A series of quality indicators for melanoma and nonmelanoma skin cancer were recently approved within a project promoted by the Healthy Skin Foundation of the Spanish AEDV. The aim of this study was to evaluate adherence to these indicators.
Material and methodsIn November 2016, an anonymous questionnaire consisting of 32 items was sent to the heads of Spanish dermatology and venereology departments listed in the AEDV's database. The questions referred to the above-mentioned quality of care indicators.
ResultsThe questionnaire was completed by 104 of the 150 people contacted (response rate, 69.3%). The lowest response rate for any given question was 56% (84 respondents). Over 85% of respondents answered 29 questions or more (91%). The most widely used indicators were those related to the use of computed tomography or magnetic resonance imaging for squamous cell carcinoma (98%), followed by the availability of a standardized melanoma pathology report (90%). The least widely used indicators were related to availability of electrochemotherapy (25%) and other invasive therapies for locoregionally advanced melanoma (20%).
ConclusionsAdherence to quality of cancer care criteria at the different hospitals evaluated varied. Our findings could be useful for identifying areas for improvement at different hospitals. Future studies should focus on measuring both process and outcome indicators.
Recientemente se han consensuado unos indicadores de calidad de la atención del cáncer de piel no melanoma y melanoma promovidos por la Fundación Piel Sana AEDV. El objetivo de este estudio es conocer la adherencia a estos criterios de calidad asistencial.
Material y métodosEn noviembre de 2016 se realizó una encuesta anónima que constaba de 32 preguntas, dirigida a los responsables de los servicios de dermatología y venereología españoles incluidos en la base de datos de la AEDV. Las preguntas de la encuesta hacían referencia a los diferentes indicadores consensuados previamente.
ResultadosFueron respondidas 104 de las 150 encuestas enviadas (69,3% de porcentaje de respuesta). El menor porcentaje conseguido de respuesta a una pregunta fue del 56% (n=84). Más del 85% de los encuestados contestaron a 29 (91%) o más preguntas. Los indicadores con mayor implantación fueron la disponibilidad de TAC o RMN para el estudio de carcinoma espinocelular (98%), seguidos de la existencia de un modelo estandarizado para la realización del informe anatomopatológico de melanoma (90%). Los indicadores con menor implantación se relacionaron con el acceso a electroquimioterapia (25%) y el acceso a otras terapias invasivas para el melanoma locorregionalmente avanzado (20%).
ConclusionesSe ha encontrado variabilidad en la adherencia de estos criterios en los diferentes centros. Con los datos obtenidos se pueden identificar posibilidades de mejora en los centros. Futuras investigaciones deberían centrarse en la medición de indicadores de proceso y resultado.
A series of quality indicators for melanoma and nonmelanoma skin cancer—basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and Merkel cell carcinoma (MCC)—were recently approved as part of a project promoted by the Healthy Skin Foundation of the Spanish Academy of Dermatology and Venereology (AEDV), with the assistance of skin cancer experts.
Quality indicators are well-defined, quantifiable elements of the health care process1 that should encompass all areas of care for a disease.2 The use of appropriate indicators leads to higher-quality patient care and helps to standardize clinical practice.1,2 Structure indicators are used to analyze the presence of physical structures, spaces, equipment, staff, and functions that form part of health care activities; this type of indicator is therefore the easiest to measure. For structure indicators, the unit of analysis is the hospital. Process indicators measure specific parts of the health care process; the data used to quantify these parts of the process are obtained from clinical records—for example, the percentage of melanomas for which the surgical margin is recorded in the patient history. Outcome indicators are those which measure health quality most directly; they rely on data not only from clinical records but also from long-term follow-up—for example, tracking patients who have undergone a lymphadenectomy in order to calculate lymphedema rates.3 For process and outcome indicators, the unit of analysis is the patient.
Indicators are closely related to clinical guidelines. In Germany, for example, in addition to the S-3 guidelines on the diagnosis, treatment and follow-up of melanoma, dermato-oncological centers certified by the German Cancer Society are also required to assess and adhere to 12 additional indicators.4 These indicators make it possible to anonymously compare a center's outcomes with those of other centers, which is useful in efforts to improve services and melanoma care. Appropriate, evidence-based indicators help to reduce variability among hospitals and improve the quality of care for specific diseases.5–8
For the development of our quality indicators, we took into account various multidisciplinary clinical practice guidelines that had previously been reviewed by a group of skin cancer experts. These guidelines were used to draw up an initial list of proposed indicators. This list was clarified and fine-tuned by a coordinating team and was eventually reduced to fewer than half of the indicators initially proposed. Subsequently, with the help of 20 Spanish skin cancer experts, consensus on the indicators was reached using the modified Delphi method (2 rounds).
