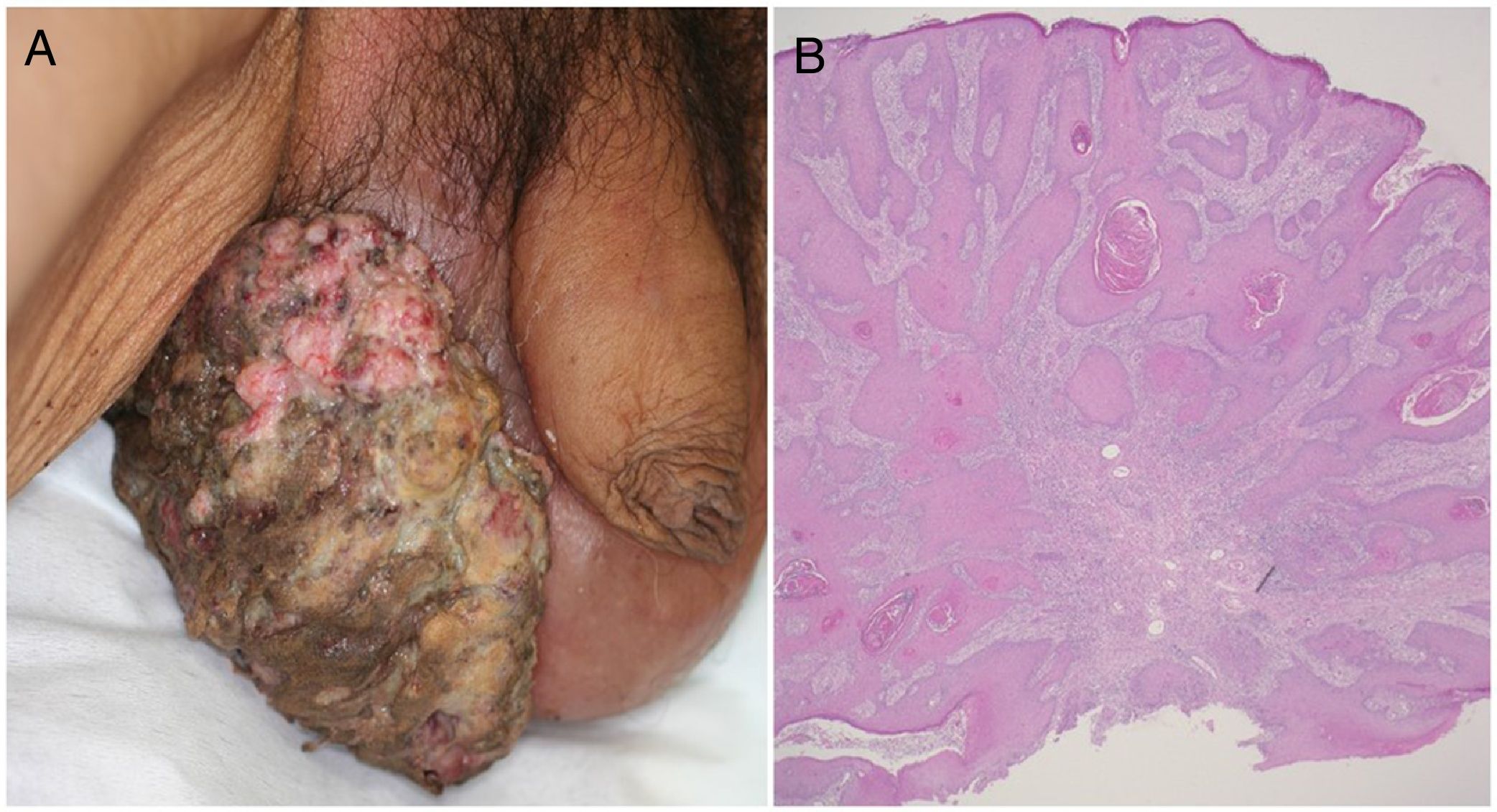
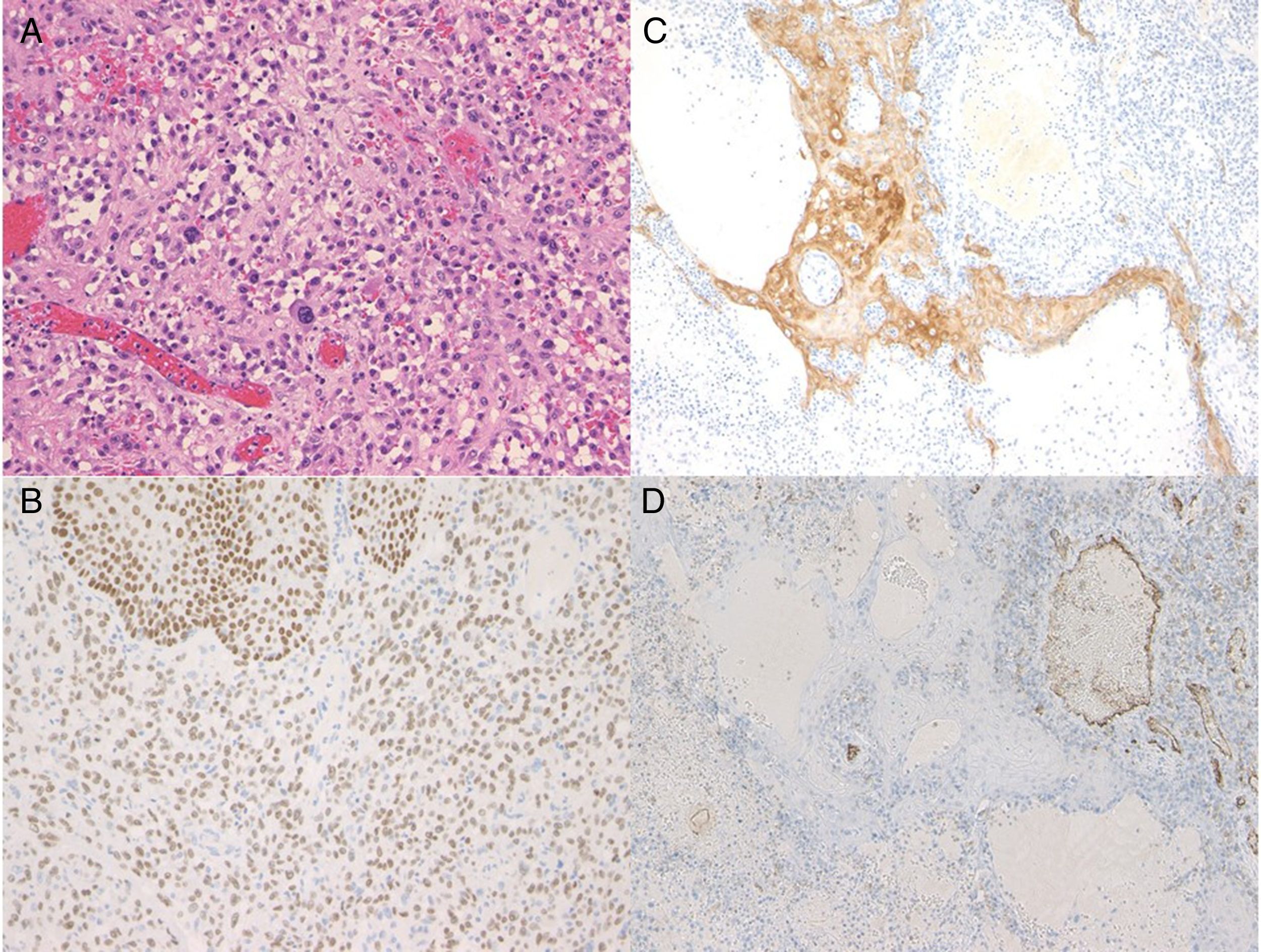
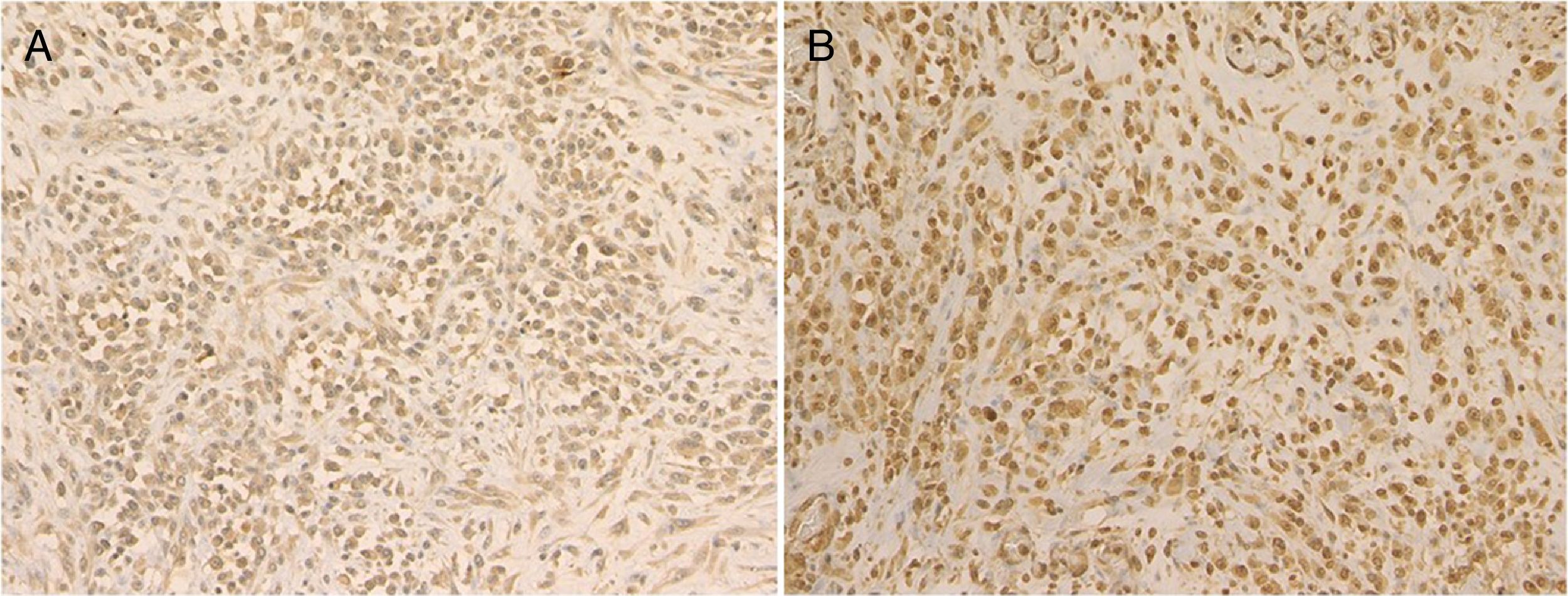
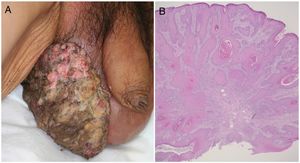
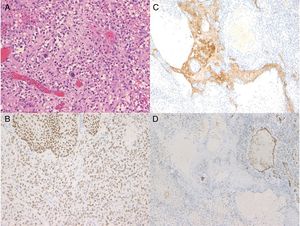
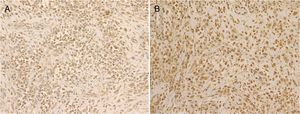
A 72-year-old man presented with a painful tumor of 1 year's duration on the scrotum. Physical examination showed a cauliflower-like 9.5×8.0-cm mass with a surface that was mostly hemorrhagic and necrotic (Fig. 1A). Biopsy showed squamous cell carcinoma (SCC) (Fig. 1B). Biopsy of the inguinal lymph nodes also showed metastasis from SCC. The white blood cell count (WBC) in blood was 19700/μL, with 86% neutrophils. Evaluation of SCC