Vismodegib is the first selective Hedgehog inhibitor approved for the treatment of locally advanced and metastatic basal cell carcinoma (BCC). In this article, we describe our experience with the use of this drug to treat advanced and/or multiple BCCs at a cancer center over 5 years.
Material and methodsWe analyzed the following variables: patient age and sex; tumor location, size, type, and characteristics; time since onset; primary or recurrent status; duration of treatment; response to treatment (complete, partial, stabilization, or absence of response); adverse effects; and recurrences.
ResultsWe treated 22 patients, of whom 20 had locally advanced BCCs and 2 had metastatic BCCs with lymph node involvement. The treatment was administered over a mean of 11.8 months. Nine patients (41%) achieved complete response and 10 (45%) partial response. The disease was stabilized in 3 (14%). Two patients relapsed after a median of 21 months. The main adverse effects were dysgeusia, alopecia, and muscle cramps, all of which were mild. None of the patients developed squamous cell carcinoma in an area treated with vismodegib, although metatypical changes were observed after treatment.
ConclusionsWith a response rate of 96%, vismodegib is a safe and effective treatment for locally advanced BCC. Adverse effects are generally mild but they need to be taken into account owing to their high frequency.
Vismodegib es el primer inhibidor selectivo de la vía de la señalización Hedgehog aprobado para el tratamiento del carcinoma basocelular (CBC) localmente avanzado y metastásico. Describimos nuestra experiencia en un centro oncológico con el vismodegib en el tratamiento de pacientes con CBC avanzados y/o múltiples durante un periodo de 5 años.
Material y métodosAnalizamos variables como la edad y el sexo del paciente, la localización, el tamaño, el tipo y las características del tumor, el tiempo de evolución, si son tumores primarios o recidivas, la duración del tratamiento, la respuesta a este (completa, parcial, estabilización o ausencia de respuesta), los efectos secundarios observados y las recidivas.
ResultadosUn total de 22 pacientes fueron tratados, 20 con CBC localmente avanzados y 2 con CBC metastásicos con afectación ganglionar. El tratamiento fue administrado durante 11,8 meses de media. El 41% (9) de los pacientes obtuvieron una respuesta completa al tratamiento, un 45% (10) respuesta parcial y en el 14% (3) de los pacientes el tratamiento consiguió estabilizar la enfermedad. Tras una mediana de 21 meses, 2 casos recidivaron. Los principales efectos secundarios observados fueron disgeusia, alopecia y calambres musculares, todos ellos de carácter leve. Ningún paciente desarrolló un carcinoma epidermoide sobre el área tratada con vismodegib, aunque sí cambios metatípicos tras el tratamiento.
ConclusionesEl vismodegib es un fármaco seguro y eficaz para el tratamiento del CBC localmente avanzado, con un porcentaje de respuesta del 86%. Los efectos adversos deben tenerse en cuenta por su alta frecuencia, aunque estos suelen ser de carácter leve.
Basal cell carcinoma (BCC) is the most common skin tumor. Approximately 80% of nonmelanoma skin tumors are BCCs,1 that is, an incidence of 113-253 cases/100 000 person-years in Spain.2 Within this group of tumors, the incidence of advanced BCC is estimated to be 0.6%-0.8%,3 or approximately 300-900 cases per year. We can differentiate between 2 types of BCCs: locally advanced BCCs, which include those with lesions for which current treatment modalities, such as surgery and radiotherapy, are considered potentially contraindicated owing to tumor- or patient-related factors4; and metastatic BCCs, which can disseminate to lymph nodes or spread to distant organs, although this is very uncommon (0.003%-0.1% of BCCs).5,6
Various genetic studies reveal alterations in the Hedgehog signaling pathway in the vast majority of BCCs.7 This was the setting that witnessed the appearance of vismodegib, the first selective Hedgehog inhibitor approved for the treatment of advanced and metastatic BCC. The drug acts by specifically binding to and inactivating the receptor of the G protein Smoothend, thus stopping activation of the transcription factor family of the glioma-associated oncogene and suppressing proliferation and growth of the tumor.8
Vismodegib was approved for the treatment of advanced BCC in January 2012 in the United States of America and in July 2013 in Europe. In June 2016, the Spanish Ministry of Health authorized funding for the drug through the National Health System for use in adults with advanced BCC or with symptoms of metastatic disease in whom the physician considered that other treatments would be inappropriate.
ObjectivesIn the present study, we describe our experience with vismodegib for the treatment of advanced and/or multiple BCCs over a 5-year period at a cancer center.
Material and MethodsWe performed a retrospective observational study of 22 patients with BCC treated in the Dermatology Department of Fundación Instituto Valenciano de Oncología, Valencia, Spain between June 2012 and October 2017.
The patients selected to receive vismodegib had advanced and/or multiple BCCs. The nonmelanoma skin cancer tumor board considered that for these patients, treatments such as surgery and radiotherapy were inappropriate owing to the patient's profile, the invasiveness of the technique, or the likelihood of a successful outcome.
All of the patients received oral vismodegib 150mg per day until the disease progressed or they developed unacceptable toxicity. Some patients also interrupted treatment because the disease had completely resolved. Patients were followed up at monthly visits, where the progress of the lesion, tolerance, and adverse effects were recorded.
The variables analyzed for each of the patients were as follows: age and sex; location, size, type, and characteristics of the tumor; time since onset; primary or recurrent status; duration of treatment; response to treatment (complete, partial, stabilization, or none); adverse effects; and recurrences after treatment. A complete response was defined as absence of tumor based on clinical, radiological, and/or histologic evidence. A partial response was defined as a 30% reduction in the diameter of the tumor (based on clinical or radiological findings). Stabilization was defined as not fulfilling the criteria for partial response, complete response, or progression. We defined progression as a ≥20% increase in the initial size of the tumor.1
In order to determine the time to response to vismodegib, we recorded the time of onset of the response, that is, the time between initiation of therapy and the first signs of response. We also recorded the time to maximum response, that is, the time until maximum reduction in the size of the tumor.
