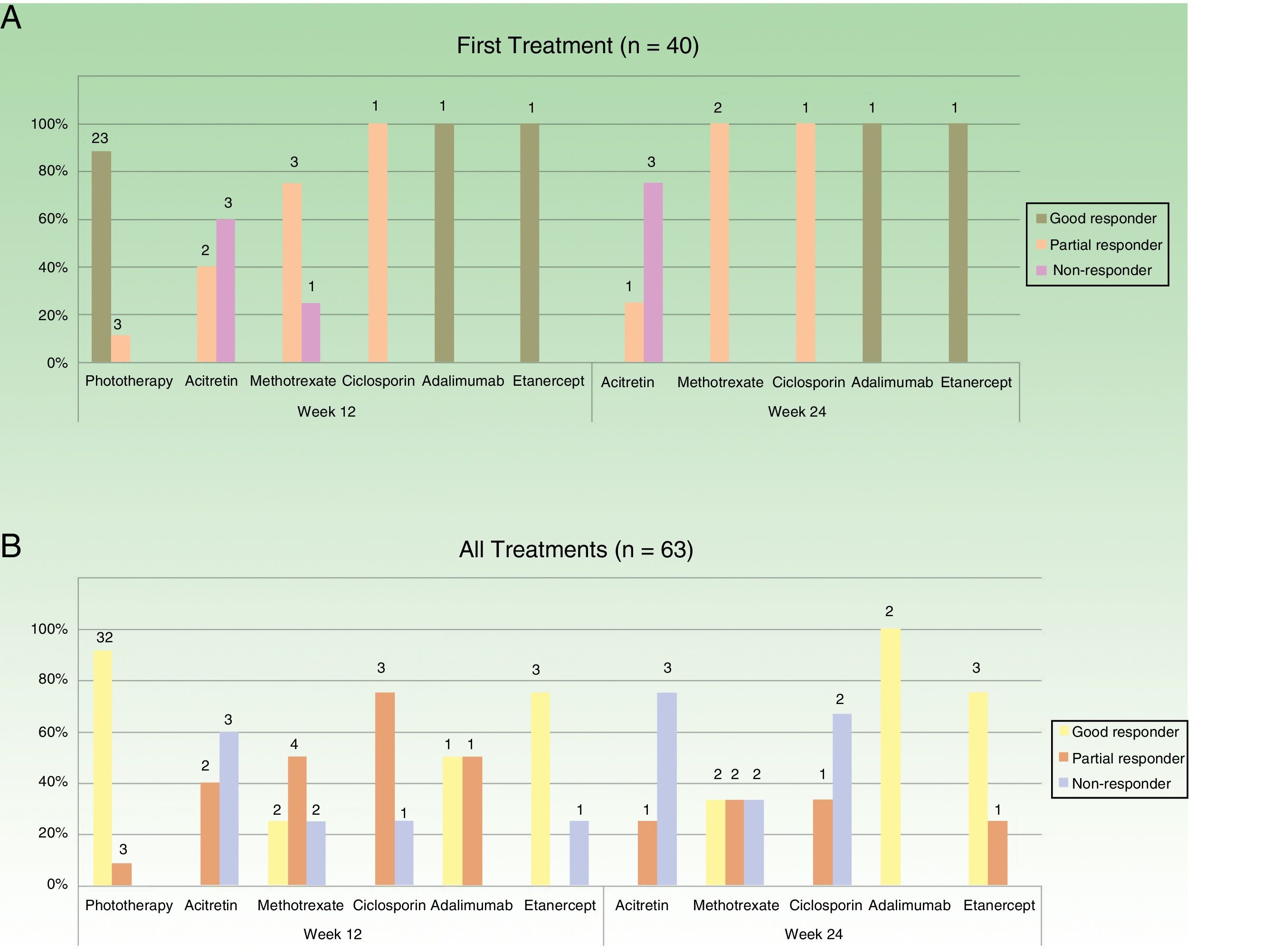
Figure 2 shows the data on response for the different treatments at weeks 12 and 24 with respect to the first treatment administered (n=40) and for all the treatment cycles (n=63). Phototherapy, ciclosporin, and biologic drugs achieved the best results in the short term (week 12). At week 24, biologic drugs obtained the best response and the next most effective treatment was methotrexate.