Multiple studies have described the development and assessment of quality indicators for the treatment of various types of cancer.9–12 In dermatology, very few studies have described the development and, in particular, the assessment of indicators for melanoma and nonmelanoma skin cancer.4,13–17
The variability among Spanish hospitals in terms of resources and care for skin cancer is currently unknown. This lack of knowledge poses an obstacle to efforts to develop recommendations and improve the health care process.
The aim of this study was to carry out a survey to determine baseline levels of adherence to the structure quality indicators defined in the AEDV's White Book of Skin Cancer in the dermatology departments of Spanish hospitals.
Materials and MethodsIn November 2018, we sent an anonymous online questionnaire to the identified correspondence addresses for the heads of the dermatology and venereology departments listed in the AEDV database (150 recipients in total). The entities included in the AEDV database ranged from dermatology departments or units at county hospitals to dermatology departments at tertiary referral centers that provide skin cancer care. We had previously reviewed the list of contacts by consulting with the secretaries of each of the AEDV's regional sections.
The survey questions were derived from the quality indicators developed for melanoma and nonmelanoma skin cancer (Appendix I. Supplementary Material). The survey covered structure indicators only and the questions were designed to be answered with dichotomous variables (yes/no).
Questions with nondichotomous answers were reformulated if possible and otherwise were rejected (Appendix I. Supplementary Material). The final questionnaire consisted of 32 questions.
Some indicators were formulated as 2 questions in order to obtain better-defined responses (Appendix I. Supplementary Material). For example, the indicator existence of a multidisciplinary team for head and neck tumors that includes a dermatologist was assessed as follows: (1) Does the hospital have a multidisciplinary team for head and neck tumors? and (2) Does the multidisciplinary team for head and neck tumors include a dermatologist?
To simplify the questionnaire, questions referring to infrastructure that could be used for both melanoma and nonmelanoma cancer care were asked just once, in a generic form (for example, questions about availability of radiotherapy and lymphadenectomy).
To increase the response rate,18 we sent each department head a cover letter describing the project, explaining the importance of participation in the project, and providing the research team's contact details for queries. The questionnaire was available via a link on the Survey Monkey website for 30 days and reminder emails were sent once a week. The questionnaire was anonymous in order to minimize social desirability bias.
Responses were stored in an Excel database and analyzed with the Stata software package.
Because the survey focused on hospital facilities and did not collect data on patients or clinical activity, we considered that it was not necessary for it to be evaluated by a clinical research ethics committee.
ResultsThe questionnaire was completed by 104 of the 150 people contacted (response rate, 69.3%). The lowest response rate for any given question was 56% (84 respondents). Over 85% of respondents answered 29 questions (91%) or more.
