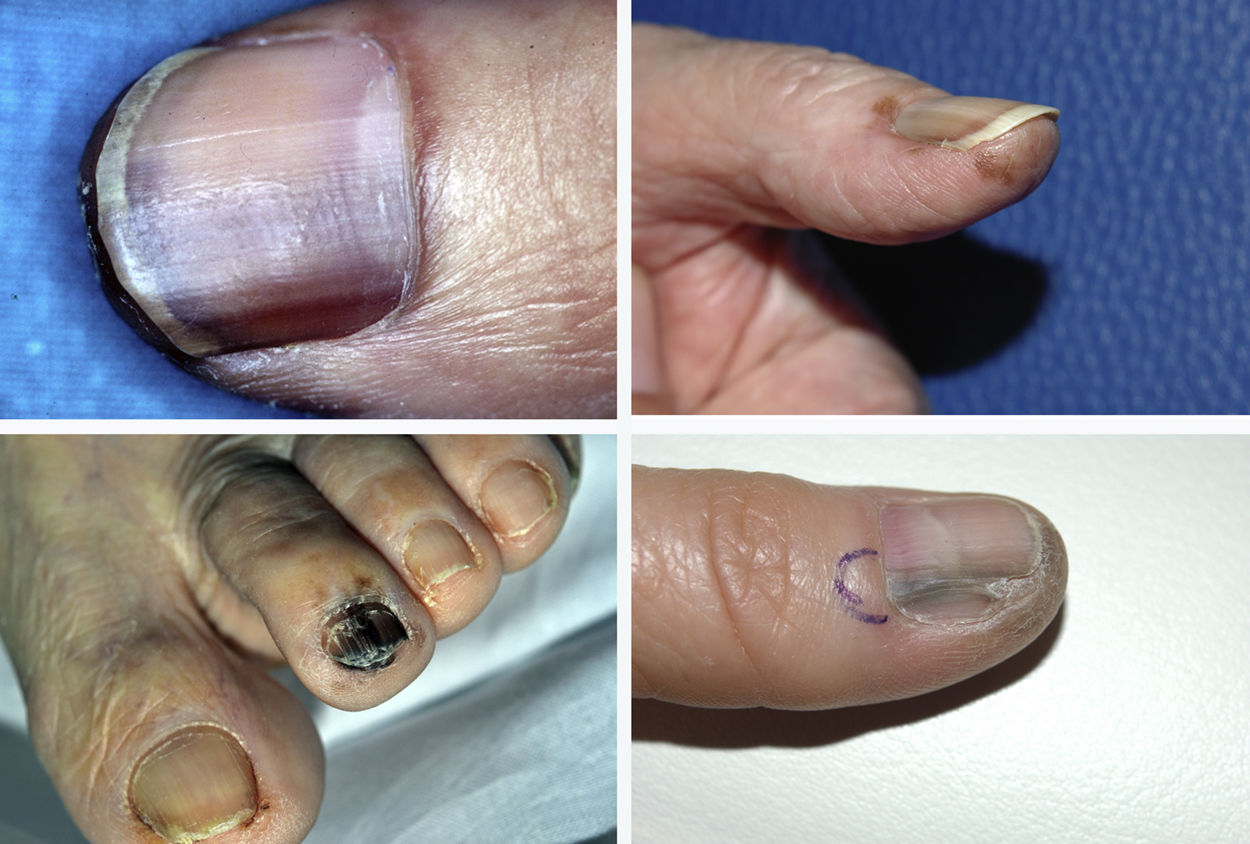
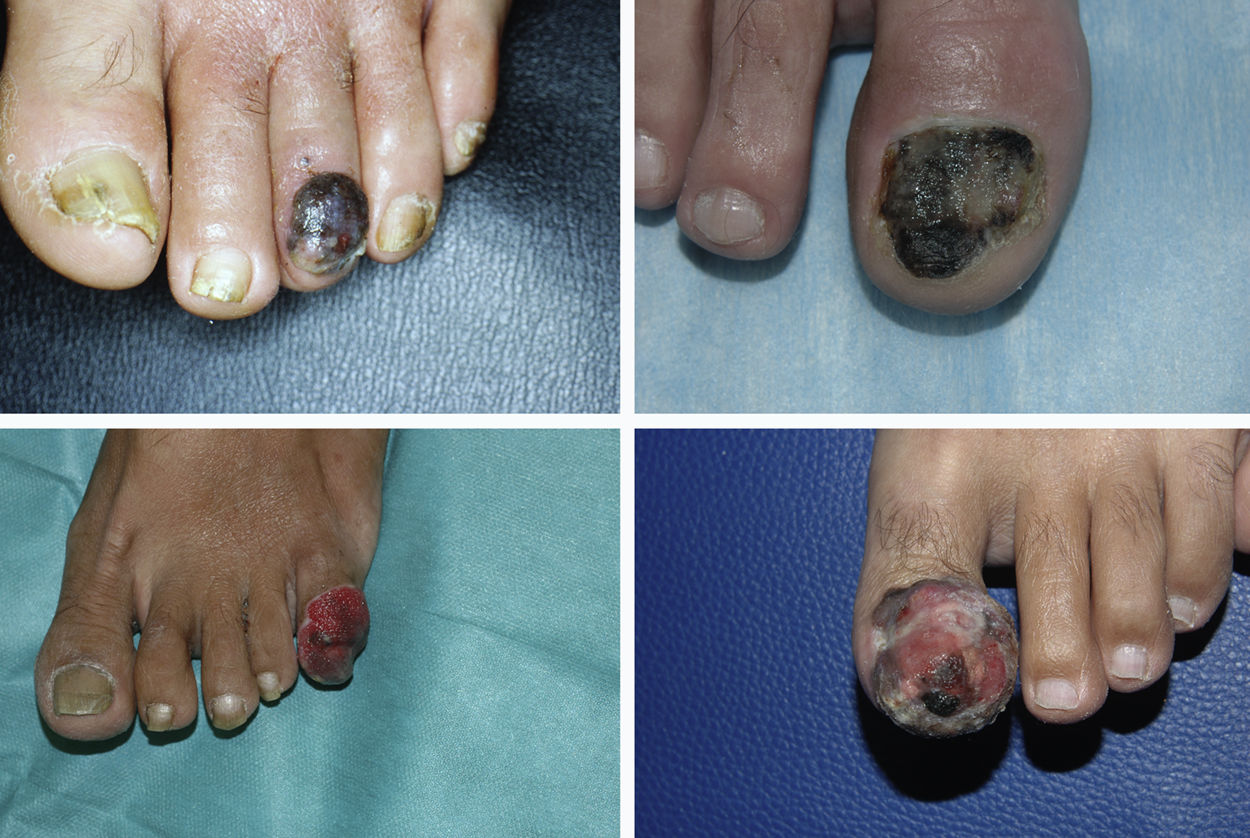
Subungual melanoma constitutes a diagnostic challenge because it often has an atypical clinical presentation. The aims of this study were to revise the clinical and pathologic characteristics of patients with subungual melanoma diagnosed at a tertiary care university hospital and analyze the factors potentially associated with a delayed diagnosis.
Material and methodsWe analyzed data for 34 patients diagnosed with subungual melanoma at our hospital over a period of 20 years.
ResultsThe study population comprised 18 women and 16 men with a median age at diagnosis of 66 years. Only 5 of the patients had longitudinal melanonychia when examined at the dermatology department. At the time of diagnosis, 19 of the 34 patients had invasive melanoma (median Breslow thickness, 3.70mm); 16 had ulceration and 8 had regional lymph node involvement. Five patients had subungual melanoma in situ at diagnosis. The median time from appearance of the lesions to consultation at a primary care center was 15 months; the corresponding time from primary care consultation to diagnosis at our hospital was 5.5 months. Lesions located on the toes were more likely to be ulcerated (P=.017) and to be accompanied by regional lymph node involvement at diagnosis (P=.012).