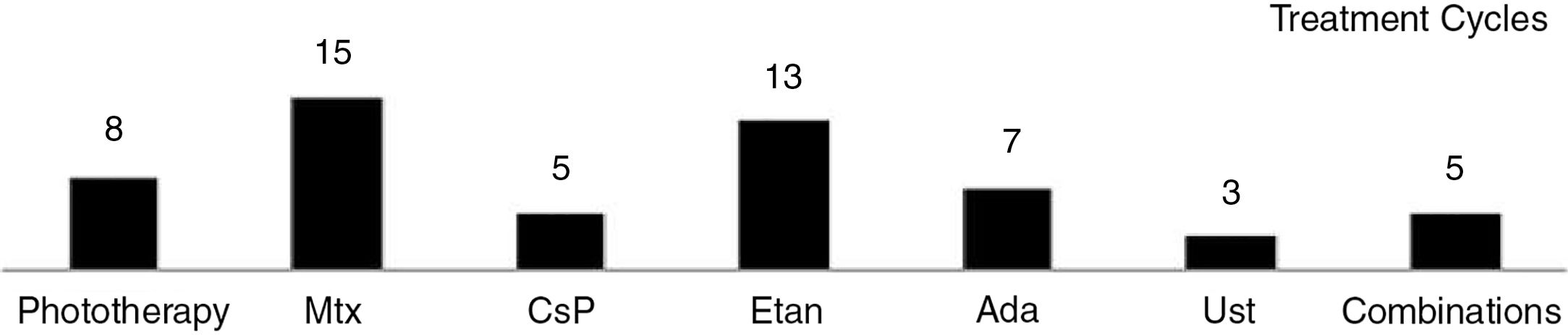
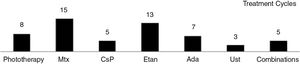
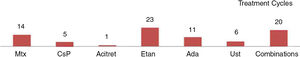
The regimens used and the data on tolerance and adverse effects for all the treatment cycles are included in the supplementary material (n=63). Etanercept and acitretin were the most prolonged treatments with mean durations of 26 and 23 months, respectively. In the case of phototherapy (94% narrow-band UV-B therapy), the average duration of a treatment cycle was 2 months. No relationship was observed between duration of treatment or number of treatment cycles and the type of psoriasis (plaque versus guttate) (P > .05). The treatments were well tolerated (97%). Adverse events were rare (11%) and in no case led to withdrawal of treatment.
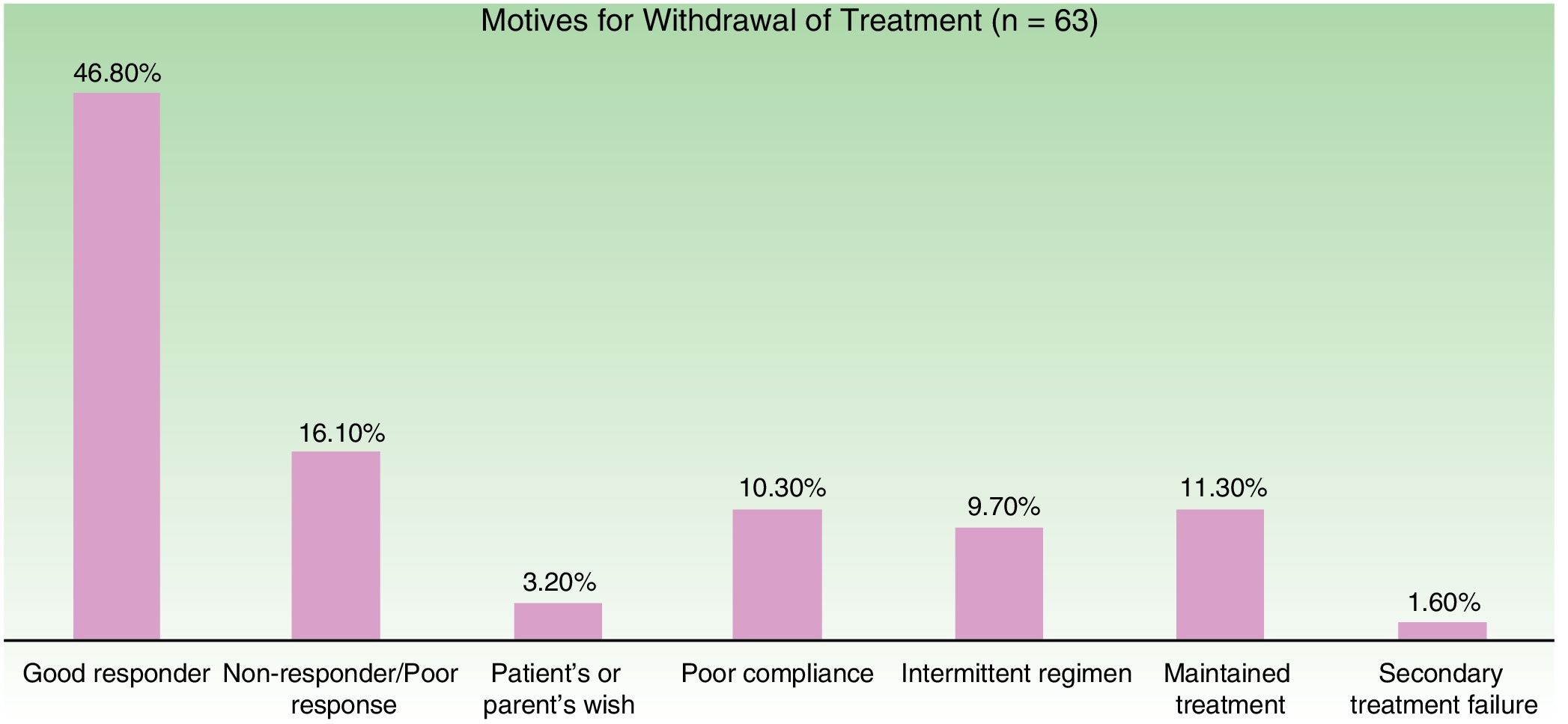
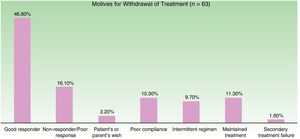
Figure 3 shows the distribution of the motives for treatment withdrawal. The most common reason for withdrawal was a good response (47%).
