In recent years, the growing interest in the role played by vitamin D in skin disease has given rise to the publication of many studies of the relationship between this vitamin and certain skin conditions. As dermatologists, we need to understand, among other aspects, how vitamin D is synthesized and the main sources in humans, as well as plasma levels and the factors that can modify them. Of particular interest are the latest discoveries about the role of vitamin D in skin diseases such as lupus erythematosus, ichthyosis, atopic dermatitis, hidradenitis suppurativa, acne, alopecia areata, androgenetic alopecia, melanoma, and nonmelanoma skin cancer. Also of interest is the importance of vitamin D as adjuvant therapy in patients on long-term treatment with corticosteroids. In this review, we provide an overview of the most important and most recent information regarding the relationship between vitamin D and skin disease and discuss the importance of assessing individual vitamin D status and correcting deficiencies.
El papel de la vitamina D en enfermedades cutáneas ha cobrado interés en los últimos años. La relación entre esta vitamina y algunas dermatosis ha supuesto la publicación de múltiples trabajos al respecto. Como dermatólogos, conocer sus principales fuentes, síntesis, niveles plasmáticos y algunos de los factores modificadores son varios de los aspectos a recordar. Asimismo, es preciso resaltar los últimos descubrimientos sobre el papel de la vitamina D en las diferentes enfermedades dermatológicas, como el lupus eritematoso, la ictiosis, la dermatitis atópica, la hidrosadenitis supurativa, el acné, la alopecia areata y androgenética, el melanoma y el cáncer cutáneo no melanoma, así como la relevancia como terapia adyuvante en pacientes en tratamiento crónico con corticoides. Acercamos al lector la información más relevante y reciente de la relación entre la vitamina D y las enfermedades de la piel, así como la importancia de conocer los niveles de esta vitamina.
Rickets, a condition caused by vitamin D deficiency, was first described by Glisson, DeBoot and Whistler in the seventeenth century. Subsequently, in 1882, Sniadecki realized the importance of exposure to sunlight for the prevention and cure of rickets. Today, we know that the role of vitamin D in the body goes beyond the regulation of bone mineral metabolism, making the function of this vitamin important to a wide range of medical specialists. Charpy1 published the first study linking vitamin D levels and lupus vulgaris in 1945. In 2016, Deschasaux et al.2 published the results of a study based on an online questionnaire designed to investigate the understanding of the role of vitamin D in the general population. They reported that over 1-third of the respondents were unaware of the role of vitamin D in the body—a significant finding in light of the fact that vitamin D levels were insufficient in 40% of a subsample of respondents in whom these were tested. The recent literature includes several interesting new studies linking vitamin D levels with a number of common skin disorders. The aim of the present review is to provide an overview of the relationship between vitamin D and skin disease based on current evidence.