ConclusionsThe factors associated with a longer diagnostic delay in patients with subungual melanoma were absence of melanonychia as a presenting feature and involvement of the toes.
El melanoma subungueal constituye un reto diagnóstico por su presentación clínica frecuentemente atípica. El objetivo del estudio fue revisar las características clínico-patológicas de los pacientes con melanoma subungueal diagnosticados en un hospital universitario de tercer nivel y analizar los factores posiblemente asociados al retraso del diagnóstico.
Material y métodosFueron analizados los datos de 34 pacientes diagnosticados de melanoma subungueal durante 20 años en nuestro centro.
ResultadosDel total de pacientes, 18 eran mujeres y 16 eran varones, con una edad mediana al diagnóstico de 66 años. Únicamente 5 de los pacientes presentaron melanoniquia longitudinal al ser visitados en nuestro Servicio de Dermatología. De los 34 pacientes, 19 presentaron melanoma invasivo al diagnóstico, con una mediana de índice de Breslow de 3,70mm; 16 presentaron ulceración y 8 invasión ganglionar regional al diagnóstico. Cinco pacientes fueron diagnosticados en fase de melanoma in situ. La mediana del tiempo de evolución de las lesiones desde su aparición hasta la consulta al Centro de Asistencia Primaria fue de 15 meses, y desde la consulta al Centro de Asistencia Primaria hasta el diagnóstico en nuestro hospital fue de 5,5 meses. Las lesiones localizadas en los dedos de los pies presentaron con mayor frecuencia ulceración (p=0,017) y una mayor probabilidad de invasión ganglionar regional al diagnóstico (p=0,012).
ConclusionesLos factores que en nuestro estudio se asociaron a un mayor retraso del diagnóstico del melanoma subungueal fueron la ausencia de melanoniquia como presentación clínica inicial y la localización de las lesiones en los dedos de los pies.
Subungual melanoma (SUM) was first described by Hutchinson in 1886. It is a rare subtype of melanoma that accounts for just 1.5% to 2% of all cases in white patients.1,2 Onset is more common between the fifth and seventh decades of life and the tumor does not appear to have any particular predilection for sex. It is extremely rare in children.3 SUM typically presents with longitudinal melanonychia.4 Atypical presentations, however, are not uncommon and can result in diagnostic delays and compromise prognosis. Just 20% of SUMs are diagnosed at stage i1 and overall, SUM is associated with shorter 5-year survival than other types of melanoma.1,5
The aim of this study was to revise the clinical and pathologic characteristics of patients with SUM diagnosed at our hospital and to analyze potential factors associated with diagnostic delays.
Material and MethodsAll patients diagnosed with SUM at a tertiary care university hospital between 1997 and 2017 were included. Their charts were retrospectively reviewed to collect the following information: sex, age at diagnosis, initial diagnosis of lesion, time from onset to first primary care center visit, diagnostic delay (time from visit to primary care center to diagnosis of melanoma at our hospital), treatments received during this time, location of SUM (hands/feet and affected fingers/toes), and prognostic factors (Breslow thickness, presence or not of ulceration, and clinical stage at the time of diagnosis).
The data were entered into a database and analyzed using SPSS software version 17.0 for Windows. Categorical variables were compared using contingency tables (χ2, Fisher exact test). Continuous variables were compared using the t test or analysis of variance for normally distributed data. Nonparametric tests were used for nonnormally distributed data.