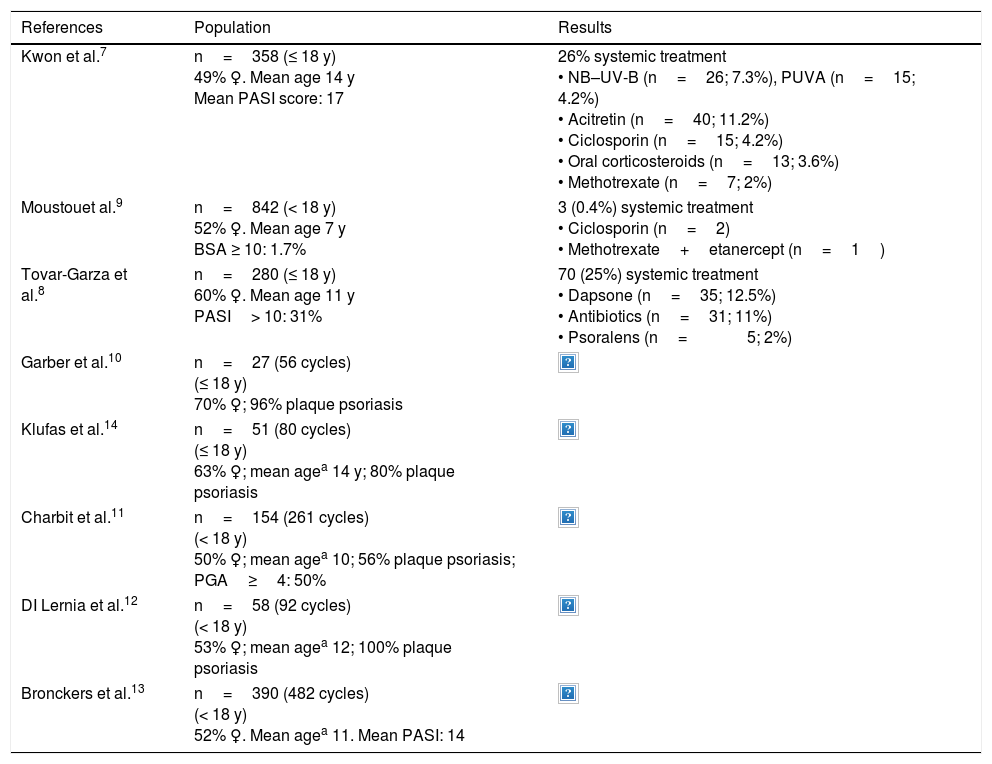
DiscussionStudies evaluating therapies used in children and adolescents to treat moderate to severe psoriasis have used differing methodologies and have reported diverse results (Table 2).
Systemic Treatments for Moderate to Severe Psoriasis in Children and Adolescents: Data From Descriptive Studies7–14
| References | Population | Results |
|---|---|---|
| Kwon et al.7 | n=358 (≤ 18 y) 49% ♀. Mean age 14 y Mean PASI score: 17 | 26% systemic treatment • NB–UV-B (n=26; 7.3%), PUVA (n=15; 4.2%) • Acitretin (n=40; 11.2%) • Ciclosporin (n=15; 4.2%) • Oral corticosteroids (n=13; 3.6%) • Methotrexate (n=7; 2%) |
| Moustouet al.9 | n=842 (< 18 y) 52% ♀. Mean age 7 y BSA ≥ 10: 1.7% | 3 (0.4%) systemic treatment • Ciclosporin (n=2) • Methotrexate+etanercept (n=1) |
| Tovar-Garza et al.8 | n=280 (≤ 18 y) 60% ♀. Mean age 11 y PASI> 10: 31% | 70 (25%) systemic treatment • Dapsone (n=35; 12.5%) • Antibiotics (n=31; 11%) • Psoralens (n=5; 2%) |
| Garber et al.10 | n=27 (56 cycles) (≤ 18 y) 70% ♀; 96% plaque psoriasis | |
| Klufas et al.14 | n=51 (80 cycles) (≤ 18 y) 63% ♀; mean agea 14 y; 80% plaque psoriasis | |
| Charbit et al.11 | n=154 (261 cycles) (< 18 y) 50% ♀; mean agea 10; 56% plaque psoriasis; PGA≥4: 50% | |
| DI Lernia et al.12 | n=58 (92 cycles) (< 18 y) 53% ♀; mean agea 12; 100% plaque psoriasis | |
| Bronckers et al.13 | n=390 (482 cycles) (< 18 y) 52% ♀. Mean agea 11. Mean PASI: 14 | |
Abbreviations: ♀, female; Acitret, acitretin; Ada, adalimumab; BSA, Body Surface Area; CsP, ciclosporin; Etan, etanercept; FAE, fumaric acid esters; Inf, infliximab; Mtx, methotrexate NB–UV-B, narrow-band UV-B; n, number of patients; PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; PUVA, UV-A+psoralen; Ust, ustekinumab.