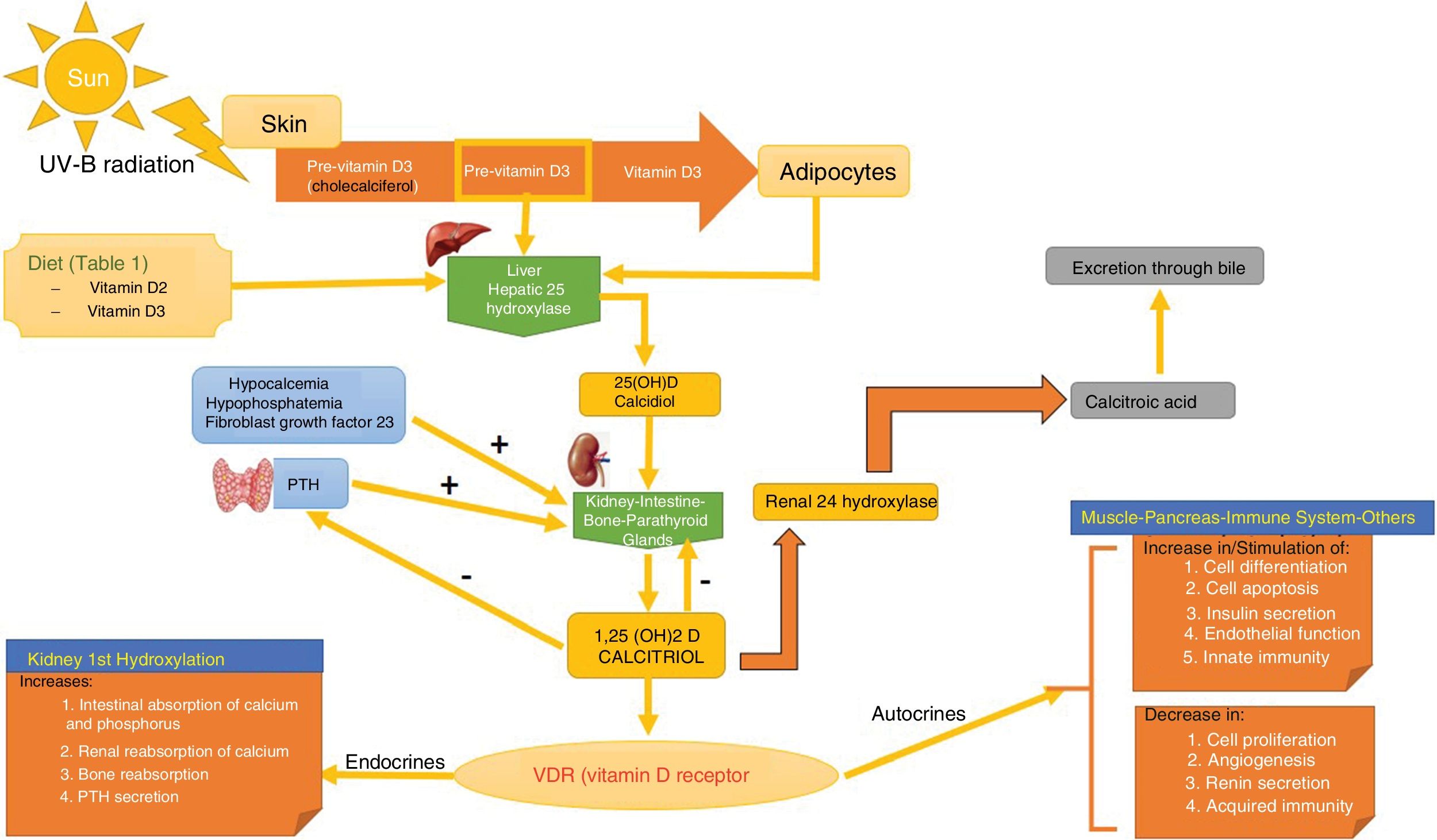
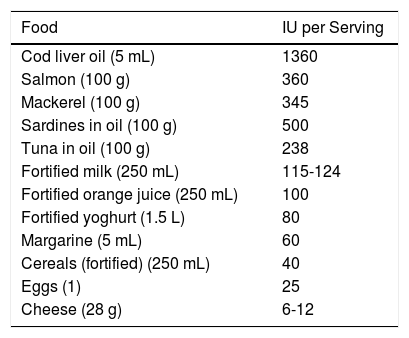
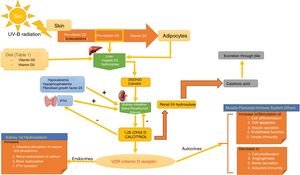
SynthesisThe body obtains vitamin D in two ways. A small amount of active vitamin D in the form of cholecalciferol can be obtained from dietary intake of animal origin: the foods with significant levels are egg yolks and certain blue fish (Table 1). Ergocalciferol, another form of pre-vitamin D, is found in small amounts in foods of vegetable origin. The other way the body obtains vitamin D is through exposure to solar radiation. Photons in UV-B radiation photo-isomerize 7-dehydrocholesterol in the epidermis to precholecalciferol, which is then rapidly converted through a heat-mediated reaction to cholecalciferol or vitamin D3. Both molecules are hydroxylated in the liver to calcifediol (25-hydroxyvitamin D [25(OH)D]), which is later hydroxylated in the kidneys through the action of the enzyme 25(OH)D-1-hydroxylase to calcitriol, the active metabolite that performs a range of different functions by binding to its cell receptor. Parathyroid hormone (PTH), calcium, and phosphorus levels are just some of the factors that play a role in the regulation of this process, in addition to transforming growth factor beta (Fig. 1).
Foods Rich in Vitamin D.
| Food | IU per Serving |
|---|---|
| Cod liver oil (5 mL) | 1360 |
| Salmon (100 g) | 360 |
| Mackerel (100 g) | 345 |
| Sardines in oil (100 g) | 500 |
| Tuna in oil (100 g) | 238 |
| Fortified milk (250 mL) | 115-124 |
| Fortified orange juice (250 mL) | 100 |
| Fortified yoghurt (1.5 L) | 80 |
| Margarine (5 mL) | 60 |
| Cereals (fortified) (250 mL) | 40 |
| Eggs (1) | 25 |
| Cheese (28 g) | 6-12 |
Source: Gilaberte et al.15
Vitamin D Metabolism. The body acquires vitamin D from certain foods and through a reaction that occurs when the skin is exposed to sunlight in which UV-B radiation converts 7-dehydrocholersterol (synthesized in the skin) to pre-vitamin D3, which then becomes inactive vitamin D. This is then converted in the liver to 25(OH)D by way of hepatic hydroxylation. In a second step, 25(OH)D is metabolized in the kidney to the active metabolite 1,25(OH)D, or calcitriol, through the action of renal alpha-1-hydroxylase. This mechanism is regulated by serum levels of calcium and inorganic phosphorus, among others, which are detected by the glands that secrete parathyroid hormone (PTH) and stimulate the activity of alpha-1-hydroxylase, when necessary. Calcitriol concentrations regulate the secretion of PTH. The 1,25(OH)D acts through the vitamin D receptors (VDR) within cells, which activate the transcription and translation of messenger RNA, leading to the synthesis of vitamin D-dependent proteins and their corresponding activity, which depend on the function of the target cell. The excretion of vitamin D through bile is mediated by the activity of 24 renal hydroxylase, which converts 1,25(OH)D to calcitroic acid.