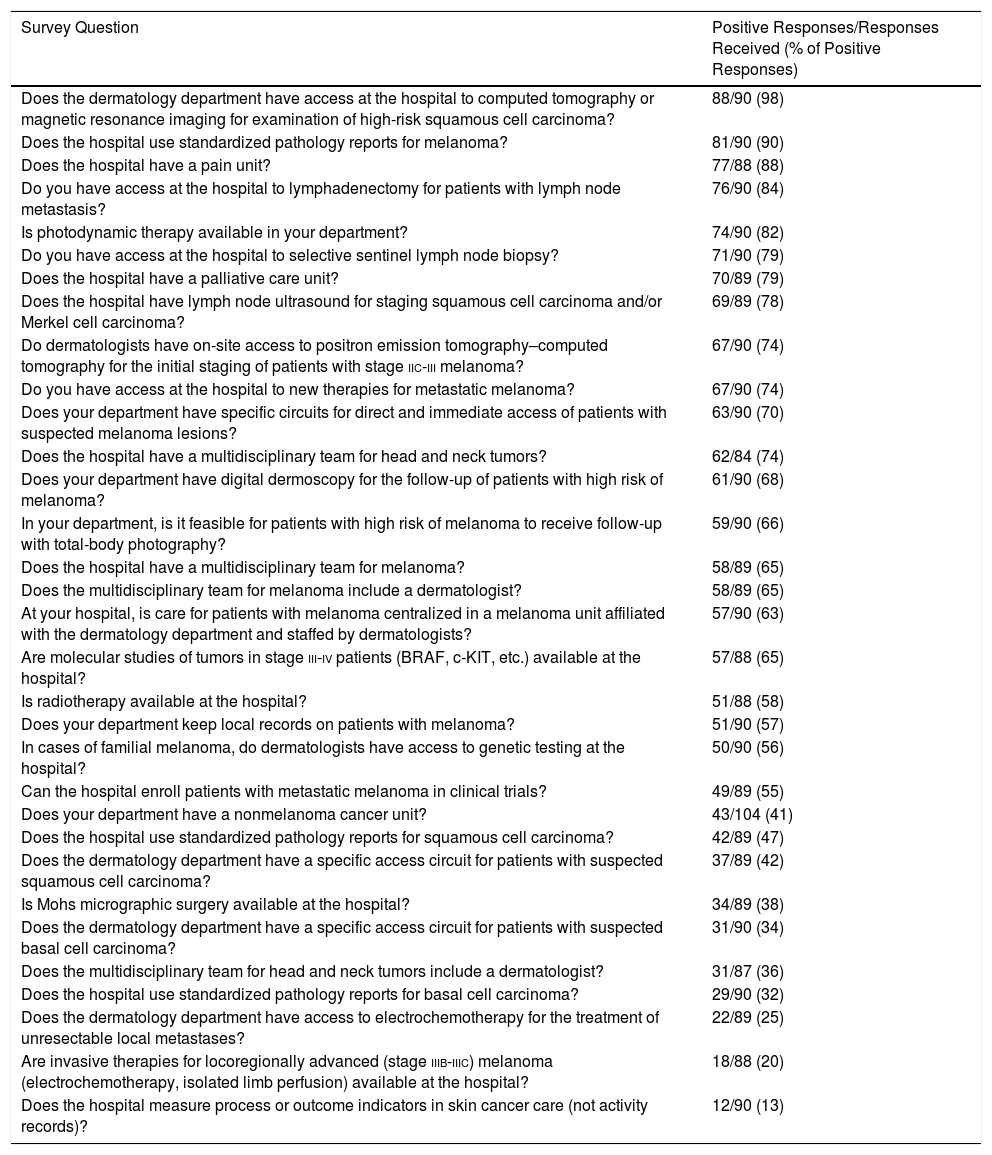
Table 1 shows the results of the responses to questions about the existence or availability of facilities for the treatment of skin cancer, sorted from highest to lowest percentage of positive responses.
Survey Responses, Ordered From Highest to Lowest Adherence.
| Survey Question | Positive Responses/Responses Received (% of Positive Responses) |
|---|---|
| Does the dermatology department have access at the hospital to computed tomography or magnetic resonance imaging for examination of high-risk squamous cell carcinoma? | 88/90 (98) |
| Does the hospital use standardized pathology reports for melanoma? | 81/90 (90) |
| Does the hospital have a pain unit? | 77/88 (88) |
| Do you have access at the hospital to lymphadenectomy for patients with lymph node metastasis? | 76/90 (84) |
| Is photodynamic therapy available in your department? | 74/90 (82) |
| Do you have access at the hospital to selective sentinel lymph node biopsy? | 71/90 (79) |
| Does the hospital have a palliative care unit? | 70/89 (79) |
| Does the hospital have lymph node ultrasound for staging squamous cell carcinoma and/or Merkel cell carcinoma? | 69/89 (78) |
| Do dermatologists have on-site access to positron emission tomography–computed tomography for the initial staging of patients with stage iic-iii melanoma? | 67/90 (74) |
| Do you have access at the hospital to new therapies for metastatic melanoma? | 67/90 (74) |
| Does your department have specific circuits for direct and immediate access of patients with suspected melanoma lesions? | 63/90 (70) |
| Does the hospital have a multidisciplinary team for head and neck tumors? | 62/84 (74) |
| Does your department have digital dermoscopy for the follow-up of patients with high risk of melanoma? | 61/90 (68) |
| In your department, is it feasible for patients with high risk of melanoma to receive follow-up with total-body photography? | 59/90 (66) |
| Does the hospital have a multidisciplinary team for melanoma? | 58/89 (65) |
| Does the multidisciplinary team for melanoma include a dermatologist? | 58/89 (65) |
| At your hospital, is care for patients with melanoma centralized in a melanoma unit affiliated with the dermatology department and staffed by dermatologists? | 57/90 (63) |
| Are molecular studies of tumors in stage iii-iv patients (BRAF, c-KIT, etc.) available at the hospital? | 57/88 (65) |
| Is radiotherapy available at the hospital? | 51/88 (58) |
| Does your department keep local records on patients with melanoma? | 51/90 (57) |
| In cases of familial melanoma, do dermatologists have access to genetic testing at the hospital? | 50/90 (56) |
| Can the hospital enroll patients with metastatic melanoma in clinical trials? | 49/89 (55) |
| Does your department have a nonmelanoma cancer unit? | 43/104 (41) |
| Does the hospital use standardized pathology reports for squamous cell carcinoma? | 42/89 (47) |
| Does the dermatology department have a specific access circuit for patients with suspected squamous cell carcinoma? | 37/89 (42) |