One group of descriptive studies analyzed clinical variables and epidemiological data from children with psoriasis of all levels of severity and—although this was not their main objective—also reported the treatments used.7–9 Kwon et al.7 studied 358 children and adolescents, 26% of whom received systemic treatment. Phototherapy and acitretin were the treatments most often prescribed, followed by ciclosporin. While the proportion of patients who received systemic treatment was similar in a study of 280 cases by Tovar-Garza et al.,8 the treatments prescribed in that study were not those recommended in the consensus statements followed in our medical setting. Dapsone was the drug most frequently prescribed, followed by antibiotics, which were prescribed for guttate psoriasis.8 The largest of these studies analyzed data from 842 children and adolescents (average age 7 years, 2% with BSA≥10), of whom only 3 received systemic treatment (2 ciclosporin and 1 methotrexate in combination with etanercept).9
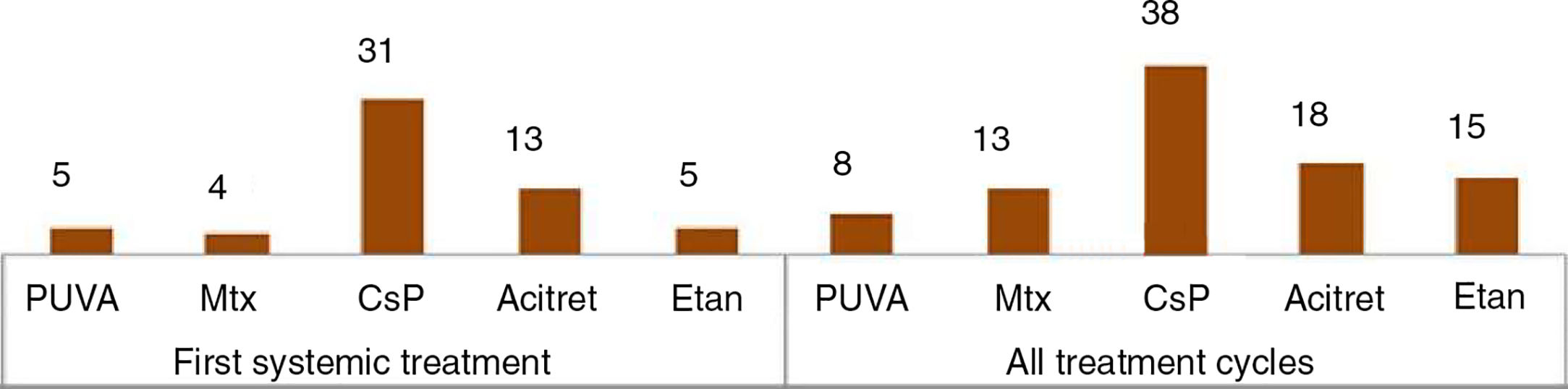
Other studies, which used a methodology more similar to that of our study, analyzed only pediatric patients with psoriasis treated with systemic drugs. Of particular interest are 5 studies with populations ranging from 27 to 390 patients (Table 2).10–14 Three included phototherapy among the treatment options assessed, as we did.10–12 Methotrexate followed by etanercept,10,13 etanercept followed by methotrexate,14 acitretin,11 and ciclosporin12 were the drugs most often prescribed, depending on the study.
The dosages of each treatment were similar across the different studies: phototherapy (narrowband UV–B and PUVA) 2 to 3 times a week; acitretin at a dose of 0.2-1mg/kg/d; methotrexate at a dose of 0.2-0.7mg/wk; ciclosporin at a dose of 2.5-5mg/kg/d; and etanercept prescribed according to the Summary of Product Characteristics (0.8mg/kg/wk up to a maximum of 50mg/wk).3,12,15,16 These data are in line with the guidelines followed in our study.
In the descriptive studies cited above,10–12,14 notwithstanding differences in study design and the way the results are expressed, the authors generally report satisfactory responses for all treatment groups. With respect to efficacy, Garber et al.10 observed better responses with biologic drugs than with classic systemic therapy and phototherapy, while Charbit et al.11 reported a higher response rate for the combination of phototherapy and acitretin, although this regimen accounted for only 10 of the 261 treatment cycles they analyzed (Table 3). In our study population, the percentage of good responders was 66% at week 12, falling to 32 at week 24. The percentages for good and partial responses combined are higher (88% at week 12 and 68% at week 24).