antigen levels in serum using the chemiluminescent enzyme immunoassay (Abbott Japan Co. Ltd.) showed elevated levels (8.7ng/dL; normal value, <1.5). No other metastases were detected. The patient was treated with complete tumor resection and bilateral inguinal lymph node dissection. Histology showed massive infiltration of undifferentiated atypical squamous cells with abundant vascularization and multiple microthrombi extending from the dermis to the subcutaneous tissue (Fig. 2A). Immunohistochemical staining was positive for p40, keratin (AE1/AE3), and vimentin, and negative for CD31 (Fig. 2B-D). The tumor cells were also positive for granulocyte colony-stimulating factor (G-CSF) and the G-CSF receptor (Fig. 3 A, B). Serum levels of the SCC antigen returned to normal after surgery, but 4 days after the operation, the patient developed a high fever. Three weeks postsurgery, the leukocyte count increased suddenly to 85700/μL and serum G-CSF levels rose to 70.4pg/mL (normal range, 4.7-18.1). Computed tomography of the chest showed multiple pulmonary metastases and the patient suddenly developed pleural effusions. His blood calcium levels were extremely high (19.5mg/dL; normal range, 8.4-10.2mg/dL). Three weeks after resection of the tumor, the patient died of respiratory failure. His WBC count was 189600/μL. An autopsy was not performed.