Daily vitamin D requirements are age dependent and different medical associations do not agree on the levels required. According to the Institute of Medicine (IoM), the daily requirement is 400 IU in the first year of life, 600 IU between 12 months and 70 years of age, and 800 IU in people over 70 years of age.3 However, the American Endocrine Society recommends between 400 and 1000 IU/d in the first year of life and 600 to 1000 IU/d thereafter.
In 2009, 25(OH)D was established as the best blood marker of vitamin D status. 4 The societies also have different opinions about desirable levels in the body. While the IoM specifies 20 ng/mL as the lower limit indicating healthy and sufficient levels of vitamin D (Appendix A), the American Endocrine Society defines a level under 20 ng/mL as deficient and a level between 20 and 30 ng/mL as insufficient, and considers values above 30 ng/mL (75 mmol/L) to be essential for maintaining good health.5 There is currently generalized alarm worldwide about vitamin D deficiency in both the healthy and the sick population. In response to this situation, an article in The New England Journal of Medicine addressed the question of whether or not there is really a pandemic of vitamin D deficiency in the world today.6 Between 35% and 70% of Europeans have 25 (OH) D levels considered insufficient by some authors.7 By contrast, in Australia and New Zealand, the percentage ranges from 6% to 9%. Publication of these findings might lead to the consideration of the usefulness of screening healthy people for vitamin D deficiency. However, since routine screening is not currently supported by the IoM or the US Preventive Services Task Force, it is not recommended at this time.
Vitamin D toxicity can cause serious health problems. Blood levels persistently above 200 ng/mL are considered toxic. The most frequent symptoms of toxicity are anorexia, weight loss, polyuria, and cardiac arrhythmias. Vitamin D toxicity also increases the risk of kidney stone formation, the calcification of blood vessels and tissues, and the complications associated with both of these alterations. Toxicity can occur when the daily intake of vitamin D exceeds 10,000 IU, giving rise to 25(OH)D levels in excess of 200 to 240 ng/mL. However, most medical societies consider 25(OH)D levels higher than 125 to 150 ng/mL to be unadvisable in order to avoid the adverse effects of a vitamin D excess.
Modifying FactorsSome people are more susceptible than others to developing vitamin D deficiency, defined as a level below the established minimum of 20 ng/mL (Table 2). Risk factors for susceptibility include obesity,8 smoking,9 a high skin phototype10 (although this is still controversial), insufficient sun exposure, and age.11 With age, the skin produces less of the precursor 7-dehydrocholesterol in the epidermis, intestinal absorption of vitamin D declines due to a reduction in the number of vitamin D receptors (VDR) in enterocytes, and the body's ability to hydroxylate vitamin D in the liver and kidney also decreases. Some clinical conditions predispose patients to inadequate vitamin D levels.12 These include osteoporosis, chronic kidney disease, liver failure, inflammatory bowel disease, hyperparathyroidism, hyperthyroidism, hypogonadism, and celiac disease. Another risk factor is long-term treatment with certain drugs, including anticonvulsants, antiretrovirals, rifampicin, hormonal treatments prescribed for breast and prostate cancer, and, above all, corticosteroids. Pregnancy can also give rise to low 25(OH)D levels.
Risk Factors Associated with Low Vitamin D Levels.