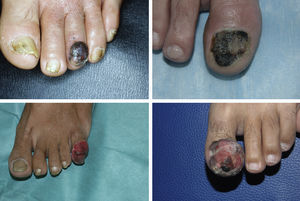
ResultsA total of 1958 patients were diagnosed with melanoma at our hospital during the study period. We identified 103 cases of acral lentiginous melanoma, and 34 of these corresponded to SUM. Of the 34 patients included in the study, 18 were women and 16 were men. The median age at diagnosis was 66 years (range, 35-84 years). The median time from onset to the primary care visit was 15 months (range, 4-60 months) and the median time from this visit to diagnosis at our hospital was 5.5 months (range, 1-36 months). Just 5 of the patients had longitudinal melanonychia when examined at the dermatology department (Fig. 1). The majority of patients had advanced-stage lesions when diagnosed (Fig. 2). The tentative primary care diagnosis was recorded for just 19 patients; this was an infection in 5 patients (1 case of osteomyelitis and 4 of onychomycosis), trauma-induced ulceration in 3 patients, and pyogenic granuloma in 2. An initial diagnosis of SUM was made in just 2 cases. The main treatments received between the primary care visit and diagnosis at our hospital were topical wound and foot care products, antifungals and antibiotics (oral and topical), and cryotherapy.
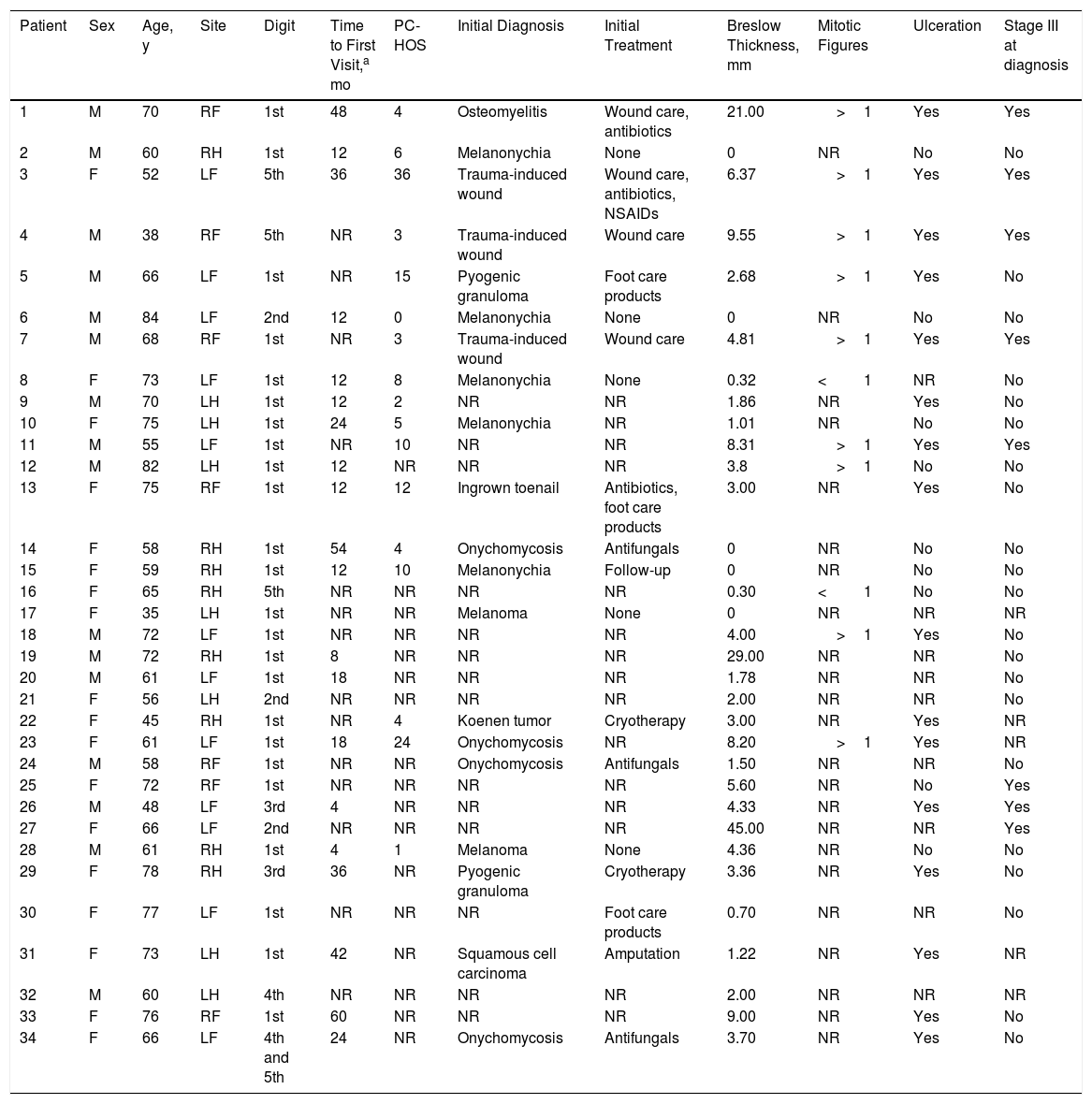
The melanoma involved the toes in 19 patients (55.9%) and the fingers in 15 (44.1%). The most commonly affected digits were the first toes (13/19, 68.4%) and the thumbs (11/15, 73.3%). Twenty-nine of the 34 patients (85.3%) had invasive melanoma at diagnosis and the median Breslow thickness was 3.70mm (range, 0.30-45.00mm). Just 5 patients (14.7%) had melanoma in situ at diagnosis. When diagnosed, 16 of the SUMs (47.1%) were ulcerated and 8 (23.5%) were accompanied by regional lymph node invasion (stage III disease) (Table 1).
Patient Characteristics.
| Patient | Sex | Age, y | Site | Digit | Time to First Visit,a mo | PC-HOS | Initial Diagnosis | Initial Treatment | Breslow Thickness, mm | Mitotic Figures | Ulceration | Stage III at diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 70 | RF | 1st | 48 | 4 | Osteomyelitis | Wound care, antibiotics | 21.00 | >1 | Yes | Yes |
| 2 | M | 60 | RH | 1st | 12 | 6 | Melanonychia | None | 0 | NR | No | No |