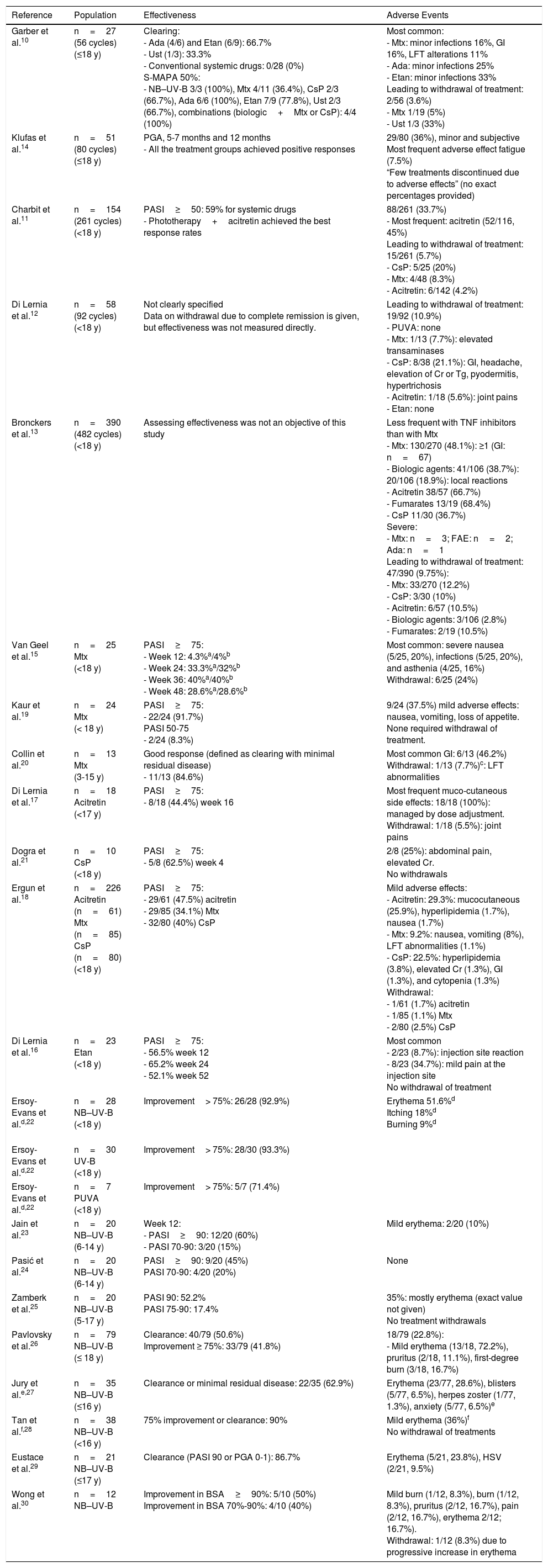
Systemic Treatment for Moderate to Severe Psoriasis in Children and Adolescents: Effectiveness and Safety According to Data from Descriptive Studies10–12,14,15,17–30
| Reference | Population | Effectiveness | Adverse Events |
|---|---|---|---|
| Garber et al.10 | n=27 (56 cycles) (≤18 y) | Clearing: - Ada (4/6) and Etan (6/9): 66.7% - Ust (1/3): 33.3% - Conventional systemic drugs: 0/28 (0%) S-MAPA 50%: - NB–UV-B 3/3 (100%), Mtx 4/11 (36.4%), CsP 2/3 (66.7%), Ada 6/6 (100%), Etan 7/9 (77.8%), Ust 2/3 (66.7%), combinations (biologic+Mtx or CsP): 4/4 (100%) | Most common: - Mtx: minor infections 16%, GI 16%, LFT alterations 11% - Ada: minor infections 25% - Etan: minor infections 33% Leading to withdrawal of treatment: 2/56 (3.6%) - Mtx 1/19 (5%) - Ust 1/3 (33%) |
| Klufas et al.14 | n=51 (80 cycles) (≤18 y) | PGA, 5-7 months and 12 months - All the treatment groups achieved positive responses | 29/80 (36%), minor and subjective Most frequent adverse effect fatigue (7.5%) “Few treatments discontinued due to adverse effects” (no exact percentages provided) |
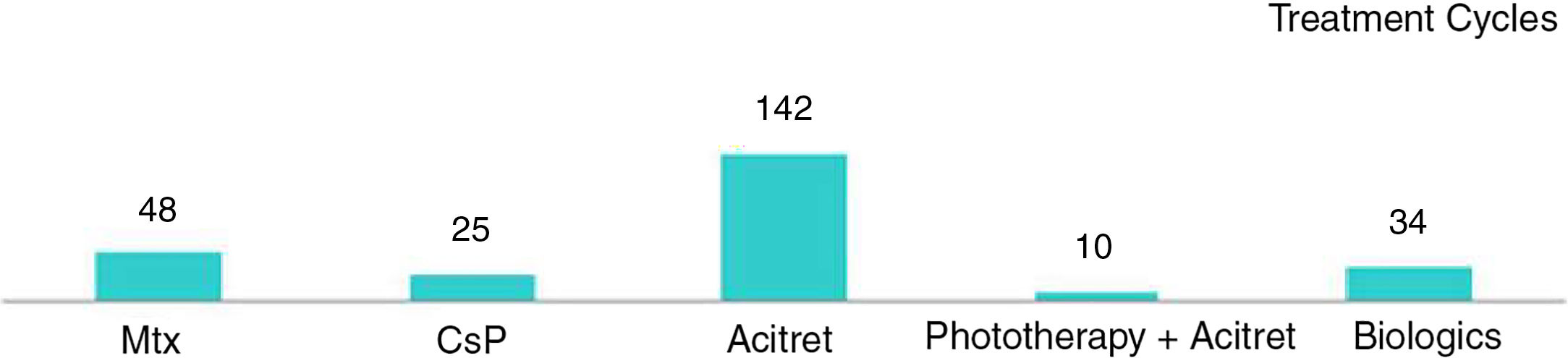
| Charbit et al.11 | n=154 (261 cycles) (<18 y) | PASI≥50: 59% for systemic drugs - Phototherapy+acitretin achieved the best response rates | 88/261 (33.7%) - Most frequent: acitretin (52/116, 45%) Leading to withdrawal of treatment: 15/261 (5.7%) - CsP: 5/25 (20%) - Mtx: 4/48 (8.3%) - Acitretin: 6/142 (4.2%) |
| Di Lernia et al.12 | n=58 (92 cycles) (<18 y) | Not clearly specified Data on withdrawal due to complete remission is given, but effectiveness was not measured directly. | Leading to withdrawal of treatment: 19/92 (10.9%) - PUVA: none - Mtx: 1/13 (7.7%): elevated transaminases - CsP: 8/38 (21.1%): GI, headache, elevation of Cr or Tg, pyodermitis, hypertrichosis - Acitretin: 1/18 (5.6%): joint pains - Etan: none |
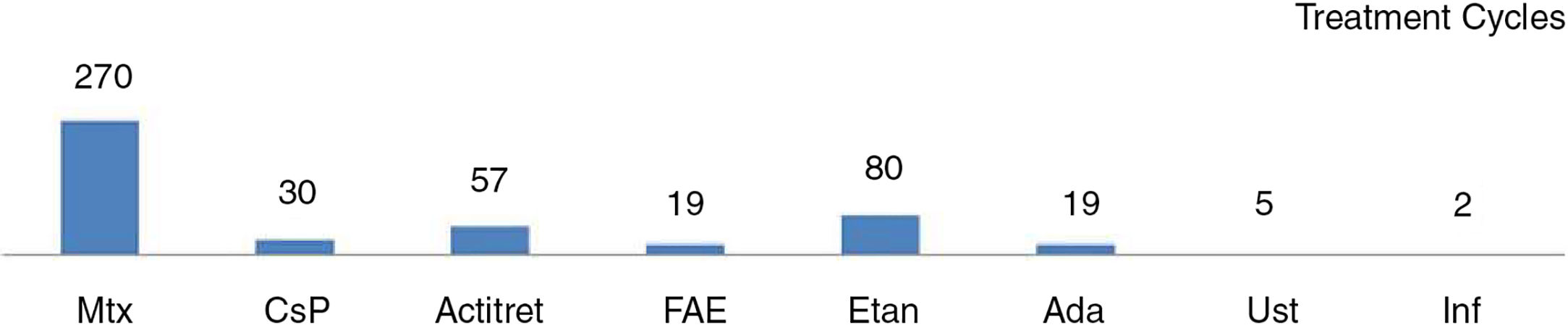
| Bronckers et al.13 | n=390 (482 cycles) (<18 y) | Assessing effectiveness was not an objective of this study | Less frequent with TNF inhibitors than with Mtx - Mtx: 130/270 (48.1%): ≥1 (GI: n=67) - Biologic agents: 41/106 (38.7%): 20/106 (18.9%): local reactions - Acitretin 38/57 (66.7%) - Fumarates 13/19 (68.4%) - CsP 11/30 (36.7%) Severe: - Mtx: n=3; FAE: n=2; Ada: n=1 Leading to withdrawal of treatment: 47/390 (9.75%): - Mtx: 33/270 (12.2%) - CsP: 3/30 (10%) - Acitretin: 6/57 (10.5%) - Biologic agents: 3/106 (2.8%) - Fumarates: 2/19 (10.5%) |