All data were recorded from the clinical history, biopsy bank of the pathology department, and the photographic archive of our department.
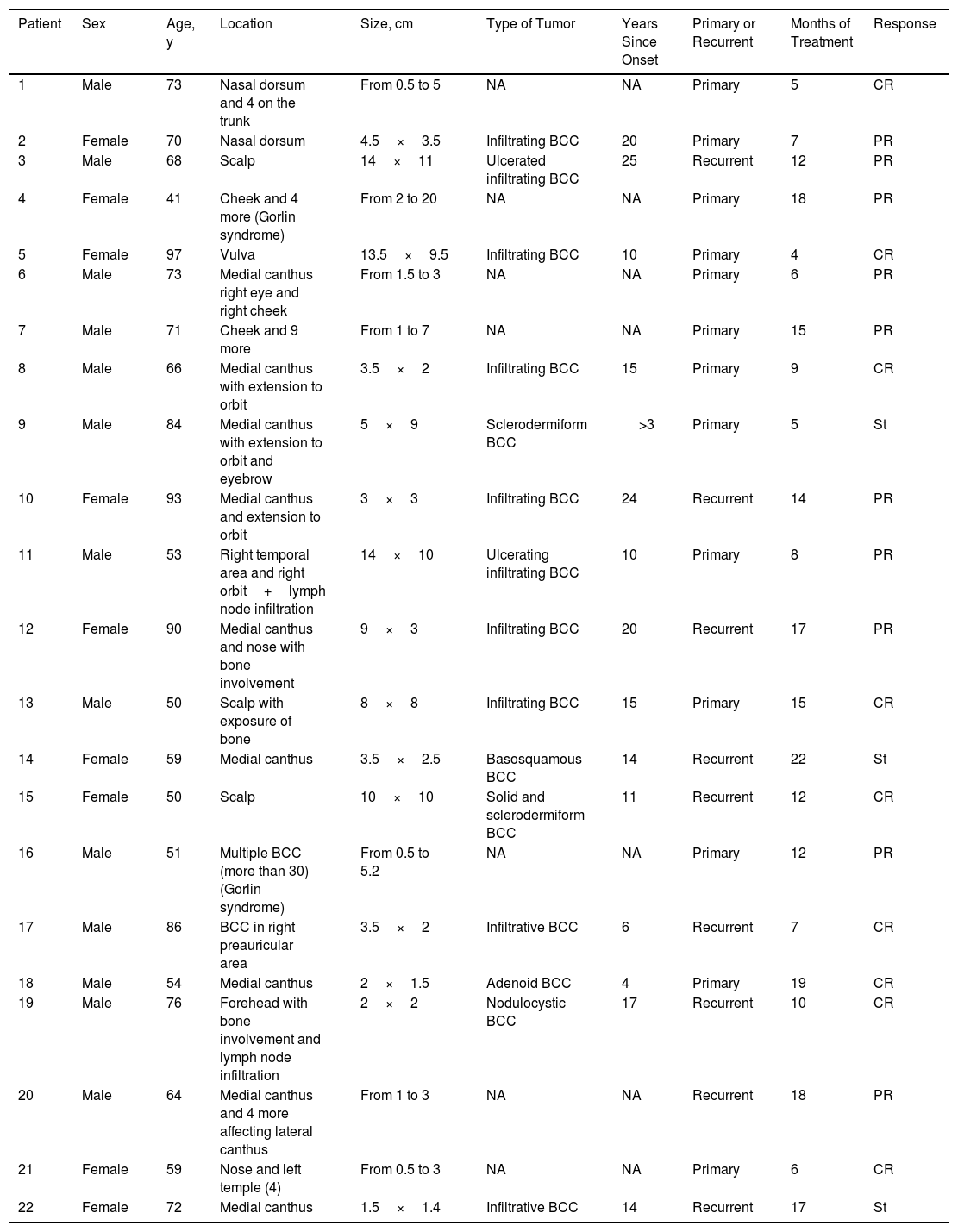
ResultsWe treated 22 patients: 20 with locally advanced basal cell carcinoma, and 2 with metastatic disease and lymph node involvement. The characteristics of these patients are summarized in Table 1.
Data on Patients and Tumors Included in the Series.
