A proliferating pilar tumor is a rare skin neoplasm that arises from the outer root sheath of a hair follicle. Presentation varies widely, as the tumor can be benign or malignant and has a high probability of recurring after excision. We report our experience managing 3 proliferating pilar tumors with different clinical presentations and pathology findings at Hospital de San José, Bogota, Colombia.
El tumor pilar proliferante es una neoplasia cutánea rara, que se deriva de la vaina de la raíz externa de los folículos pilosos con un amplio espectro en su presentación dado a que puede variar desde la benignidad hasta la malignidad con alta probabilidad de recurrencia posterior a su escisión. En este artículo describimos la experiencia del Hospital de San José en Bogotá, Colombia en el manejo de 3 tumores pilares proliferantes, con una presentación clínica y un comportamiento patológico distinto.
Proliferating pilar tumors (PPT) are rare skin tumors derived from the outer root sheath of hair follicles. They mainly affect women older than 40 years and 90% of cases occur on the scalp. Presentation varies widely, as PPT can be benign or malignant; it can also metastasize and recur after surgical excision. Although excision is the treatment of choice for PPT, Mohs micrographic surgery (MMS) has emerged as a useful alternative.
We describe our experience with the treatment of 3 cases of PPT at Hospital de San José in Bogotá Colombia and review the literature on this rare tumor, the treatment options available, and recurrence rates, which remain high.
Clinical Cases, Methods, and ResultsCase 1A 59-year-old woman presented with a mobile, asymptomatic lesion on the scalp of 6 years’ duration. Her past medical history was unremarkable. The physical examination showed a hard erythematous nodule measuring 18×15mm in diameter in the left parietal region. A skin biopsy was performed with a tentative diagnosis of an infundibular cyst. Histology showed a well-circumscribed, partially cystic dermal scalp lesion formed by a wall comprising a cord of atypical, squamous, anastomotic cells and lamellar keratin in the lumen. The histopathologic findings were consistent with a diagnosis of PPT. The lesion was treated by wide local excision with lateral margins of 2cm and deep margins extending down to the muscle fascia. Histology confirmed absence of disease in the margins. The patient did not experience recurrence over a 12-month follow-up period.
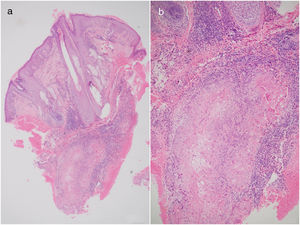
Case 2A 29-year-old woman was evaluated for a progressive, painful lesion on the scalp that had appeared 4 years earlier. She had no relevant past medical history. Previous skin biopsy of the lesion at another hospital had shown a proliferation of keratinocytes with moderate atypia, trichilemmal keratinization, dyskeratosis, and occasional mitotic figures. These findings were suggestive of a number of entities, including invasive squamous cell carcinoma (SCC) and PPT. Physical examination of the patient on admission to our hospital showed a semi-soft, tumor-like, exophytic nodule that was painful on palpation in the left parietal region. As the diagnosis was unclear, it was decided to perform a second skin biopsy, which showed a dense lymphocytic, neutrophilic infiltrate with predominantly periadnexal hemorrhage in the dermis and nests of slightly pleomorphic squamous keratinocytes, occasional mitotic figures, and keratin pearls in the deep dermis (Figure 1 A and B). Immunohistochemical staining was positive for cytokeratin (CK) AE1, AE3, and epithelial membrane antigen. There was also focal peripheral staining for CK8/CK18 (CAM 5.2). The results were negative for CK7, smooth muscle actin, CD34, BER EP4, S100, and p53. The Ki67 proliferation index was 20%. Both morphologic and immunophenotypic findings were consistent with a diagnosis of PPT. The tumor was excised using MMS and clear margins were obtained after the second stage. No recurrences were observed over 17 months of follow-up.
Case 3A 71-year-old man presented with a recurrent asymptomatic lesion of 3 years’ duration on the scalp. The lesion had been excised 3 years earlier with a diagnosis of infundibular cyst. Physical examination revealed a nodule measuring 50 x 30mm attached to the deep planes in the left occipital region. The patient was scheduled for surgical excision of what was suspected to be a recurrent infundibular cyst. Surgery revealed an irregular mass attached to the deep planes and difficulties were experienced in securing hemostasis. Histology showed a tumor affecting the skin and subcutaneous tissue formed by multiple nodules with exophytic and endophytic proliferations of squamous epithelial cells with abrupt keratinization and other areas of trichilemmal keratinization. Wide islands of compact keratin, stromal invasion, and areas of growth were also observed. There were no signs of lymphovascular or perineural invasion. The clinical and histologic findings were consisted with PPT. The tumor was treated with MMS and tumor-free margins were achieved after the second stage. No evidence of tumor recurrence was observed over 10 months of follow-up.
DiscussionPPT is a rare skin tumor derived from the outer root sheath of hair follicles.1 It appears to be induced by trauma, infection, inflammation, or irritation of a preexisting trichilemmal cyst.
PPT has a predilection for women aged between 40 and 80 years, with a peak in incidence during the sixth decade; 90% of cases occur on the scalp.1
Tumor size varies from 2cm to 15cm but a maximum size of 24cm has been reported.2 The tumor presents as an exophytic nodule or papule that often has an ulcerated surface and affects sun-exposed hair-bearing areas. It can, however, also affect the forehead, neck, mammary region, and vulva.3
Typical histologic features include abrupt amorphous keratinization of the epithelium lining the cyst wall, without a granular cell layer.4
Ye et al.5 studied 76 cases of PPT and classified them into 3 groups based on histologic criteria (Table 1).