| 3 | F | 52 | LF | 5th | 36 | 36 | Trauma-induced wound | Wound care, antibiotics, NSAIDs | 6.37 | >1 | Yes | Yes |
| 4 | M | 38 | RF | 5th | NR | 3 | Trauma-induced wound | Wound care | 9.55 | >1 | Yes | Yes |
| 5 | M | 66 | LF | 1st | NR | 15 | Pyogenic granuloma | Foot care products | 2.68 | >1 | Yes | No |
| 6 | M | 84 | LF | 2nd | 12 | 0 | Melanonychia | None | 0 | NR | No | No |
| 7 | M | 68 | RF | 1st | NR | 3 | Trauma-induced wound | Wound care | 4.81 | >1 | Yes | Yes |
| 8 | F | 73 | LF | 1st | 12 | 8 | Melanonychia | None | 0.32 | <1 | NR | No |
| 9 | M | 70 | LH | 1st | 12 | 2 | NR | NR | 1.86 | NR | Yes | No |
| 10 | F | 75 | LH | 1st | 24 | 5 | Melanonychia | NR | 1.01 | NR | No | No |
| 11 | M | 55 | LF | 1st | NR | 10 | NR | NR | 8.31 | >1 | Yes | Yes |
| 12 | M | 82 | LH | 1st | 12 | NR | NR | NR | 3.8 | >1 | No | No |
| 13 | F | 75 | RF | 1st | 12 | 12 | Ingrown toenail | Antibiotics, foot care products | 3.00 | NR | Yes | No |
| 14 | F | 58 | RH | 1st | 54 | 4 | Onychomycosis | Antifungals | 0 | NR | No | No |
| 15 | F | 59 | RH | 1st | 12 | 10 | Melanonychia | Follow-up | 0 | NR | No | No |
| 16 | F | 65 | RH | 5th | NR | NR | NR | NR | 0.30 | <1 | No | No |
| 17 | F | 35 | LH | 1st | NR | NR | Melanoma | None | 0 | NR | NR | NR |
| 18 | M | 72 | LF | 1st | NR | NR | NR | NR | 4.00 | >1 | Yes | No |
| 19 | M | 72 | RH | 1st | 8 | NR | NR | NR | 29.00 | NR | NR | No |
| 20 | M | 61 | LF | 1st | 18 | NR | NR | NR | 1.78 | NR | NR | No |
| 21 | F | 56 | LH | 2nd | NR | NR | NR | NR | 2.00 | NR | NR | No |
| 22 | F | 45 | RH | 1st | NR | 4 | Koenen tumor | Cryotherapy | 3.00 | NR | Yes | NR |
| 23 | F | 61 | LF | 1st | 18 | 24 | Onychomycosis | NR | 8.20 | >1 | Yes | NR |
| 24 | M | 58 | RF | 1st | NR | NR | Onychomycosis | Antifungals | 1.50 | NR | NR | No |
| 25 | F | 72 | RF | 1st | NR | NR | NR | NR | 5.60 | NR | No | Yes |
| 26 | M | 48 | LF | 3rd | 4 | NR | NR | NR | 4.33 | NR | Yes | Yes |
| 27 | F | 66 | LF | 2nd | NR | NR | NR | NR | 45.00 | NR | NR | Yes |
| 28 | M | 61 | RH | 1st | 4 | 1 | Melanoma | None | 4.36 | NR | No | No |
| 29 | F | 78 | RH | 3rd | 36 | NR | Pyogenic granuloma | Cryotherapy | 3.36 | NR | Yes | No |
| 30 | F | 77 | LF | 1st | NR | NR | NR | Foot care products | 0.70 | NR | NR | No |