| Patient | Sex | Age, y | Location | Size, cm | Type of Tumor | Years Since Onset | Primary or Recurrent | Months of Treatment | Response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 73 | Nasal dorsum and 4 on the trunk | From 0.5 to 5 | NA | NA | Primary | 5 | CR |
| 2 | Female | 70 | Nasal dorsum | 4.5×3.5 | Infiltrating BCC | 20 | Primary | 7 | PR |
| 3 | Male | 68 | Scalp | 14×11 | Ulcerated infiltrating BCC | 25 | Recurrent | 12 | PR |
| 4 | Female | 41 | Cheek and 4 more (Gorlin syndrome) | From 2 to 20 | NA | NA | Primary | 18 | PR |
| 5 | Female | 97 | Vulva | 13.5×9.5 | Infiltrating BCC | 10 | Primary | 4 | CR |
| 6 | Male | 73 | Medial canthus right eye and right cheek | From 1.5 to 3 | NA | NA | Primary | 6 | PR |
| 7 | Male | 71 | Cheek and 9 more | From 1 to 7 | NA | NA | Primary | 15 | PR |
| 8 | Male | 66 | Medial canthus with extension to orbit | 3.5×2 | Infiltrating BCC | 15 | Primary | 9 | CR |
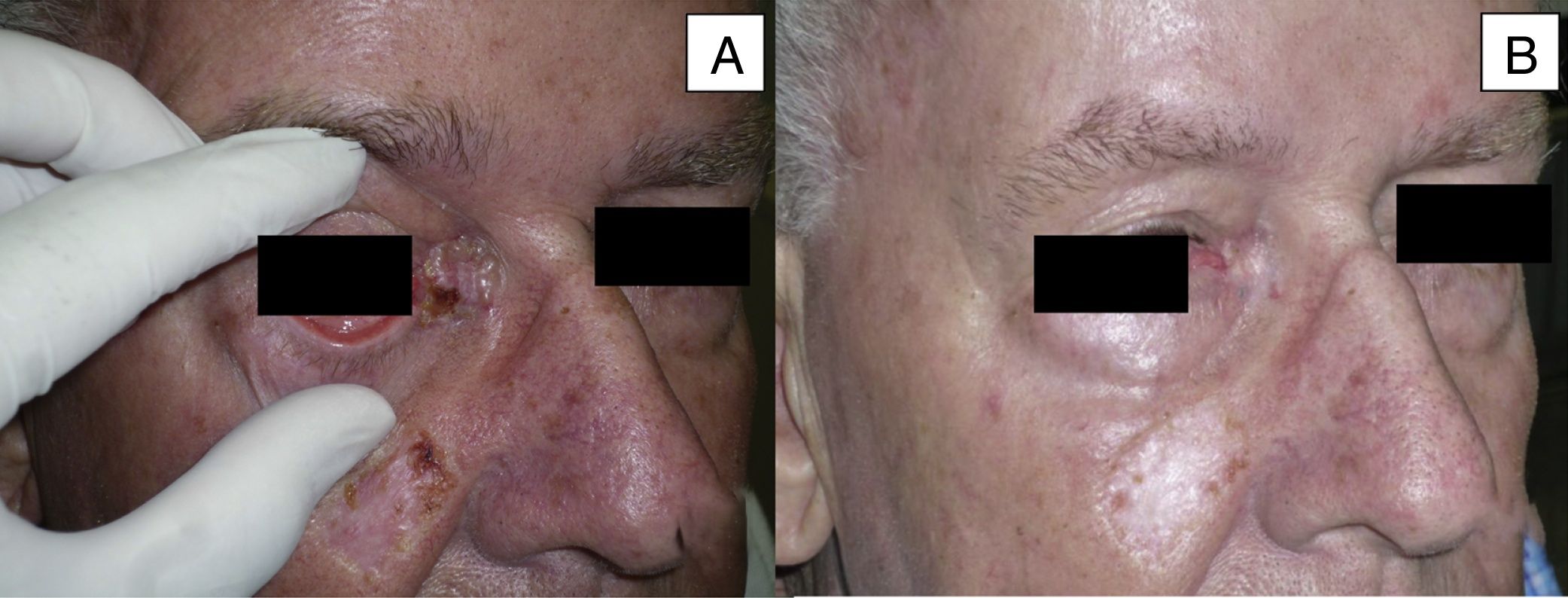
| 9 | Male | 84 | Medial canthus with extension to orbit and eyebrow | 5×9 | Sclerodermiform BCC | >3 | Primary | 5 | St |