| Van Geel et al.15 | n=25 Mtx (<18 y) | PASI≥75: - Week 12: 4.3%a/4%b - Week 24: 33.3%a/32%b - Week 36: 40%a/40%b - Week 48: 28.6%a/28.6%b | Most common: severe nausea (5/25, 20%), infections (5/25, 20%), and asthenia (4/25, 16%) Withdrawal: 6/25 (24%) |
| Kaur et al.19 | n=24 Mtx (< 18 y) | PASI≥75: - 22/24 (91.7%) PASI 50-75 - 2/24 (8.3%) | 9/24 (37.5%) mild adverse effects: nausea, vomiting, loss of appetite. None required withdrawal of treatment. |
| Collin et al.20 | n=13 Mtx (3-15 y) | Good response (defined as clearing with minimal residual disease) - 11/13 (84.6%) | Most common GI: 6/13 (46.2%) Withdrawal: 1/13 (7.7%)c: LFT abnormalities |
| Di Lernia et al.17 | n=18 Acitretin (<17 y) | PASI≥75: - 8/18 (44.4%) week 16 | Most frequent muco-cutaneous side effects: 18/18 (100%): managed by dose adjustment. Withdrawal: 1/18 (5.5%): joint pains |
| Dogra et al.21 | n=10 CsP (<18 y) | PASI≥75: - 5/8 (62.5%) week 4 | 2/8 (25%): abdominal pain, elevated Cr. No withdrawals |
| Ergun et al.18 | n=226 Acitretin (n=61) Mtx (n=85) CsP (n=80) (<18 y) | PASI≥75: - 29/61 (47.5%) acitretin - 29/85 (34.1%) Mtx - 32/80 (40%) CsP | Mild adverse effects: - Acitretin: 29.3%: mucocutaneous (25.9%), hyperlipidemia (1.7%), nausea (1.7%) - Mtx: 9.2%: nausea, vomiting (8%), LFT abnormalities (1.1%) - CsP: 22.5%: hyperlipidemia (3.8%), elevated Cr (1.3%), GI (1.3%), and cytopenia (1.3%) Withdrawal: - 1/61 (1.7%) acitretin - 1/85 (1.1%) Mtx - 2/80 (2.5%) CsP |
| Di Lernia et al.16 | n=23 Etan (<18 y) | PASI≥75: - 56.5% week 12 - 65.2% week 24 - 52.1% week 52 | Most common - 2/23 (8.7%): injection site reaction - 8/23 (34.7%): mild pain at the injection site No withdrawal of treatment |
| Ersoy-Evans et al.d,22 | n=28 NB–UV-B (<18 y) | Improvement> 75%: 26/28 (92.9%) | Erythema 51.6%d Itching 18%d Burning 9%d |
| Ersoy-Evans et al.d,22 | n=30 UV-B (<18 y) | Improvement> 75%: 28/30 (93.3%) | |
| Ersoy-Evans et al.d,22 | n=7 PUVA (<18 y) | Improvement> 75%: 5/7 (71.4%) | |