| 31 | F | 73 | LH | 1st | 42 | NR | Squamous cell carcinoma | Amputation | 1.22 | NR | Yes | NR |
| 32 | M | 60 | LH | 4th | NR | NR | NR | NR | 2.00 | NR | NR | NR |
| 33 | F | 76 | RF | 1st | 60 | NR | NR | NR | 9.00 | NR | Yes | No |
| 34 | F | 66 | LF | 4th and 5th | 24 | NR | Onychomycosis | Antifungals | 3.70 | NR | Yes | No |
Abbreviations: F, female; LF, left foot; LH, left hand; RF, right foot; RH, right hand; M, male; NR, not reported; NSAIDs, nonsteroidal anti-inflammatory drugs; PC-HOS, time from first primary care visit to diagnosis of melanoma at university hospital.
Compared with finger lesions, toe lesions were more frequently ulcerated (85.7% vs. 36.4%, P=.017) and had a greater Breslow thickness (median, 7.77mm vs. 4.72), although the difference was not statistically significant, probably due to the small number of patients. They were also more likely to be associated with regional lymph node invasion at diagnosis (80% vs. 0%, P=.012).
Median time from appearance of the lesion to the primary care visit was significantly longer for lesions that did not present with melanonychia (25.9 months vs. 14.4 months for those with melanonychia; P=.048). Median time from the primary care visit to diagnosis at our hospital was longer for lesions involving the toes (12.8 months vs. 4.6 months for finger lesions), although the differences were not statistically significant (P=.06), possibly because of the few patients analyzed.
DiscussionSUM has certain characteristics that set it apart from other forms of cutaneous melanoma. Unlike with other melanomas, no clear link has been established with exposure to UV-B radiation.2 The tumor is rarely associated with BRAF mutations and is characterized by a higher incidence of c-KIT mutations.2 Overall, 66% of SUMs are lentiginous acral melanomas, although other variants, including nodular and desmoplastic melanoma, may be seen.2
SUM also has a distinctive age of onset compared with other melanomas. The median age at diagnosis in our study was 66 years (range, 35-84 years). While this age is similar to that reported in the literature for SUM,6–8 it is considerably higher than the mean age of all the patients diagnosed with melanoma (and superficial spreading melanoma in particular) at our hospital.