| 10 | Female | 93 | Medial canthus and extension to orbit | 3×3 | Infiltrating BCC | 24 | Recurrent | 14 | PR |
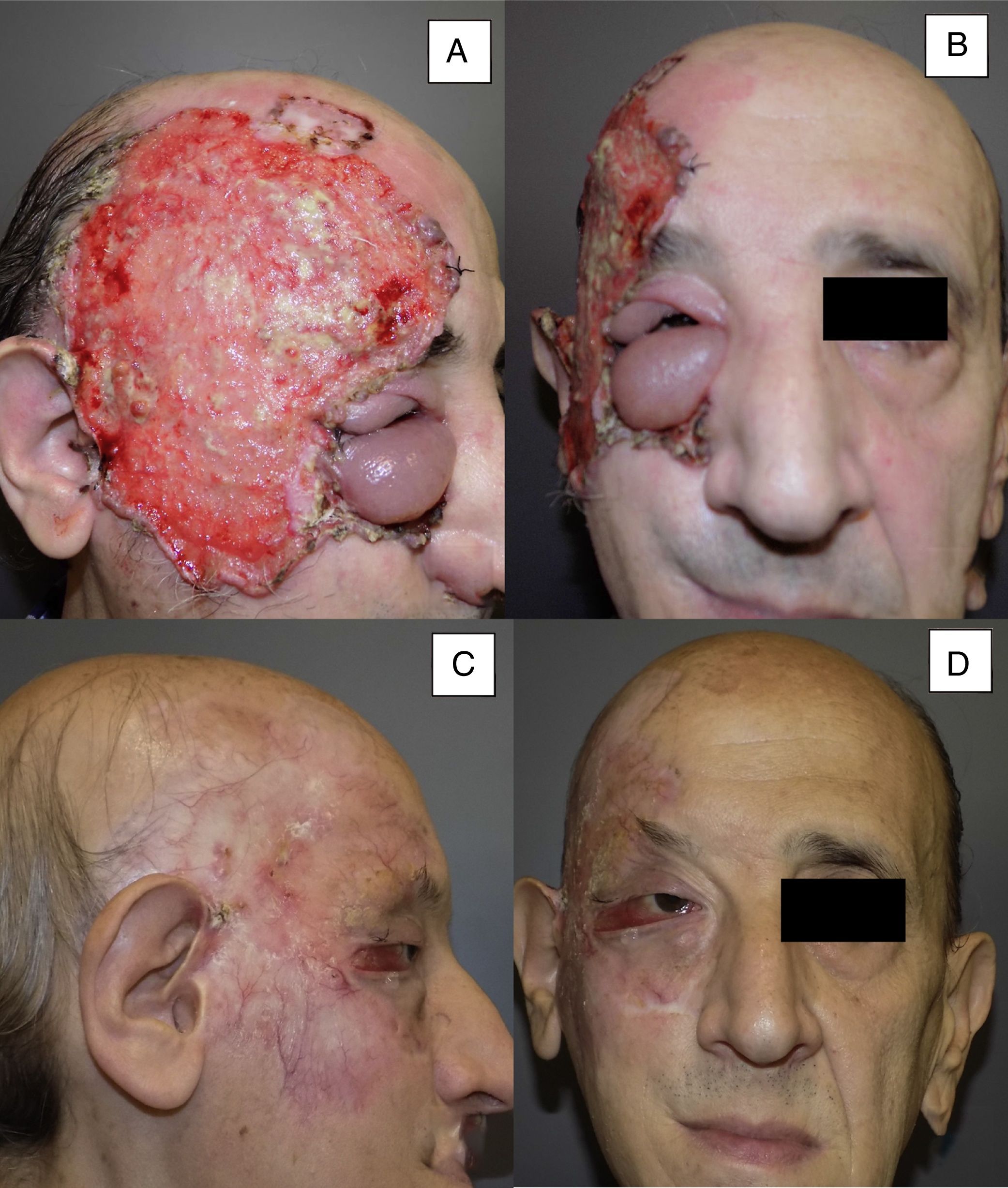
| 11 | Male | 53 | Right temporal area and right orbit+lymph node infiltration | 14×10 | Ulcerating infiltrating BCC | 10 | Primary | 8 | PR |
| 12 | Female | 90 | Medial canthus and nose with bone involvement | 9×3 | Infiltrating BCC | 20 | Recurrent | 17 | PR |
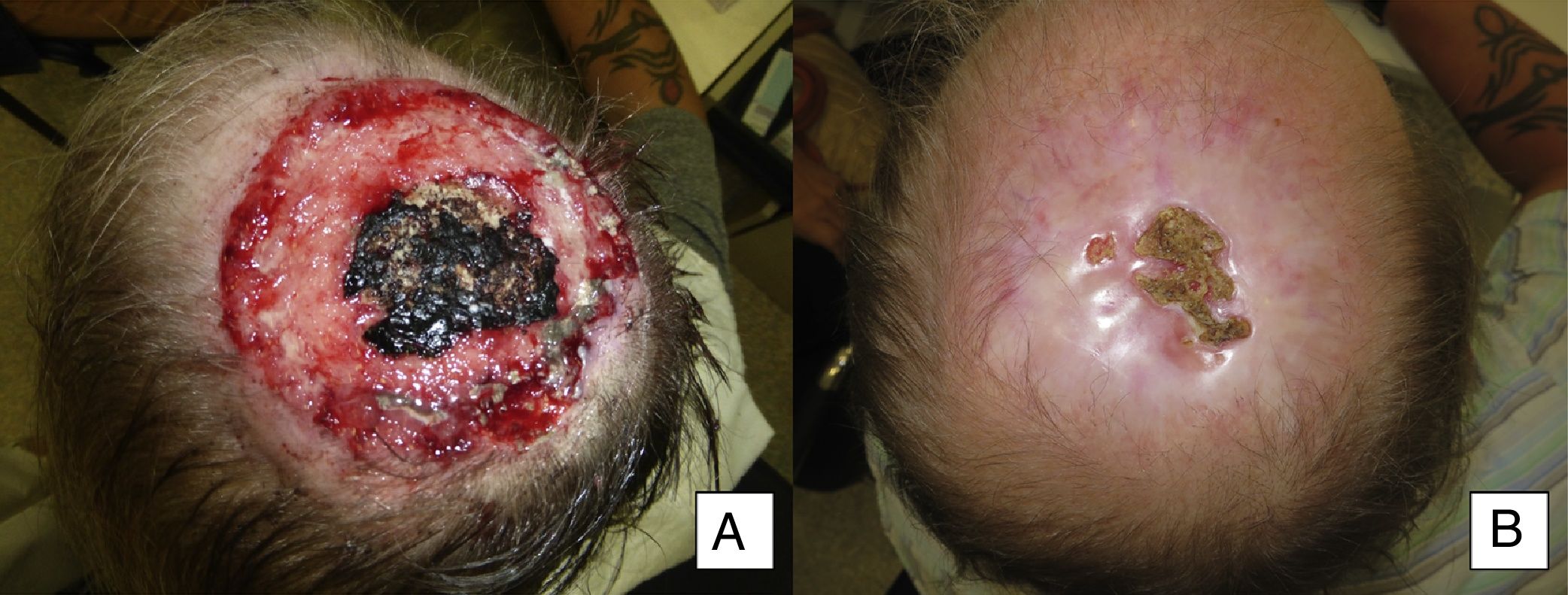
| 13 | Male | 50 | Scalp with exposure of bone | 8×8 | Infiltrating BCC | 15 | Primary | 15 | CR |
| 14 | Female | 59 | Medial canthus | 3.5×2.5 | Basosquamous BCC | 14 | Recurrent | 22 | St |
| 15 | Female | 50 | Scalp | 10×10 | Solid and sclerodermiform BCC | 11 | Recurrent | 12 | CR |
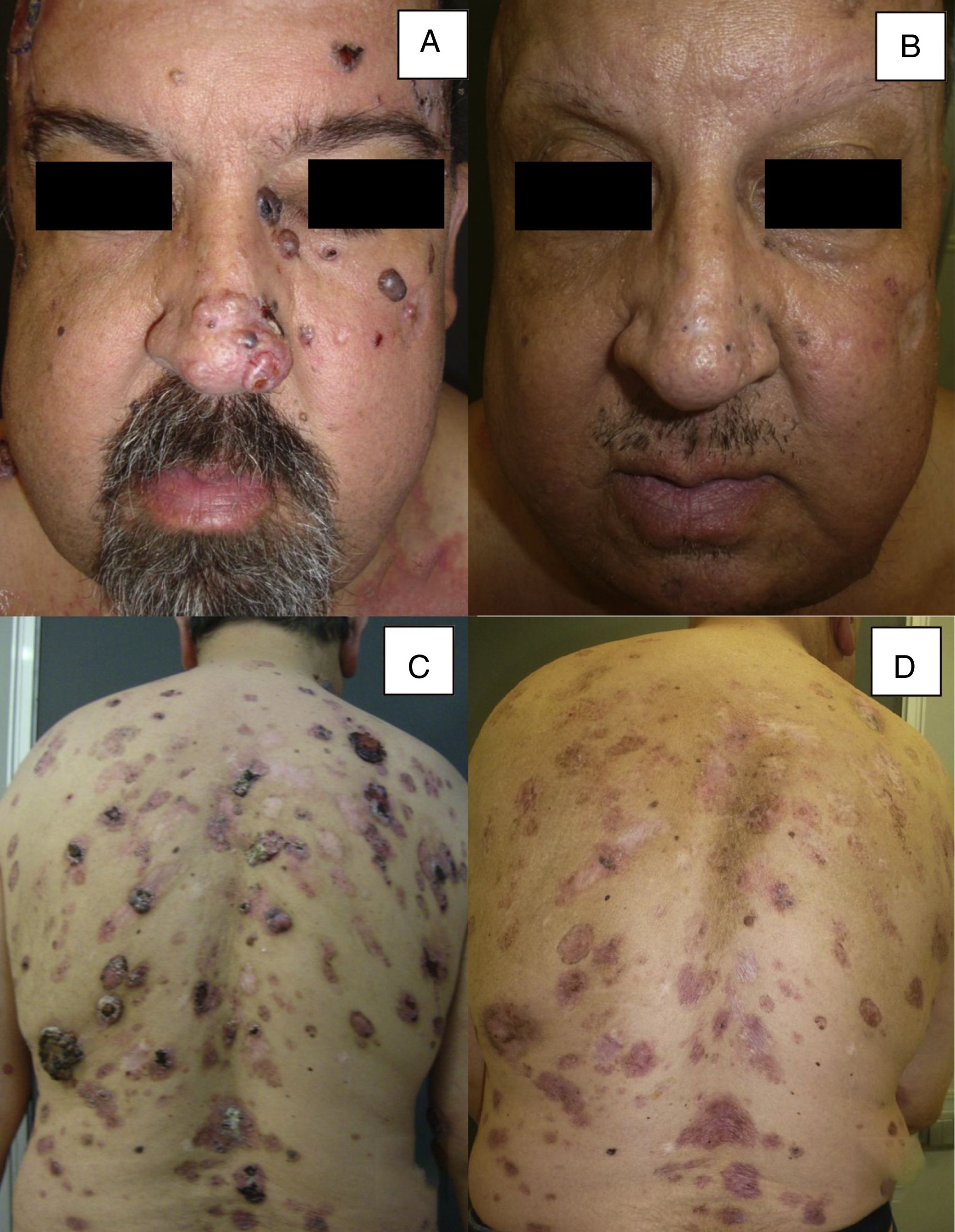
| 16 | Male | 51 | Multiple BCC (more than 30) (Gorlin syndrome) | From 0.5 to 5.2 | NA | NA | Primary | 12 | PR |
| 17 | Male | 86 | BCC in right preauricular area | 3.5×2 | Infiltrative BCC | 6 | Recurrent | 7 | CR |
| 18 | Male | 54 | Medial canthus | 2×1.5 | Adenoid BCC | 4 | Primary | 19 | CR |