| Is Mohs micrographic surgery available at the hospital? | 34/89 (38) |
| Does the dermatology department have a specific access circuit for patients with suspected basal cell carcinoma? | 31/90 (34) |
| Does the multidisciplinary team for head and neck tumors include a dermatologist? | 31/87 (36) |
| Does the hospital use standardized pathology reports for basal cell carcinoma? | 29/90 (32) |
| Does the dermatology department have access to electrochemotherapy for the treatment of unresectable local metastases? | 22/89 (25) |
| Are invasive therapies for locoregionally advanced (stage iiib-iiic) melanoma (electrochemotherapy, isolated limb perfusion) available at the hospital? | 18/88 (20) |
| Does the hospital measure process or outcome indicators in skin cancer care (not activity records)? | 12/90 (13) |
The indicators with the highest percentages of positive responses were those related to the availability of computed tomography or magnetic resonance imaging for examination of SCC (88 hospitals, 98%), followed by the use of standardized melanoma pathology reports (81 hospitals, 90%) and the existence of a pain unit (76 hospitals, 88%). The indicators with the lowest percentages of positive responses were those related to the use of standardized pathology reports for BCC (29 hospitals, 32%), access to electrochemotherapy (22 hospitals, 25%), and access to other invasive therapies for melanoma (18 hospitals, 20%).
Fifty-seven respondents (63%) said their hospital had a melanoma unit. Fifty-eight respondents (65%) said their hospital had an interdisciplinary melanoma team and 51 (57%) said their hospital kept local melanoma records.
Twenty-one of the 35 hospitals with a nonmelanoma cancer unit (60%) had specific access for BCC. However, only 18% of the hospitals without a nonmelanoma cancer unit (n=10) had specific access for BCC. This difference is statistically significant (P<.001).
The difference between direct access for SCC in hospitals with a nonmelanoma cancer unit and in hospitals without such a unit was also statistically significant: 71% (n=25) vs 22% (n=12) (P<.001).
The head and neck tumor team included a dermatologist at 56% of hospitals with a nonmelanoma cancer unit (n=19), compared with 23% of hospitals without such a unit (n=12) (P=.002).
Of the 57 hospitals with a melanoma unit (63%), 89% had specific direct access for cases in which there is suspicion of melanoma (n=51), compared with 36% of hospitals without such a unit (n=12) (P<.001). Likewise, 84% of hospitals with a melanoma unit (n=47) had a multidisciplinary melanoma team that included a dermatologist, compared with 33% of hospitals without such a unit (n=11) (P<.001). Finally, 81% of hospitals with a melanoma unit (n=46) were equipped to use digital dermoscopy in follow-up, compared with 46% of hospitals without such a unit (n=15) (P<.001).
Twelve hospitals (13.33%) evaluated process or outcome indicators in skin cancer care, while 42 (46.67%) did not. Thirty-six respondents (40%) either did not know whether their hospital evaluated said indicators or chose not to respond.