| Jain et al.23 | n=20 NB–UV-B (6-14 y) | Week 12: - PASI≥90: 12/20 (60%) - PASI 70-90: 3/20 (15%) | Mild erythema: 2/20 (10%) |
| Pasić et al.24 | n=20 NB–UV-B (6-14 y) | PASI≥90: 9/20 (45%) PASI 70-90: 4/20 (20%) | None |
| Zamberk et al.25 | n=20 NB–UV-B (5-17 y) | PASI 90: 52.2% PASI 75-90: 17.4% | 35%: mostly erythema (exact value not given) No treatment withdrawals |
| Pavlovsky et al.26 | n=79 NB–UV-B (≤ 18 y) | Clearance: 40/79 (50.6%) Improvement ≥ 75%: 33/79 (41.8%) | 18/79 (22.8%): - Mild erythema (13/18, 72.2%), pruritus (2/18, 11.1%), first-degree burn (3/18, 16.7%) |
| Jury et al.e,27 | n=35 NB–UV-B (≤16 y) | Clearance or minimal residual disease: 22/35 (62.9%) | Erythema (23/77, 28.6%), blisters (5/77, 6.5%), herpes zoster (1/77, 1.3%), anxiety (5/77, 6.5%)e |
| Tan et al.f,28 | n=38 NB–UV-B (<16 y) | 75% improvement or clearance: 90% | Mild erythema (36%)f No withdrawal of treatments |
| Eustace et al.29 | n=21 NB–UV-B (≤17 y) | Clearance (PASI 90 or PGA 0-1): 86.7% | Erythema (5/21, 23.8%), HSV (2/21, 9.5%) |
| Wong et al.30 | n=12 NB–UV-B | Improvement in BSA≥90%: 5/10 (50%) Improvement in BSA 70%-90%: 4/10 (40%) | Mild burn (1/12, 8.3%), burn (1/12, 8.3%), pruritus (2/12, 16.7%), pain (2/12, 16.7%), erythema 2/12; 16.7%). Withdrawal: 1/12 (8.3%) due to progressive increase in erythema |
Abbreviations: Ada, adalimumab; BSA, Body Surface Area; Cr, creatinine; CsP, ciclosporin; FAE, fumaric acid esters; Etan, etanercept; GI, gastrointestinal; HSV: herpes simplex virus; LFT, liver function tests; Mtx, methotrexate; NB-UV-B: narrow-band UV-B; n, number of patients; PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; PUVA, UV-A and psoralen; S–MAPA, Simple Measure for Assessing Psoriasis Activity: a product of the PGA times the % BSA; Tg, triglycerides; TNF, tumor necrosis factor; Ust, ustekinumab.
a As treated.
b Last observation carried forward
c 2/18 (11.1%) taking into account all the treatment cycles (n=18).
d,e,f Studies including patients with psoriasis and other skin conditions (n=113d, n=77e, n=116f); adverse effects corresponding to all the patients in those studies (and not the subgroup of patients with psoriasis).