| 19 | Male | 76 | Forehead with bone involvement and lymph node infiltration | 2×2 | Nodulocystic BCC | 17 | Recurrent | 10 | CR |
| 20 | Male | 64 | Medial canthus and 4 more affecting lateral canthus | From 1 to 3 | NA | NA | Recurrent | 18 | PR |
| 21 | Female | 59 | Nose and left temple (4) | From 0.5 to 3 | NA | NA | Primary | 6 | CR |
| 22 | Female | 72 | Medial canthus | 1.5×1.4 | Infiltrative BCC | 14 | Recurrent | 17 | St |
Abbreviations: BCC, basal cell carcinoma; CR, complete response (all tumors in patients with multiple BCC); NA, not assessed; PR, partial response; St, stabilization.
The median age at initiation of treatment was 69 years; 13 patients were men and 9 women. Thirteen of the tumors evaluated were primary and 9 recurrent, with a median of 13 years (1-25 years) since onset.
Most tumors were located around the eye (12 cases). Other frequently affected sites were the scalp (5 cases), nose (3 cases), forehead (2 cases), cheek (2 cases), and vulva (1 case). Two of the patients included in the present series were diagnosed with Gorlin syndrome (patients 4 and 16). The size of the tumors ranged from 1.5cm to 20cm, with a median size of 4cm.