DiscussionThe study assessed some of the indicators developed by the AEDV for the White Book of Skin Cancer. Previous studies have noted the difficulty of measuring all quality indicators for skin cancer.19 For example, a study in the United States found that only 10 of 26 proposed quality indicators were readily assessable using data from the National Cancer Database, which records most cases of cancer diagnosed in the United States.13
Our results show that there is room for improvement in structure indicators at all of the hospitals studied. For the indicators associated with the best outcomes, the following results were obtained: 63 hospitals (73%) had immediate access for patients with suspected melanoma; 58 hospitals (65%) had a multidisciplinary melanoma team that included a dermatologist; 81 hospitals (90%) used standardized pathology reports. Notably, less than 70% of the dermatology departments surveyed had implemented certain widely accepted standards: digital dermoscopy20–23 (evidence level ii), total-body photography24,25 (evidence level iii), multidisciplinary melanoma team,26 and Mohs micrographic surgery27–30 (evidence level i).
Important areas for improvement include creating multidisciplinary melanoma teams that include dermatologists and providing direct access for suspected cases of melanoma, SCC, or BCC, which is currently available at 63 (70%), 37 (42%), and 31 (34%) hospitals, respectively. Some potential improvements—such as the introduction of standardized pathology reports—would be especially simple to introduce.
Our findings show that hospitals with a melanoma unit or nonmelanoma cancer unit comply with a larger number of structure indicators for the treatment of these tumors.
Sixty-two hospitals (74%) had a head and neck tumor team. However, these teams only included dermatologists at 31 hospitals (50%). Studies have shown that certain patients with complex or recurring cases of nonmelanoma cancer31 benefit from decisions made by a multidisciplinary cancer team.32–34 As for melanoma, hospitals with a melanoma team (n=58) that includes a dermatologist (58 hospitals, 65%) benefit from a multidisciplinary approach to the disease.35,36 Multidisciplinary melanoma care has been shown to be more efficient than traditional treatment approaches.37
Studies of adherence to indicators in melanoma treatment have revealed similar levels of variability to that found in our study.12 A study in the United States found that adherence to process indicators ranged from 12% (use of lactate dehydrogenase tests in stage iv melanoma) to 97% (use of pathology reports that document the number of lymph nodes examined and the number containing metastases), and that these discrepancies translate into differences in the treatment received by patients at different hospitals.13
Spanish hospitals that receive skin cancer referrals may eventually implement a certification system similar to that used in German hospitals4; the indicators described in the present study could be incorporated into the hospital certification requirements.
The strengths of this study include the large number of dermatology departments invited to take part in the survey and the high response rate, which supports the conclusion that the results are representative of reality. The main limitation of the study is social desirability bias; some respondents may have provided an optimistic assessment of the real situation. Moreover, when interpreting the results, it is important to consider the hierarchical structure of the Spanish hospital system: smaller facilities refer their patients to tertiary referral hospitals that use techniques not available elsewhere. Because of this hierarchical structure, it is not strictly necessary or desirable for all hospitals to have every type of infrastructure (100% positive responses). To guarantee anonymity, respondents were not specifically asked about hospital type or number of dermatologists. Future studies could include questions about these aspects, as this information could be useful in the interpretation of survey results.
In conclusion, we have observed that hospitals with melanoma and nonmelanoma units comply with a larger number of structure indicators. We have found that adherence rates could be increased for certain indicators, in some cases quite easily. This description of the situation of structure indicators for assessing skin cancer quality of care in Spanish dermatology departments provides a sense of the current situation in Spain and can help each hospital identify its weaknesses and areas for improvement. Future research should include the design and quantification of process and outcome indicators. Such studies would make it possible to quantify the quality of care provided for certain groups of diseases—particularly skin cancer—and to design improvement strategies.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
FundingThis study was funded by the Healthy Skin Foundation (AEDV) as part of the AEDV's White Book of Skin Cancer project.
This study would not have been possible without the participation and collaboration of Spanish hospitals in our survey of heads of dermatology and venereology departments.
Please cite this article as: Kueder-Pajares T, Descalzo MA, García-Doval I, Ríos-Buceta L, Moreno-Ramírez D. Evaluation of Structure Indicators for Assessing Skin Cancer Quality of Care in Dermatology Departments. Actas Dermosifiliogr. 2018;109:807–812.