| Risk Factors for Hypovitaminosis D |
|---|
| Inadequate exposure to sunlight (controversial) |
| Smoking |
| Obesity |
| High skin phototype (IV or greater) |
| Older age |
| Institutionalization |
| Current treatment with corticosteroids, antiepileptics, hormone therapies for breast or prostate cancer, immunosuppressants, or antiretrovirals |
| History of osteoporosis |
| Chronic kidney disease |
| Liver failure |
| Inflammatory bowel disease |
| Hyperparathyroidism |
| Hypogonadism |
| Celiac disease |
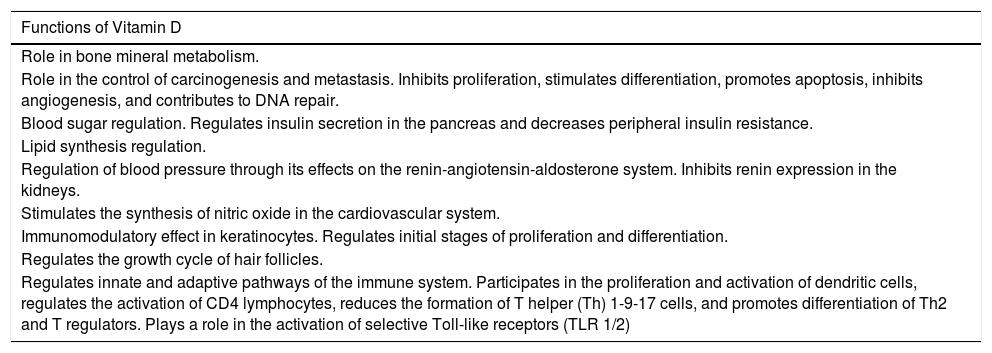
In 2016, Bikle13 described the principal functions of vitamin D in the body. A more recent article discussed the relationship between vitamin D and the skin.14 Vitamin D has been shown to have antiproliferative properties, including the inhibition of angiogenesis and metastatic capacity in some tumors, and it also participates in DNA repair. A relationship has even been found between certain mutations in the VDR gene and cancer. In the metabolic system, vitamin D plays an important role in glucidic, lipid, arterial, and vascular homeostasis. It also modulates immune responses by regulating the differentiation of T helper (Th) 1-9-17 cells and by inducing the formation of antimicrobial peptides such as cathelicidin peptides. In dermatology, it is important because of its immunomodulatory effects in keratinocytes, as described by Gilaberte et al.15 Vitamin D plays an important role in the homeostasis of glucose by promoting insulin secretion in pancreatic beta cells,16 which express alpha-1-hydroxylase through cell stimulation by way of high affinity VDR. Moreover, it decreases peripheral insulin resistance by activating peroxisome proliferator-activated gamma receptors in the liver, muscle, and fatty tissue. In view of this relationship, it has been postulated that vitamin D may be a protective factor in the development of type 2 diabetes mellitus.17 In the case of lipid metabolism, vitamin D can also have an indirect effect, not only because it may improve peripheral insulin resistance18 and reduce PTH levels (shown to have a lipolytic effect),19 but also because it favors higher serum calcium levels, thereby decreasing hepatic triglyceride production.20 In 2010, Pittas et al.21 published a systematic review on the relationship between blood pressure and vitamin D levels. Their conclusion was that deficient vitamin D levels (< 20 ng/mL) were associated with an increased risk of arterial hypertension. Other studies have shown that vitamin D supplementation improved blood pressure in hypertensive patients.22 This improvement is mediated by various effects: direct effects on vitamin D receptors, which decrease vascular inflammation and correct endothelial dysfunction; regulation of the proliferation, migration, and mineralization of vascular muscle cells23; and finally, indirectly by decreasing serum levels of PTH, a hormone that acts on the renin-angiotensin-aldosterone system. Furthermore, recent studies have shown that vitamin D stimulates the release of nitric oxide, thereby reducing oxidative stress in the cardiovascular system.24 It is, therefore, clear that vitamin D not only plays a role in bone metabolism but is also involved in many other functions in the body (Table 3).
Functions of Vitamin D in the Body.
| Functions of Vitamin D |
|---|
| Role in bone mineral metabolism. |
| Role in the control of carcinogenesis and metastasis. Inhibits proliferation, stimulates differentiation, promotes apoptosis, inhibits angiogenesis, and contributes to DNA repair. |
| Blood sugar regulation. Regulates insulin secretion in the pancreas and decreases peripheral insulin resistance. |
| Lipid synthesis regulation. |
| Regulation of blood pressure through its effects on the renin-angiotensin-aldosterone system. Inhibits renin expression in the kidneys. |
| Stimulates the synthesis of nitric oxide in the cardiovascular system. |
| Immunomodulatory effect in keratinocytes. Regulates initial stages of proliferation and differentiation. |
| Regulates the growth cycle of hair follicles. |
| Regulates innate and adaptive pathways of the immune system. Participates in the proliferation and activation of dendritic cells, regulates the activation of CD4 lymphocytes, reduces the formation of T helper (Th) 1-9-17 cells, and promotes differentiation of Th2 and T regulators. Plays a role in the activation of selective Toll-like receptors (TLR 1/2) |
Vitamin D has been implicated in the pathogenesis and treatment of a wide variety of skin diseases. These are discussed below.