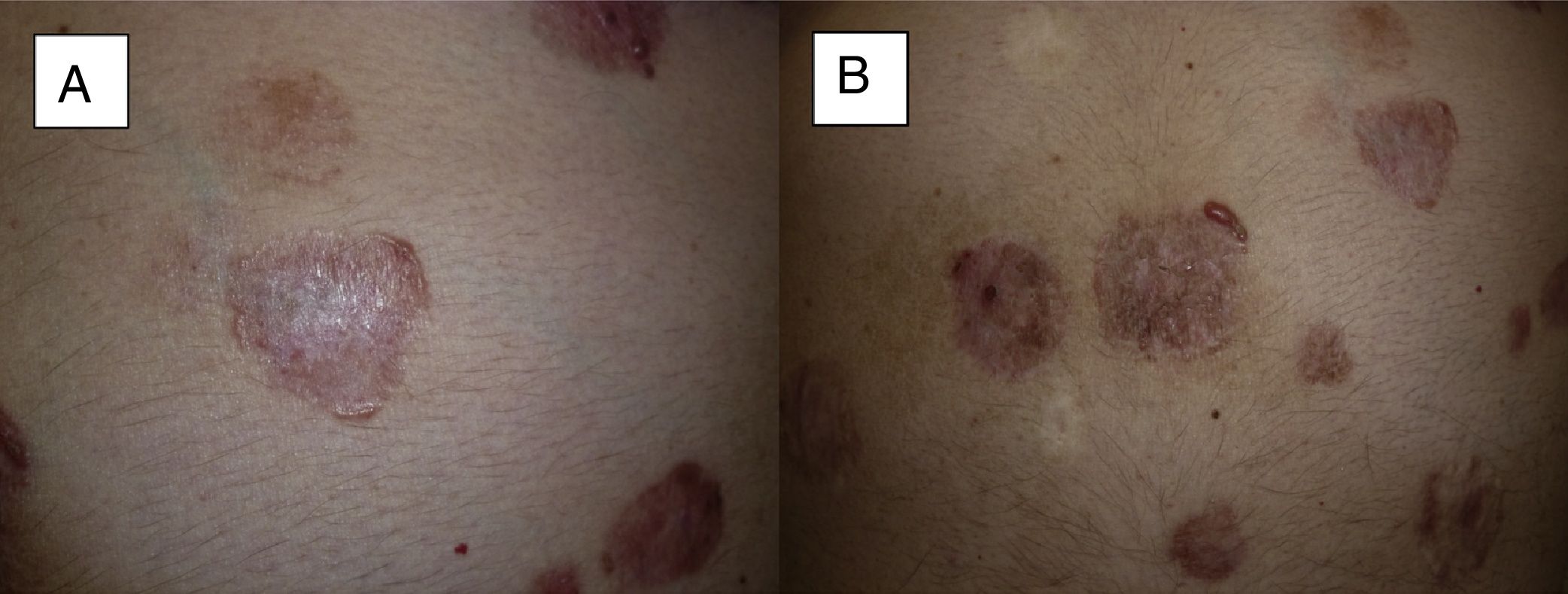
Treatment was administered for a mean of 11.8 months. A complete response was recorded in 9 patients (41%) (Fig. 1A and B), and a partial response was recorded in 10 patients (45%) (Fig. 2A and B, Figs. 3 and 4A-D). The disease stabilized with vismodegib in 3 cases (14%).
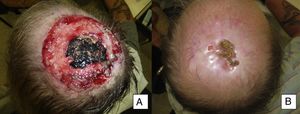
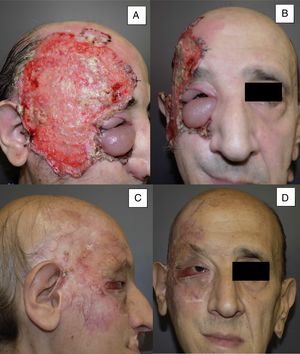
Man aged 50 years with infiltrating basal cell carcinoma measuring 8×8cm on the scalp with exposed bone (A). Treatment with vismodegib led to a response during the first month. The maximum response was observed at 8 months (B). The patient has been disease-free for 13 months (patient 13 in Table 1).
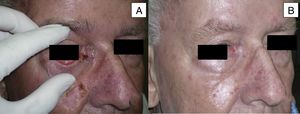
Man aged 73 years with sclerodermiform basal cell carcinoma measuring 3×3cm on the medial canthus (A). A partial response was observed after 6 months of treatment (B) (patient 6 in Table 1).
Man aged 53 years with an ulcerated infiltrating basal cell carcinoma measuring 14×10cm on the right temporal area and right orbit with associated palpebral edema and infiltration of lymph nodes (A and B). Clinical signs of a complete response are observed after 8 months of treatment (C and D). Nevertheless, a follow-up biopsy revealed residual tumor, and the response was classified as partial (patient 11 in Table 1).
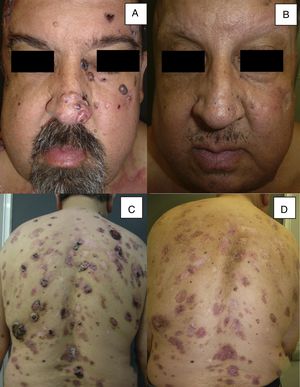
Man aged 51 years affected by Gorlin syndrome with multiple basal cell carcinomas (more than 30) (A and C). A partial response was achieved after 12 months of treatment (B and D) (patient 16 in Table 1).
The median time to onset of response was 1 month (1-5 months). The median time to maximum response was 5 months (1-15 months).
Histologically, complete response was characterized by replacement of tumor tissue by dermis with abundant hyaline stroma, which was sometimes accompanied by a mild inflammatory infiltrate. Partial response was characterized by 2 tendencies: one in which the size and ulceration of the tumor was reduced, with the morphology of BCC maintained; and another, in which the tumor became metatypical, with larger and eosinophilic cells with large and pleomorphic nuclei, that is, more undifferentiated. Furthermore, in 1 case the subtype of the tumor changed from infiltrating BCC to basosquamous cell carcinoma.