In most of these case series, side effects were mild and rare. The percentage of adverse events leading to withdrawal of treatment ranged from 4% to 11% (Table 3)10–14 In a recent retrospective study of 390 children aged under 18 years, Bronckers et al.13 observed a lower percentage of adverse effects overall with biologic agents than with methotrexate, although the percentage of infections was higher in the group of patients on biologic therapy. The most significant adverse events were injection site reactions in patients on biologic drugs (20/106) and gastrointestinal symptoms in patients on methotrexate (67/270). Six patients (2%) developed a serious adverse event: 3 associated with methotrexate, 2 with fumaric acid esters, and 1 with adalimumab.
Other studies provide data on effectiveness and adverse effects for a single treatment or a few different drugs (Table 3).15,17,18 The number of patients included, the timing of response assessment, and the scales used to measure effectiveness vary, but the percentage of good responders was around 40% for methotrexate, acitretin, and ciclosporin in all these studies. Earlier studies reported better responses with methotrexate.19,20 Response rates of 62% have been reported for ciclosporin21; but this result should be evaluated with caution, since the study enrolled only 10 patients and the effectiveness of ciclosporin was evaluated at week 4. Di Lernia et al., 16 who analyzed the effectiveness of etanercept in clinical practice in a group of patients under 18 years of age, reported a PASI 75 response at week 24 in 65%.
Once again, all of these authors report very few adverse effects, and these led to withdrawal of treatment in fewer than 4% of cases.15–21In the case of phototherapy, total clearing or an improvement of more than 70% has been documented in over 60% of cases, and only minor side effects have been reported (erythema, itching or burning sensation, blisters, and reactivation of the herpes simplex virus).22–30 In our study population, phototherapy achieved a good response in over 80% of treatment cycles, with minimal adverse effects.
Our study is affected by certain limitations. The study population was small and the data was analyzed retrospectively. Consequently, the results depend on the information recorded in medical records. Further research is needed to confirm our findings and provide more data, preferably prospective studies analyzing larger groups of patients.
ConclusionIn our setting, in the population under 18 years of age with psoriasis, phototherapy was the treatment most often prescribed, followed by methotrexate. The treatments studied had a good safety profile and achieved a good response in 66% of the patients assessed at week 12 (including phototherapy) and 32% at week 24 (systemic drugs without phototherapy).
Conflicts of InterestAna Batalla has received honoraria for training activities from Abbvie, Leo-Pharma, Novartis and Pierre-Fabre and has participated in clinical trials from Lilly and Novartis.
Rosa Fernández-Torres has received honoraria for training activities from Abbvie, Leo-Pharma, Janssen, Novartis and Pfizer and has participated in clinical trials from Janssen and Novartis.
Laura Rodríguez-Pazos has participated in clinical trials from Novartis.
Romina Rodríguez Lojo has received honoraria for training activities from Abbvie, Bristol-Myers, and Roche, and has participated in clinical trials from Janssen and Novartis.
Ander Zulaica has received honoraria for training activities, clinical trials, and consultancy work from Abbvie, Almirall, Celgene, Janssen, Leo-Pharma, MSD, Novartis, and Pfizer.
Miguel Cabanillas has received honoraria for training activities from Abbvie, Janssen, Leo-Pharma, and Pfizer, and has participated in clinical trials from Abbvie and Novartis.
Eduardo Fonseca Capdevila has received honoraria for training activities and clinical trials from Abbvie, Almirall, Celgene, Janssen, Novartis, and Pfizer.
Álvaro León has received honoraria for training activities from Leo-Pharma, Novartis, and Pierre-Fabre.
Luisa Fernández-Díaz has received honoraria for training activities, research, and consultancy from Abbvie, Celgene, Gebro-Pharma, Janssen, Leo-Pharma, Lilly, MSD, Novartis, and Pfizer.
María José Seoane-Pose has participated in clinical trials from Abbvie and Novartis.
Hugo Vázquez-Veiga has received honoraria for his participation in research projects, training and consulting activities from Almirall, Celgene, Leo-Pharma, Lilly, Janssen, MSD, and Novartis.
Teresa Abalde has received honoraria for training activities from Celgene, LeoPharma, Janssen, MSD, Novartis, and Pfizer and has participated in clinical trials from Janssen and Novartis.
Laura Salgado-Boquete has received honoraria for training activities and scientific consultancy from Abbvie, Celgene, Janssen, Lilly, Medea, MSD; Novartis, and Pfizer, and has participated in clinical trials from Janssen and Novartis.
Ignacio Suárez-Conde has participated in clinical trials from Novartis.
Ángeles Flórez has received honoraria for participation in research projects, training and consulting activities from Almirall, Celgene, Leo-Pharma, Lilly, Janssen, MSD, and Novartis.
The other authors report no conflicts of interest.
Please cite this article as: Batalla A, Fernández-Torres R, Rodríguez-Pazos L, Monteagudo B, Pardavila-Riveiro R, Rodríguez-Lojo R, et al. Tratamiento sistémico de la psoriasis moderada-grave en edad pediátrica en Galicia: estudio descriptivo. Actas Dermosifiliogr. 2018;109:722–732.