PsoriasisKrafka25 and Thacker26 were the first scientists to postulate a role for vitamin D in psoriasis. The use of oral vitamin D as a treatment for psoriasis failed because of the risk of hypercalcemia. The development of topical vitamin D analogs, such as calcipotriol and tacalcitol, later revolutionized the treatment of the condition. Bergler-Czop and Brzezińska-Wcislo27 observed a correlation between Psoriasis Area and Severity Index (PASI) scores and serum levels of 25(OH)D and reported that patients with lower vitamin D levels had higher PASI scores. Orgaz-Molina et al.28 demonstrated that patients with psoriasis and a body mass index higher than 27 were more likely to have insufficient 25(OH)D levels. The comorbidities of psoriasis (diabetes mellitus, obesity, and metabolic syndrome) have also been linked to diets low in vitamin D.29 Clinicians should recommend diets that provide vitamin D in view of its beneficial effect on many of the comorbidities associated with psoriasis. The use of vitamin D in psoriasis is currently a grade B recommendation with IIa level of evidence.
Atopic DermatitisAtopic dermatitis (AD) is a chronic and recurrent inflammatory disease, initially characterized by pruritus and eczematous lesions. It affects up to 20% of the pediatric population and around 7.2% of adults.30 AD is caused by an alteration in the cutaneous immune system and a defect in the synthesis of the epidermal barrier, which affects mainly filaggrin.31,32 Many authors have studied the association between vitamin D and AD. Kim et al.33 published a meta-analysis and systematic review of 11 articles, 7 observational studies, and 4 clinical trials. Compared to healthy controls, patients with AD had lower levels of vitamin D, especially in the pediatric population. Oral vitamin D supplements have been associated with reductions in SCORAD and Eczema Area and Severity Index scores in pediatric patients and is considered to be a possible adjuvant treatment in patients with a deficiency (levels below 20 ng/mL). In a series of 210 adult patients with AD, Van der Schaft et al.34 reported that vitamin D supplementation in patients with deficient levels did not lead to any significant improvement in eczematous lesions. Several studies in the literature report that vitamin D supplementation in children decreased colonization with Staphylococcus aureus, a microorganism that plays a role in outbreaks of DA.35–37 Despite the evidence that vitamin D levels tend to be insufficient in adult patients with AD,38 there is still debate about whether vitamin D supplementation is useful in this age group. In children the use of vitamin D is a grade B recommendation with a IIa level of evidence.
Ichthyosis CongenitaSethuraman et al.39 reported low levels of 25(OH)D and high levels of PTH in children with ichthyosis congenita. In that study, vitamin D supplementation with 60,000 IU of oral cholecalciferol for 10 days followed by a maintenance regimen of 400 to 600 IU/d achieved a rapid response, with noticeable improvement in the dermatological symptoms by the fifth day in terms of both scaling and stiffness. Of the 7 children studied, 6 had serum 25(OH)D levels under 4 ng/mL. While more studies are needed, these findings open up interesting possibilities in the management of ichthyosis congenita. Lucker et al.40 published the results achieved with topical calcipotriol in patients with ichthyosis congenita (Netherton syndrome, lamellar, ichthyosis bullosa of Siemens, and epidermolytic ichthyosis of Brocq). Scaling and associated redness improved in all but the bullous form of the disease. In ichthyoses in which epidermal hyperproliferation and retention hyperkeratosis do not predominate—such as X-linked ichthyosis, ichthyosis vulgaris, and acquired ichthyosis—topical application of vitamin D analogs has not been shown to be effective.41 Neema et al.42 raised the possibility of combining vitamin D with oral retinoid treatment in patients with low vitamin levels. Despite the published findings, the use of vitamin D supplementation in patients with ichthyosis is a grade C recommendation with a IIIa level of evidence.
AcneInterest in the role of vitamin D in acne first emerged in 1938.43 A comparative study of serum levels of 25(OH)D in adolescents with and without acne was published in PloS One in 2016.44 The patients with acne had lower vitamin D levels than the healthy controls and an inverse relationship was observed between vitamin D levels and the severity and number of inflammatory acne lesions. Following 2 months of vitamin D supplementation, the number of inflammatory lesions decreased but no change was observed in non-inflammatory lesions. The role of vitamin D in acne could be explained by a relationship between the vitamin and reduced synthesis of interleukin (IL) 6, IL-8 and metalloproteinase-945 as well as to a reduction in the expression of IL-17 because of its action on Th17 cells.46 Zouboulis et al.47 described the effect of vitamin D on the sebaceous glands and the increase in lipogenesis when vitamin D levels are insufficient, an effect that promotes an increase in inflammatory lesions. The authors of a recent study reported that 25(OH)D levels were higher following treatment than at baseline in patients treated with oral isotretinoin, concluding that the role of vitamin D in the inflammatory pathogenesis of acne is important in the development of lesions.48 The use of vitamin D in acne is currently a grade B recommendation with a IIb level of evidence.