Patients were followed for a mean of 38 months and a median of 21 months (3-59 months). Of the patients who achieved a complete response (9 cases), 2 (22%) experienced a recurrence (based on clinical, histological, and/or radiological findings), with a time to recurrence of 2 and 10 months (Fig. 5A and B). In our series, no patients developed squamous cell cancer on an area treated with vismodegib. Nevertheless, squamous cell cancer was observed at a site other than that treated 3 years after completion of treatment.
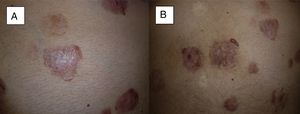
Recurrence at 4 months after completion of treatment. We can see that in 2 of the tumors located on the abdomen, local recurrence begins from the periphery of the tumor (A and B) (patient 16 in Table 1).
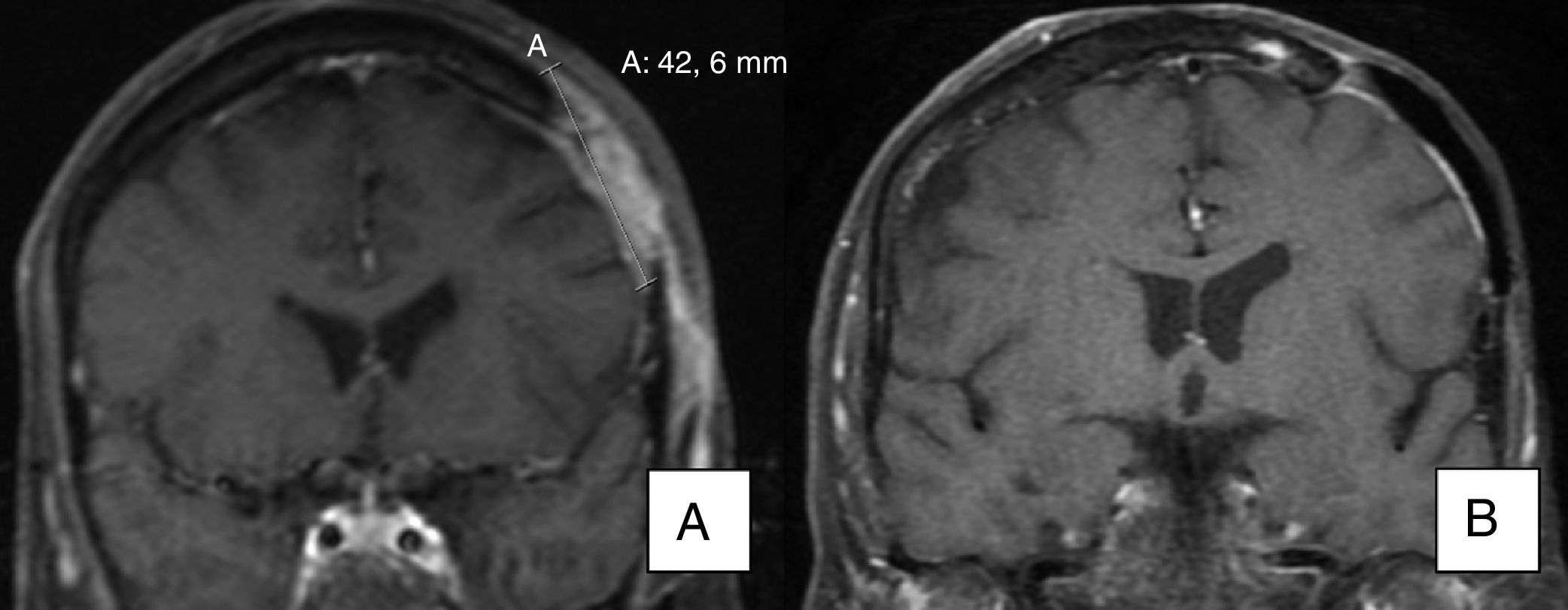
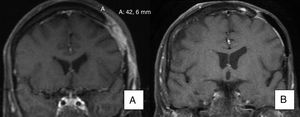
As for patients with metastatic BCC, one (patient 11) achieved a partial response, with a reduction in the size of a cervical lymph node affected by the tumor, as seen on a magnetic resonance image taken 3 months after initiation of the drug. Another patient with metastatic BCC (patient 19) achieved a complete response with no new lymph node involvement according to the imaging tests ordered during follow-up (the lymph node affected in the parotid region was removed before initiation of vismodegib).
All of the patients experienced adverse effects, mainly dysgeusia, alopecia, and muscle cramps. Although these were all mild, they led to temporary interruption of treatment due to intolerance in 5 cases.
DiscussionDespite the lack of an established definition for locally advanced BCC, this is the term proposed for BCC classed as stageII by the American Joint Committee on Cancer (tumors>2cm and with at least 2 high-risk factors, such as depth of invasion>2mm, Clark levelIV, perineural invasion, location in the H-zone of the face, and poor tumor differentiation).9 Subsequent publications, such as that of a British multidisciplinary group, support this definition and add a series of tumor-dependent factors (giant tumor size, location in the H-zone of the face, high number of tumors, histologic subtype, and possibility of curative treatment) and patient-dependent factors (age, patient's general status, diminished quality of life as a consequence of treatment, patient's opinion, and the presence of genodermatosis or major comorbidities).10
This is the setting in which vismodegib appeared as a targeted therapy with the ability to selectively inhibit the molecular signaling pathway of BCC, thus offering an alternative approach to surgery or radiotherapy for the treatment of advanced BCCs.
