Acute Generalized Exanthematous Pustulosis (AGEP) is an acute eruption considered as a severe cutaneous adverse reaction (SCAR) related to drugs. Although antibiotics are the most common triggers, many other drugs have been associated with AGEP. More rarely, cases of nun-drug-related AGEP have been reported, due to viral or bacterial infections, spider bites or mercury hypersensitivity.1 Here, we present the case of an AGEP triggered by the kinase-inhibitor Sorafenib and we expose a brief review of similar cases reported.
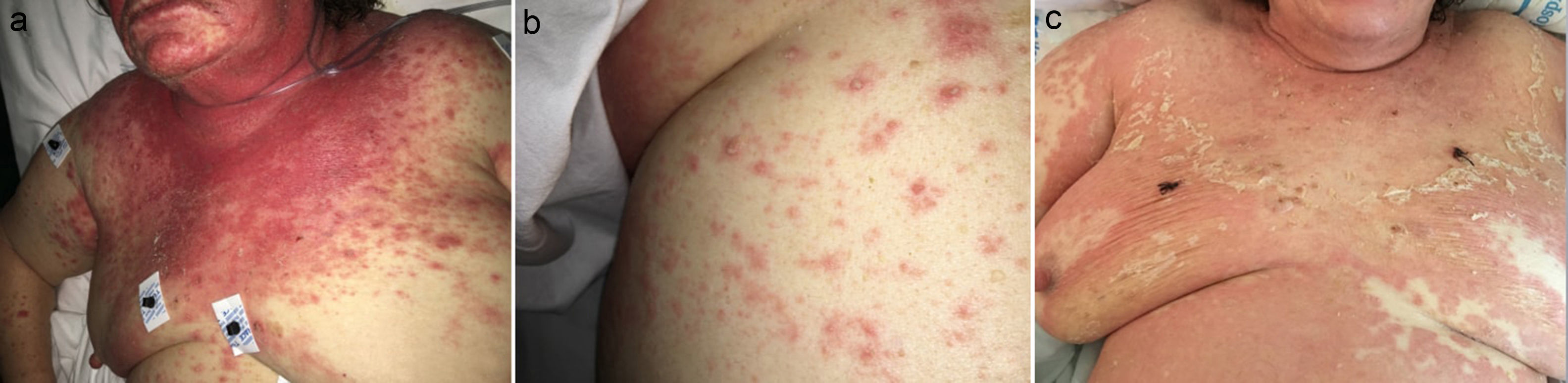
A 78-year-old woman presented to the Emergency Department with a 6-week history of an extensive, pruriginous exanthema consisting of erythematous and edematous plaques in the face, chest and upper extremities, with small isolated pustules (Fig. 1). Mucous membrane involvement was confined to the lips. Neither fever nor other systemic symptoms were evident. Two months before, she had initiated treatment with sorafenib due to an unresectable hepatocarcinoma. Skin reaction developed gradually two days after initiating the treatment.
Clinical evolution. (a) Exanthema affecting mainly face, upper trunk, superior limbs with isolated patches on abdomen and lower extremities. (b) Closer image showing isolated pustules surrounded by erythema. (c) Evolution of the exanthema 5 days after Sorafenib discontinuation. Mild erythema and desquamation on upper trunk with pustules resolution.