Hidradenitis SuppurativaHidradenitis suppurativa (HS) is a chronic inflammatory skin disease with a significant impact on the patients’ quality of life. Several studies have been published on the relationship between HS and vitamin D levels.49,50 In those studies, the patients with HS had lower vitamin D levels than controls and an inverse correlation was observed between vitamin D levels and disease severity. Following 6 months of vitamin D supplementation, inflammatory nodules improved in 79% of patients (out of the 14 treated); however, as with acne, no significant changes were observed in non-inflammatory lesions. In the opinion of the authors of another study, the endocrinological and metabolic alterations associated with HS make vitamin D a therapeutic target because of its relationship with metabolic syndrome, among other reasons.51 Well-designed studies with a sufficient number of patients are needed to determine the role of vitamin D in HS. The use of vitamin D in HS is currently a grade B recommendation with a III level of evidence.
VitiligoThere are more than 300 articles in the literature on the relationship between vitamin D and vitiligo. Karagün et al.52 observed lower serum vitamin D levels in 50 patients with vitiligo compared to healthy controls and an inverse correlation with the extent of disease. In a meta-analysis, Upala and Sanguankeo53 concluded that the evidence did indicate a clear relationship between vitiligo and low vitamin D levels. Variable results have been reported in cases in which vitiligo was treated with vitamin D analogs either alone54 or in combination with other treatments.55 However, there are no clinical trials that demonstrate the efficacy of vitamin D supplements in these patients. Khurrum and AlGhamdi56 found no significant differences in vitamin D levels between patients with vitiligo and healthy controls; in the analysis by subgroups, however, it was observed that in the group of patients with vitiligo, the lowest vitamin D levels were associated with male sex, younger age, a short duration of vitiligo, and not having received treatment with phototherapy. At present, the use of vitamin D in patients with vitiligo is a grade B recommendation with a IIa level of evidence.
Systemic Lupus ErythematosusSeveral studies have found a higher prevalence of vitamin D deficiency in patients with systemic lupus erythematosus (SLE) than in the healthy population.57,58 The authors of a study published in PloS One, which investigated the relationship between vitamin D levels and the severity of SLE, reported that 71.4% of the patients had vitamin D levels under 30 ng/mL.59 They also observed an association between lower vitamin D levels and poorer scores on the Systemic Lupus Erythematosus Disease Activity Index. However, supplementation with 400 to 1,000 IU/d of vitamin D failed to raise levels above 30 ng/mL. In a comparative study with placebo by Karimzadeh et al.,60 patients with SLE who received vitamin D supplementation showed a significant increase in serum 25(OH)D levels but this was not associated with any effect on disease activity. Some authors defend the usefulness of vitamin D supplementation in these patients on the basis of the protective cardiovascular effect and the possible improvement in systemic symptoms and cognitive development.61 The authors of a recent review article on this topic defend the usefulness of vitamin D in SLE on the basis of its immunomodulatory effects.62 The use of vitamin D in patients with SLE is a grade B recommendation with a IIa evidence level.
Polymorphic Light EruptionPolymorphic light eruption (PLE) is an idiopathic photosensitivity disorder related primarily with exposure to UV-A and less often with UV-B radiation. It is found more frequently in young women. Schweintzger et al.63 found no significant relationship between vitamin D levels and the number and function of regulatory T cells, concluding that other factors affect the immune response that occurs in PLE. Nevertheless, in another study patients with PLE had lower vitamin D levels than controls and when these were increased by phototherapy with UV-B (311 nm) a correlation was observed between the increase and clinical improvement of the lesions.64 In a placebo-controlled clinical trial, Gruber-Wackernagel et al.65 observed that topical application of calcipotriol 1 week before exposure reduced symptoms in almost 1-third of patients exposed to photoprovocation and suggested this treatment as a possible prophylactic measure. However, there is no consensus in photobiology units on the use of this treatment for PLE. The use of vitamin D in PLE is a grade B recommendation with a IIb level of evidence.
Alopecia AreataThe role of vitamin D in alopecia areata (AA) has been explored in several studies.66–69 Thompson et al.70 analyzed data from a cohort of 55,929 women and found no association between vitamin D supplementation and incident AA. However, other studies have found that vitamin D levels tend to be lower in patients with AA than in healthy controls71 and even lower in patients with universal AA compared to those with the ophthalmic or plaque forms. Vitamin D levels have not been shown to differ significantly according to age, sex, disease duration, disease recurrence, or family history of AA. However, patients with AA have been found to have a lower number of vitamin D receptors in the blood and tissues than the group of healthy controls.72
While topical vitamin D analogs can be used to treat AA,72,73 no studies have been carried out which demonstrate that oral supplements with vitamin D are effective in this setting.
The use of vitamin D in patients with AA is currently a grade B recommendation with a IIb level of evidence.