In their pivotal ERIVANCE study, Sekulic et al.1 analyzed 104 patients (71 with locally advanced BCC and 33 with metastatic BCC) who received vismodegib at 150mg per day. In this first trial, they obtained responses of 48.5% for metastatic BCC and 60.3% for locally advanced BCC. Subsequent trials were designed, such as STEVIE, with a total of 1232 patients and the main objective of monitoring drug safety, and MIKIE, which included patients with multiple BCCs. The response rate in STEVIE was 68.5%,11 and that of MIKIE ranged from 54% to 62%.12
The rate of response to vismodegib in our series was 41% for complete response and 45% for partial response, that is, a joint response rate of 86%, which is considerably higher than that of ERIVANCE (60.3%), STEVIE (68.5%), and MIKIE (54%-62%). Furthermore, the drug stabilized the disease in 14% of cases. We believe that this difference in response rates between our series and published trials could be due to the small number of patients in our series (n=22), compared with the 104 patients in ERIVANCE, 229 in MIKIE, and 1232 in STEVIE.
With respect to the time until the drug achieved its initial results, we must remember that the analysis of time to response yielded a median time to onset of response of 5 months (1-5 months) and a median time to maximum response of 5 months (1-15 months). In this case, our results are similar to those of ERIVANCE, in which the time to maximum reduction of the tumor was 5.5-6.7 months.1 Therefore, with vismodegib, our results were clearly visible at the first check-ups, and the maximum response was often reached within a few months of initiation of therapy. Such was the of case of patient 1, a 73-year-old man with multiple BCCs in whom, at his first follow-up visit (first month), all of the lesions were healing, with almost no clinical evidence of residual tumor (Table 1, patient 1).
Vismodegib is taken as a tablet at a daily dose of 150mg. Since pregnancy is contraindicated, it is important to implement a prevention program for both men and women. Pregnancy should be avoided during treatment and for 2 months after completion of treatment in men and up to 2 years in women. Where possible, a biopsy should be performed to confirm the diagnosis, and magnetic resonance imaging should be performed to measure the subsequent radiological response. Similarly, the patient should be followed up monthly with laboratory assessment including liver and muscle function.
As for adding vismodegib to existing therapy, our experience indicates that concurrent administration reveals a synergetic effect with radiotherapy, possibly owing to its radiosensitizing effect on the tumor. Patient 15 in the present series was a 50-year-old woman with sclerodermiform BCC on the scalp measuring 10×10cm who underwent surgery on 15 occasions because of multiple recurrences. She even underwent craniotomy with subsequent radiotherapy. She achieved a complete response after 12 months of therapy with vismodegib (Fig. 6A and B) and remains recurrence-free (disease-free interval, 17 months). The literature contains reports of similar experience with the combination of vismodegib and radiotherapy showing an excellent response with no additional adverse effects other than those of the drug itself and good tolerance by the patient.13
A) Magnetic resonance image showing invasion of bone by the tumor. B) Evidence of radiological resolution after 12 months of treatment (patient 15 in Table 1).
Vismodegib has been reported to increase the risk of transformation to squamous cell carcinoma in BCC treated with the drug and to lead to the development of squamous cell carcinoma at other sites. Mohan et al.14 reported an increase in the risk of tumors other than BCC after treatment with vismodegib (hazard ratio of 6.37) and in the case of squamous cell carcinoma (hazard ratio of 8.12). However, the study was widely criticized by authors such as Puig et al.,15 mainly because of its poor design (retrospective cohort study, as opposed to a case-control study, as stated in the article). Furthermore, the study included all tumors that appeared, some of which were diagnosed only 15 days after initiation of treatment.15 As mentioned above, no patients in the present series developed squamous cell carcinoma on the area treated with vismodegib, although one did 3 years later and at another site. The most recently published articles report similar experience. In 2017, Bhutani et al.16 reported the results of a retrospective study of 1675 patients treated with vismodegib in the STEVIE and ERIVANCE trials and concluded that treatment was not associated with development of squamous cell carcinoma. Nevertheless, during treatment, a histological change can be observed to metatypical basal cell carcinoma or even basosquamous cell carcinoma.
As for the safety and tolerability profile, many adverse events were associated with the mechanism of action of the drug. Most were grade 1 or 2.17 Almost all of the patients in the present study experienced adverse effects, the most frequent being dysgeusia, alopecia, and muscle cramps, all of which were mild. Other adverse effects observed were asthenia, anorexia, and weight loss. In 5 patients (22.7%), the adverse effects led treatment to be interrupted temporarily, since they were disabling, especially dysgeusia and ageusia. This percentage is similar to that reported in the ERIVANCE trial, in which 21.2% of patients had to interrupt treatment.18 An important observation associated with the findings reported above is that interrupting treatment does not seem to compromise efficacy11; in fact, the ERIVANCE trial allowed interruptions of up to 8 weeks, without these having any effect on the response to therapy.1
ConclusionsVismodegib is a safe and effective drug for the treatment of advanced BCCs, with response rates of 54%-86%. The response to vismodegib is fast (median of 1 month), and the maximum response is achieved in under 6 months (median of 5 months). None of our patients developed squamous cell cancer on the area treated with vismodegib. However, given the possibility of metatypical changes in histology, it is advisable to follow patients carefully and to biopsy areas that seem to resist treatment. Given their high frequency, adverse effects should be taken into account, although these are usually mild.
Conflicts of InterestB. Llombart, C. Serra-Guillén, and O. Sanmartín have been paid for talks by Hoffmann-La Roche. The remaining authors declare that they have no conflicts of interest.
Please cite this article as: Bernia E, Llombart B, Serra-Guillén C, Bancalari B, Nagore E, Requena C, et al. Experiencia con vismodegib en carcinoma basocelular avanzado en un centro oncológico. Actas Dermosifiliogr. 2018;109:813–820.