Histopathologic Classification of Proliferating Pilar Tumor.
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Histologic findings | Trichilemmal keratinization and modest nuclear atypia with underlying tissue showing infiltration by mononuclear, plasma, and giant cells with an absence of pathologic mitotic figures, necrosis, and nerve and vessel invasion. | Moderate cellular atypia, with foci of unicellular necrosis, involvement of the deep dermis, and abrupt keratinization. | Marked nuclear atypia with pathologic mitotic figures and a desmoplastic stroma. |
| Description | Benign | Locally aggressive | Malignant |
| Recurrence rate | 0% | 15% | 50% |
Based on reports in the literature, the first case in our series would be a group 1 PPT, while the other 2 would belong to group 2. None of our patients experienced recurrence or required additional treatment.
Diagnosis of PPT is primarily based on histologic features, but immunohistochemistry can aid the differential diagnosis as group 1 PPTs stain strongly for CK10 and involucrin, while malignant PPTs express nuclear proliferation antigens and CK16 and show a loss of CD34 immunoreactivity.6
Although imaging studies are not commonly used for diagnosis, magnetic resonance imaging (MRI) can be useful as it shows a solid mass or a cystic tumor. Solid tumors are generally malignant.7
Histologic features to aid the differential diagnosis are presented in Table 2. Clinically, PPT should be distinguished from scalp nodules that can become ulcerated, such as basal cell carcinoma, cylindroma, dermatofibrosarcoma protuberans, Merkel cell carcinoma, and skin metastasis.
Histological Differential Diagnoses.
| Differential Diagnosis | Histopathologic Findings | Immunohistochemistry |
|---|---|---|
| - Trichilemmoma- Trichilemmal keratosis- Clear-cell syringoma- Hidradenoma | Absence of invasive growth pattern and characteristic cellular atypia seen in proliferating pilar tumorCertain degree of nuclear atypia with several mitotic figures and slight architectural disorder possibly present in trichilemmal keratosis | CD34+Cytokeratin (CK) 8+ |
| - Squamous cell carcinoma | Cells with a pale or clear cytoplasm, possibly reflecting degenerative changes may be found in the epithelial lobules of squamous cell carcinoma | AE13 and AE14 antibodiesCK 34βE12/CK 903+CK8-CK14+ |
| - Malignant hidradenoma | Absence of peripheral palisading and presence of acinar or ductal differentiation | Immunoreactive carcinoembryonic antigen |
| - Sebaceous carcinoma | Differentiated sebaceous cells with foamy cytoplasm, rich in lipids, and centrally located indented nuclei | Immunoreactive forepithelial membrane antigenAdipophilinProgesterone receptor membrane component 1 (PGRMC1)Squalene synthasealpha/beta hydrolase domain- containingProtein 5 (ABHD5)CK7 |
Surgical excision with lateral margins of at least 1cm is the treatment of choice for low-grade malignant PPT, but MMS can also be considered in this setting. Although no comparative studies supported by scientific evidence have been conducted, MMS helps spare tissue as it involves step-by-step evaluation of tumor margins.11
Lymph node dissection is required for patients with malignant PPT and metastasis. Good results have been described for radiotherapy used as an adjuvant and even in isolation. Radiotherapy is an important option for elderly patients or patients with tumors in cosmetically or functionally sensitive areas. Palliative radiotherapy is also used in patients with metastatic disease.12 Systemic chemotherapy with cisplatin and 5-fluorouracil can be attempted, as regimens of cisplatin, adriamycin, and vindesine have shown limited results in the treatment of advanced SCC.13
Jo et al.14 described a case in which topical immunomodulatory treatment with imiquimod 5% for 8 months as an alternative to surgery provided good results, with the patient experiencing no recurrences over 16 months’ follow-up.
Recurrence rates ranging from 3.7% to 6.6% have been described for local metastasis and from 1.2% to 2.6% for regional lymph node metastasis over a period of between 6 months and over 10 years.6 Sau et al.15 reported a recurrence rate of 1.7% over 87 months of follow-up. One of the patients in their series had regional lymph node metastasis but did not develop distant metastasis or experience recurrence in the 7 years following surgical excision.
The metastatic rate for group 3 PPTs is 25%,6,15 highlighting the importance of additional tests following diagnosis. Contrast-enhanced computed tomography (CT) of the brain is the technique of choice for evaluating local bone invasion and erosion, while CT of the neck is used to evaluate regional and lymph node metastasis at the base of the skull and in the neck; MRI, in turn, is useful for assessing soft tissue invasion or involvement of the dural sinuses in patients with scalp lesions.11
The literature on the value of sentinel lymph node biopsy in PPT is scant and inconclusive. Just one case of its use in recurrent malignant PPT has been reported and the result was negative.16 Due to the lack of appropriate studies analyzing the value of sentinel lymph node biopsy in PPT, we cannot recommend it as a routine procedure.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We acknowledgment to the the pathology service of Hospital de San José and Dr. Samuel Morales for the photographs.
Please cite this article as: Pérez CEA, Ángulo DG, Pérez MO, Hernández OM, Morales SD. Experiencia en el manejo de 3 tumores pilares proliferantes: definición, diagnósticos diferenciales y alternativas terapéuticas. 2019;110:850–854.