Melanoma and Melanoma MetastasisThe role of vitamin D in melanoma has been investigated in a number of studies.74–77 Orlow et al.78 identified certain haplotypes of single nucleotide polymorphisms (SNPs) of the vitamin D receptor gene related to increased survival in patients with melanomas in photoexposed sites (rs1544410/BsmI and rs731236/TaqI). Subsequent studies have reported an inverse correlation between Breslow thickness and vitamin D levels79 and a possible relationship between low serum vitamin D levels and ulceration80 in patients with melanoma. In fact, a worse prognosis was observed in the patients whose vitamin D levels were low when melanoma was diagnosed and also when, even after starting supplementation, the patient did not achieve levels above 20 ng/mL. In a recent study of 3578 patients, however, no significant relationship was found between melanoma survival, determined by the Breslow thickness, number of mitosis, ulceration, and SNPs found in the pathway of vitamin D and its receptor.81 This debate has led to new studies to determine the possible advantage of recommending vitamin D supplementation in patients with melanoma in sun-exposed and sun-shielded sites.82 The use of vitamin D in patients with melanoma is currently a grade B recommendation with a IIb level of evidence.
Nonmelanoma Skin CancerThe role of vitamin D in carcinogenesis83 led to the study of its relationship with nonmelanoma skin cancer (NMSC).84 The cells of basal cell and squamous cell tumors express VDRs. Eide et al.85 reported an association between high vitamin D levels and an increased risk of keratinocyte carcinoma. Vitamin D is also able to inhibit the Hedgehog signaling pathway in basal cell carcinoma.86 Research has shown that patients with the Bsml SNP of the vitamin D receptor have a higher risk of developing NMSC.87 However, in a prospective study with more than 60,000 participants, vitamin D supplementation was not associated with squamous cell carcinoma risk or the incidence of basal cell carcinoma.88 Confounding factors such as sun exposure and phototype, among others, have given rise to controversies on this topic and clinical trials are required to gain a better understanding of the role of vitamin D in NMSC. The use of vitamin D in patients with NMSC is a grade B recommendation with a IIb level of evidence.
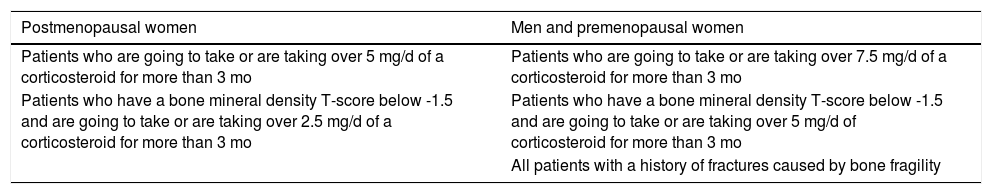
Vitamin D Supplementation in Patients on Long-Term Treatment with Systemic CorticosteroidsChronic use of systemic corticosteroids gives rise to complications, which include osteoporosis. This is due to the osteolytic activity observed in the short term, associated with osteoblast and osteocyte apoptosis in long-term treatment. The overall relative risk of fractures associated with corticosteroid treatment is 1.33. The risk is higher for vertebral fractures (RR 2.60), hip fractures (RR 1.60), and forearm or Colles fractures (RR 1.09). Associated risk factors in each patient must be taken into account; these include advanced age, family history of fracture or osteoporosis, early menopause, smoking, and a low body mass index determined by bone densitometry.89 The usefulness of vitamin D supplementation during prolonged treatment with oral corticosteroids is supported by the findings of a number of studies.90,91 A meta-analysis by Reid et al.92 revealed a correlation between fracture risk and deficient 25(OH)D levels. In another meta-analysis of 11 clinical trials, which included people over 65 years of age (mostly women) showed that supplementation with 800 IU/d of cholecalciferol reduced the risk of hip fracture by 30% and the risk of non-vertebral fracture by 14%, regardless of whether the patients were also taking calcium supplements.93 In several studies, treatment with anti-resorptive agents, such as risedronate or alendronate, in combination with cholecalciferol supplementation for 13 months was associated with an increase in bone mineral density assessed by densitometry.94,95Table 4 shows the indications for vitamin D supplementation in patients on chronic corticosteroid treatment. Table 5 shows the available forms of vitamin D supplements and Table 6 the recommended dosage according to serum vitamin D levels.
Indications for Vitamin D Supplementation During Corticosteroid Therapy.
| Postmenopausal women | Men and premenopausal women |
|---|---|
| Patients who are going to take or are taking over 5 mg/d of a corticosteroid for more than 3 mo | Patients who are going to take or are taking over 7.5 mg/d of a corticosteroid for more than 3 mo |
| Patients who have a bone mineral density T-score below -1.5 and are going to take or are taking over 2.5 mg/d of a corticosteroid for more than 3 mo | Patients who have a bone mineral density T-score below -1.5 and are going to take or are taking over 5 mg/d of corticosteroid for more than 3 mo |
| All patients with a history of fractures caused by bone fragility |
Vitamin D Formulations Available in Spain.