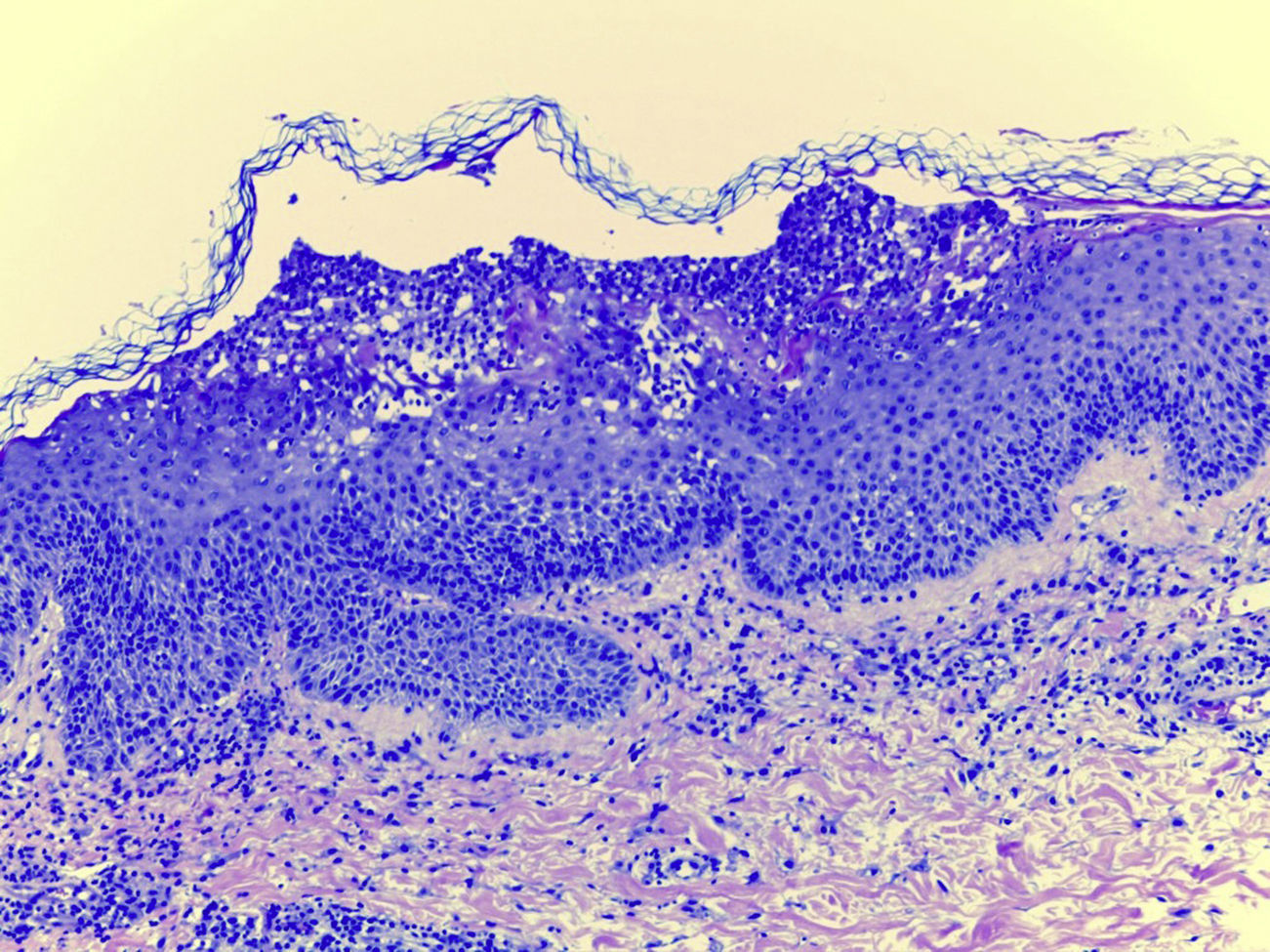
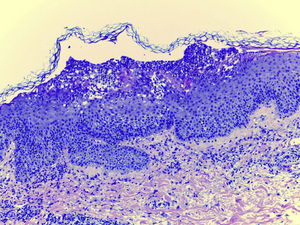
Laboratory examination revealed a hypereosinophilia of 1.4×109/L. A skin biopsy was performed, rendering typical histological features of AGEP (Fig. 2). Attending to the validated EuroSCAR scoring system,2 our case was then classified as a Probable AGEP. Sorafenib was discontinued immediately, and treatment with intravenous methylprednisolone 1mg/kg was initiated. Cutaneous lesions improved rapidly and were almost totally resolved after a week. This clinical evolution strongly supports the diagnosis of sorafenib-induced AGEP.