SUM also has a distinctive clinical presentation. It generally arises in the nail matrix4 and the classic presentation is longitudinal melanonychia. This band of pigment, however, can be caused by numerous benign lesions.1 The following features of melanonychia are suggestive of SUM: a pigment band broader than 3mm, heterogeneous pigmentation, irregular borders, a fissured nail plate, rapid lesion growth, triangular pigmentation (broader proximal part), and extension of pigmentation to periungual skin (Hutchinson sign).1 Up to 25% of SUMs, however, can be amelanotic, complicating diagnosis even further.2 SUM can be difficult to diagnose because it often has atypical presentations that mimic other conditions, such as paronychia, pyogenic granuloma, hemangioma, chronic infections, and tumors such as squamous cell carcinoma.5 This diagnostic difficulty explains another particularity of SUM: diagnostic delay. The median time from onset of lesions to the first visit with a primary care physician in our series was 15 months (range, 4-60 months), while the median time from the primary care visit to diagnosis at our hospital was 5.5 months (range, 1-36 months). In a study of acral lentiginous melanoma, Pereda et al.9 observed a diagnostic delay attributable to the patient (time from when the patient first noticed the lesion to when they sought care) in 30.4% of cases and a delay attributable to the physician (time between the first medical consultation and biopsy) of at least 6 months in almost a third of cases. Diagnostic delays in SUM mean that tumors are frequently diagnosed at an advanced stage. Just 5 of the 34 patients in our study (14.7%) had melanoma in situ, while 29 had invasive melanoma (85.3%), with a median Breslow thickness of 3.70mm (range, 0.30-45.00mm). This thickness was greater than that detected in a series of patients with malignant melanoma at our hospital.10 At the time of diagnosis, 16 of the tumors (47.1%) were ulcerated and 8 (23.5%) had associated regional lymph node involvement.
Based on reports in the literature, SUM is more common on the fingers (62%-67.7%) than on the toes, and is most common on the largest of the digits (first toe and thumb).11,12 Contrasting with previous reports, finger lesions were more common in our series (19 vs. 15), although there have been isolated reports of a similar predominance.7 Similarly to previous reports, the most common digits affected by SUM were the thumb and the first toe (13/19 and 11/15 cases, respectively). Toe lesions were more frequently ulcerated than finger lesions (85.7% vs. 36.4%, P=.017). They were also thicker (median Breslow thickness, 7.77mm vs. 4.72mm for finger lesions), although the difference was not significant, probably because of the small number of patients. Finally, they were more likely than finger lesions to be associated with regional lymph node involvement at diagnosis (80% vs. 0%, P=.012). Similarly to in the study by Fanti et al.,12 our findings appear to support the hypothesis that finger location might be a good prognostic factor for SUM, as this location was associated with a lower incidence of ulceration, a lower Breslow thickness, and a lower probability of lymph node involvement at diagnosis. The most feasible explanation is that finger lesions are easier to detect by patients and lead to earlier medical consultation due to their greater visibility.
In our analysis of factors associated with greater diagnostic delay, we observed that median time from onset to primary care visit was significantly longer for lesions that did not present with melanonychia (25.9 vs. 14.4 months for patients with melanonychia, P=.048). We also saw that median time from the first primary care visit to diagnosis at our hospital was longer for hand lesions (12.8 vs. 4.6 months for foot lesions). These data show that lesions located on the foot are particularly difficult to diagnose by primary care professionals.
Our results indicate that atypical clinical presentations of SUM are common and are associated with a considerable diagnostic delay. The factors associated with a longer diagnostic delay were absence of melanonychia as a presenting sign and SUM involving the toes. Because atypical presentations are common, it is important to contemplate SUM when investigating chronic or subungual lesions or lesions that do not respond to conventional treatment. The diagnostic delay observed with SUM and particularly that involving the feet shows that diagnosis of SUM at the primary care level needs to be improved and highlights the need for greater awareness among the general population and better training of those involved in the diagnosis and treatment of nail lesions to ensure earlier diagnosis and better prognosis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Talavera-Belmonte A, Bonfill-Ortí M, Martínez-Molina L, Fornons-Servent R, Bauer-Alonso A, Ferreres-Riera JR, et al. Melanoma subungueal: estudio descriptivo de 34 pacientes. Actas Dermosifiliogr. 2018;109:801–806.