G-CSF-producing tumors are rare and have been reported for SSC, anaplastic large-cell lymphoma,1 and angiosarcoma.2 Just 5 cases of G-CSF-producing SCCs, including ours, have been described to date (Table 1).3–6 All the patients had WBC counts of over 20 000/μL, but the count in our case was much higher. Hypercalcemia is a common complication of SCC and is believed to be caused by the stimulation of peptide parathyroid hormone–related peptide (PTH-rP) by tumor cells. In our patient, the immunohistochemistry study showed weak expression of PTH-rP (data not shown), but unfortunately, serum levels were not measured.
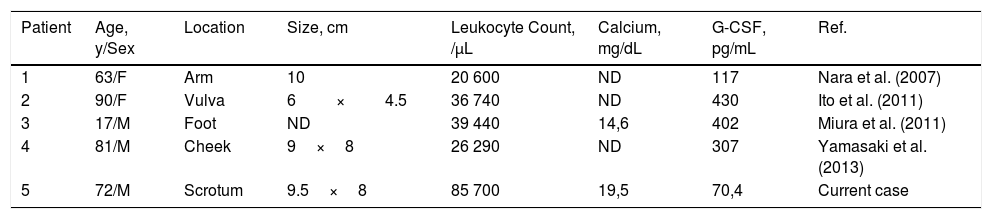
Cases of Granulocyte Colony-Stimulating Factor (G-CSF)–Producing Squamous Cell Carcinomas in the English-Language Literature.
| Patient | Age, y/Sex | Location | Size, cm | Leukocyte Count, /μL | Calcium, mg/dL | G-CSF, pg/mL | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 63/F | Arm | 10 | 20 600 | ND | 117 | Nara et al. (2007) |
| 2 | 90/F | Vulva | 6×4.5 | 36 740 | ND | 430 | Ito et al. (2011) |
| 3 | 17/M | Foot | ND | 39 440 | 14,6 | 402 | Miura et al. (2011) |
| 4 | 81/M | Cheek | 9×8 | 26 290 | ND | 307 | Yamasaki et al. (2013) |
| 5 | 72/M | Scrotum | 9.5×8 | 85 700 | 19,5 | 70,4 | Current case |
Abbreviations: F, female; M, male; ND, no data.
According to the criteria for leukocytosis in cancer,7 WBC counts should improve after tumor resection. In the case reported, however, the count did not improve. What is more, it remained high. Some authors have reported that primary tumors do not produce G-CSF, unlike recurrent tumors, which produce large amounts of this growth factor.8 In our patient, however, both the primary lesion and the lymph node metastasis showed high G-CSF expression. We unfortunately did not measure serum G-CSF levels during the course of the patient's disease so are unable to determine whether or not there was a correlation with disease activity. In addition, further studies of G-CSF levels in genital SCC are needed. G-CSF-producing SCCs often have an undifferentiated histologic phenotype, with a loss of epithelial features and epithelial-mesenchymal transition.6 In our case, the tumor cells were positive for keratin and vimentin, indicating induction of this transition. According to an earlier study, G-CSF gene expression can result in autocrine/paracrine stimulation of local invasion and angiogenesis in SCC.9 Serum SCC antigen levels were high in our patient, coinciding with previous reports for cutaneous SCC.10,11 Angiosarcoma was ruled out in all cases, as even though the tumors were highly vascularized, they were negative for CD31 staining. In addition, the tumor cells expressed both G-CSF and its receptor, indicating autocrine proliferation. In conclusion, and as indicated by the case described, G-CSF-producing tumors carry a poor prognosis.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Takako M, Yasunobu K, Toshiyuki Y. Carcinoma en escroto de células escamosas gigantes productor de factor estimulante de colonias de granulocitos (G-CSF). Actas Dermosifiliogr. 2019;110:57–59.