AGEP is characterized by numerous, small, non-follicular and sterile pustules arising within extensive areas of edematous erythema. Fever and leukocytosis are common findings and severe cases of AGEP can associate visceral involvement. Mucous membrane might also be affected. Time interval between drug administration and the skin eruption onset is typically 48h, although it varies from 1 day to 4 weeks. It typically resolves in two weeks after drug discontinuation.1,2 Main differential diagnoses of AGEP are other pustular diseases, such as pustular psoriasis, and other severe cutaneous adverse reactions, such as Stevens Johnson syndrome (SJS) or Toxic Epidermal Necrolysis. Compared to AGEP, in SJS/NET, mucosal involvement and skin detachment (Nikolsky sign) are a constant. However, the possibility of overlap of different severe cutaneous reactions have been recently discussed in the literature.3
Sorafenib is a multikinase inhibitor that mainly target tumor cell angiogenesis, first used in advanced renal cell cancer and unresectable hepatocellular carcinoma with recent indications in other cancers.4 It inhibits multiple tyrosine-kinases, such as RAF-1 and B-RAF, and it blocks tyrosine-kinases receptors, such as platelet-derived growth factor receptor (PDGFR), related with carcinogenesis.7
Patients under treatment with multikinase inhibitors usually develop cutaneous reactions. In a prospective study, up to 72% of the patients taking sorafenib presented dermatological symptoms.4,7 Most of these reactions are maculopapular rashes and other grade 1–2 severity adverse reactions that can be easily managed. Other non-severe skin reactions that can be found are hand–foot skin reaction, stomatitis, subungual splinter hemorrhages, facial erythema or alopecia.4,7
However, some of these patients develop SCARs related to sorafenib, such as Drug Reaction with Eosynophilia and Systemic Symptoms (DRESS), SJS or AGEP.5,6,8,9
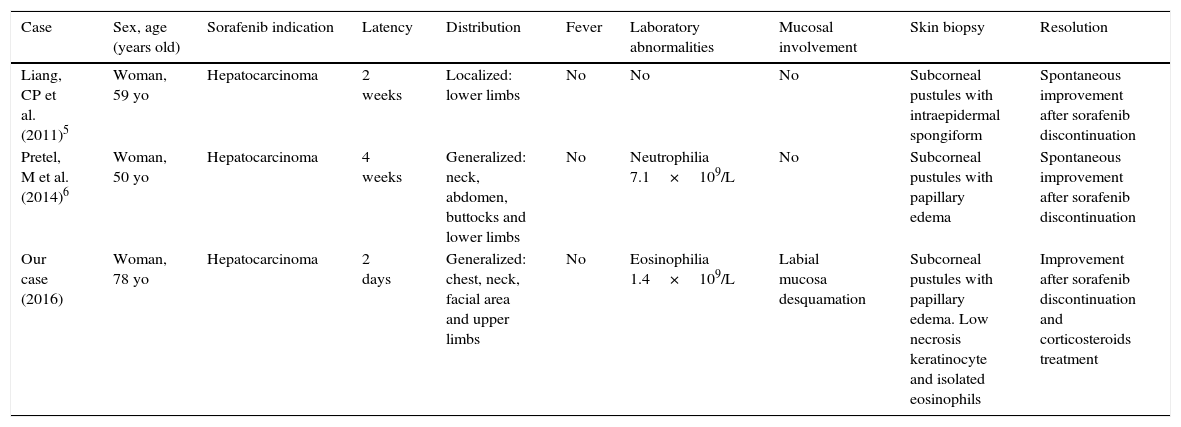
To our knowledge, only 2 more cases of sorafenib-induced acute exanthematous pustulosis have been published, being one of them an acute localized exanthematous pustulosis (ALEP) and the other one a complete AGEP.5,6 The main features of these cases are presented in Table 1. Interestingly, all the three cases occurred in women who were under treatment with sorafenib due to unresectable hepatocarcinoma. Cases of AGEP have also been reported associated with other multikinase inhibitors such as imatinib.10 Therefore; we believe that the association between kinase-inhibitors and AGEP might be an under-reported entity with an increasing incidence due to the appearance of new drugs of this nature.
Demographical and clinical features of sorafenib-induced AGEP/ALEP cases.
| Case | Sex, age (years old) | Sorafenib indication | Latency | Distribution | Fever | Laboratory abnormalities | Mucosal involvement | Skin biopsy | Resolution |
|---|---|---|---|---|---|---|---|---|---|
| Liang, CP et al. (2011)5 | Woman, 59 yo | Hepatocarcinoma | 2 weeks | Localized: lower limbs | No | No | No | Subcorneal pustules with intraepidermal spongiform | Spontaneous improvement after sorafenib discontinuation |
| Pretel, M et al. (2014)6 | Woman, 50 yo | Hepatocarcinoma | 4 weeks | Generalized: neck, abdomen, buttocks and lower limbs | No | Neutrophilia 7.1×109/L | No | Subcorneal pustules with papillary edema | Spontaneous improvement after sorafenib discontinuation |
| Our case (2016) | Woman, 78 yo | Hepatocarcinoma | 2 days | Generalized: chest, neck, facial area and upper limbs | No | Eosinophilia 1.4×109/L | Labial mucosa desquamation | Subcorneal pustules with papillary edema. Low necrosis keratinocyte and isolated eosinophils | Improvement after sorafenib discontinuation and corticosteroids treatment |
In conclusion, we report a new case of sorafenib-induced AGEP. We should be aware of this cutaneous side effect of sorafenib and other multikinase inhibitors, given that drug discontinuation is the key point on AGEP treatment.
Conflict of interestsThe authors declare no conflict of interest.