| Cholecalciferol |
|---|
| Drops. 10 mL vial (20,000 IU): 0.05 mg = 400 IU = 6 drops |
| 2.5mL oral solution (25,000 IU) equivalent to 0.625mg |
| Calcifediol |
|---|
| Drop: 0.1 mg/mL (6.000 IU); 240 IU (4 μg) = 1 drop |
| Liquid in ampoules: |
| 0.266 mg (16,000 IU) |
| 3 mg (180,000 IU) |
| Soft capsules 0.266mg (16,000 IU) |
Vitamin D Supplement Dosages According to 25(OH)D Serum Levels as Specified in the Summary of Product Characteristics.
| Treatment of deficiency (10-19 ng/mL) |
| Cholecalciferol 25,000 IU weekly for 8 wks and then every 15 d for a further 8 wks, followed by a maintenance regimen of 25,000 IU every 2-4 wks |
| Calcifediol 16,000 IU weekly for 10 wks and monthly thereafter. |
| Treatment of severe deficiency (< 10 ng/mL) |
| Cholecalciferol 25,000 IU twice weekly for 6 wks, and then 25,000 IU weekly for 4 wks, followed by a maintenance dose of 25,000 IU every 2-4 wk thereafter, depending on serum levels |
| Shock treatment with calcifediol 3mg (180,000 IU) in a single dose followed by 16,000 IU monthly, depending on serum levels (with a 2-month loading period) |
Vitamin D has a multiplicity of functions on the cellular level in the many organs and tissues where there are VDR. It would appear that this hormone is fundamental in dermatology, not only because it is synthesized in the skin, but also because of its multiple actions, which are reflected in the variety of diseases in which it appears to play a role. Table 7 summarizes the diseases in which serum vitamin D levels should be measured and vitamin D supplementation should be considered, depending on the results and taking into account the level of scientific evidence in each case (Table 8).
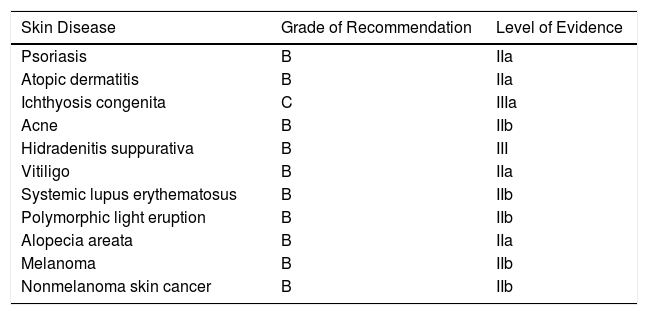
Vitamin D Supplementation in Skin Diseases: Grades of Recommendation and Levels of Evidence.
| Skin Disease | Grade of Recommendation | Level of Evidence |
|---|---|---|
| Psoriasis | B | IIa |
| Atopic dermatitis | B | IIa |
| Ichthyosis congenita | C | IIIa |
| Acne | B | IIb |
| Hidradenitis suppurativa | B | III |
| Vitiligo | B | IIa |
| Systemic lupus erythematosus | B | IIb |
| Polymorphic light eruption | B | IIb |
| Alopecia areata | B | IIa |
| Melanoma | B | IIb |
| Nonmelanoma skin cancer | B | IIb |
The authors declare that they have no conflicts of interest.
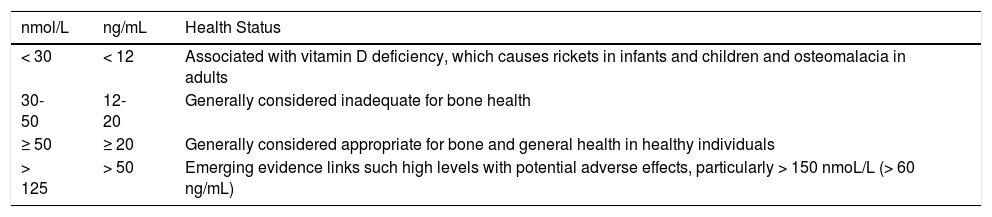
| nmol/L | ng/mL | Health Status |
|---|---|---|
| < 30 | < 12 | Associated with vitamin D deficiency, which causes rickets in infants and children and osteomalacia in adults |
| 30-50 | 12-20 | Generally considered inadequate for bone health |
| ≥ 50 | ≥ 20 | Generally considered appropriate for bone and general health in healthy individuals |
| > 125 | > 50 | Emerging evidence links such high levels with potential adverse effects, particularly > 150 nmoL/L (> 60 ng/mL) |
1 nmol/L/L equals 0.4 ng/mL.
Source: Institute of Medicine, Food and Nutrition Board.3
Please cite this article as: Navarro-Triviño FJ, Arias-Santiago S, Gilaberte-Calzada Y. Vitamina D y la piel. Una revisión para dermatólogos. Actas Dermosifiliogr. 2019;110:262–272.