It is now known that all biologic drugs, even those that are fully human, are immunogenic, that is, they have the ability to induce an immune response in the treated patient. Since the presence of antidrug antibodies may influence the levels and function of the drug in the body, this immune response can alter the efficacy of the biologic treatment and even its safety profile, depending on the mechanism of action (neutralizing or nonneutralizing) and/or an accelerated clearance of the drug. Immunogenicity is a dynamic factor that should be taken into account when prescribing biologic therapy in psoriasis, especially in the case of long-term treatment and when assessing secondary loss of response. An understanding of the immunogenicity of biologic therapies and how this can be managed is useful not only for optimizing the treatment strategy used with each drug, but also for designing predictive models of response and even for tailoring therapy on a case-by-case basis.
En la actualidad se conoce que todos los fármacos biológicos, incluidos los de estructura totalmente humana, son capaces de inducir una respuesta inmune por parte del huésped, conocida como inmunogenicidad. La presencia de anticuerpos antifármaco puede condicionar los niveles y la función del fármaco y, por lo tanto, el efecto terapéutico e incluso el perfil de seguridad en función de su mecanismo de acción —neutralizante o no neutralizante—y/o de un aclaramiento acelerado. La inmunogenicidad es un factor dinámico a tener en cuenta en la terapia biológica de la psoriasis, en particular en el tratamiento a largo plazo, y en la evaluación de la pérdida secundaria de respuesta. El conocimiento y manejo de la inmunogenicidad de los distintos tratamientos biológicos representa un instrumento útil no solo en la optimización de las estrategias terapéuticas para cada fármaco, sino también en el diseño de modelos predictivos de respuesta, e incluso en la personalización de la terapia.
As we fine tune the management of biologic therapy in psoriasis, new aspects are emerging that may, in the long term, be at least as important as the intrinsic efficacy of the biologic agent during the first weeks of treatment. Thanks to the results of the initial clinical trials—now completed or in the open-label phase—and to our growing clinical experience with biologics, we now know that, in some patients, the impressive results obtained during the first months of treatment are followed by a loss of effect, even though the molecule and receptor are the same. In such cases, it becomes necessary to modify dosing regimens, add or change adjunctive therapies, and sometimes even to switch the patient to another treatment. It is reasonable in this situation to ask ourselves what has happened since the start of treatment that might explain this change. Then again, the special nature of biologics—from which they derive their unique characteristics and advantages in terms of efficacy and safety—also implies a constant, dynamic interaction between 2 biologic entities: the drug receptor and the drug molecule. Immunogenicity is one of the factors that determine what happens in this dynamic interaction, although certainly not the only determinant and not always the most important one.
Although the concept of immunogenicity and its implications may seem rather uninteresting to the clinical dermatologist, the fact is that today immunogenicity is an important question and essential to our understanding of certain key aspects of the management of biologic regimens in psoriasis and other conditions. It not only explains, at least partially, some of the dynamic aspects of the drug-receptor interaction that influence efficacy and safety in biologic therapy, but may also even provide an opportunity to optimize therapeutic response in the not so distant future.
ConceptThe term immunogenicity refers to the ability of a molecule to induce a specific humoral or cellular immune response, which is triggered by differences between the 3-dimensional structures of the exogenous drug molecules and the body's natural proteins.1
Antibodies, which are glycoproteins secreted by plasma cells, are part of the adaptive immune system. They form part of a defense system that has evolved over millions of years and are capable of recognizing even small molecular differences in exogenous substances, which they strive to eliminate using various mechanisms, including direct neutralization or tagging to facilitate subsequent phagocytosis or complement activation.
While several antibody isotypes exist, immunoglobulin (Ig) G antibodies are the immune system's greatest defense against exogenous drugs.
Immunogenicity in Biologic Therapy: The Targets of Antidrug AntibodiesThe immunogenicity of biologics is influenced by numerous factors related not only to the drug used but also to the patient's disease and individual characteristics, as well as the dosing schedule and route of administration; many of these factors are not yet well understood.2
A patient's genetic background, for example, will determine the likelihood and nature of the immune response to the exogenous molecules. The underlying disease is also a factor, and certain diseases, such as rheumatoid arthritis, are known to be particularly associated with immunogenicity.
Obviously, the molecular structure of the drug is also very important, and this is determined by its design and the way the molecule is synthesized. Immunogenicity will vary depending on whether the biologic agent is a fusion protein or a chimeric, humanized, or fully human antibody.
In chimeric antibodies, such as infliximab, 25% of the protein is of murine origin, and, unsurprisingly, it is these sequences that are the main target of the antidrug antibodies (ADAs) produced by the immune system. However, experience has shown that ADAs develop in response to treatment with all types of biologics, including humanized monoclonal antibodies (> 90% human), fusion proteins (etanercept), and even fully human antibodies such as adalimumab and ustekinumab, which contain no murine proteins.
The mode of administration is also important. In the EXPRESS II study, anti-infliximab antibodies were detected in 35.8% of patients treated at a dose of 5mg/kg as compared to 51.5% of those treated with 3mg/kg.3 Furthermore, after 66 weeks of treatment, the proportion of patients with ADAs was higher in the group treated with an as-needed regimen (and who therefore received lower doses of infliximab after week 14) than in the continuous treatment group (41.5% vs 35.8%).3 In the PHOENIX 1 and 2 studies, ADAs were also more common in patients who received 45-mg doses of ustekinumab (18.4% and 13.6%) than in those treated with 90mg (2.2% and 6.7%).4
Subcutaneous administration is also more immunogenic than oral administration or intravenous infusion as it permits prolonged contact between the molecule and dendritic cells.2
The final outcome in the struggle between the biologic molecule and its achievement of the desired therapeutic effect and the efforts of the immune system to eliminate it will, therefore, depend not only on whether the patient's immune system can develop an effective response to the intruder, but also on whether the drug molecule can evade the systems designed to eliminate it, a factor that will be influenced by its design. The third determining factor will be the success of the administration regimen in ensuring that the available drug molecules outnumber the ADAs capable of neutralizing them. The key to treatment success is the maintenance of sufficiently high therapeutic drug levels to achieve the desired effect, whatever the impact of immunogenicity or other factors.
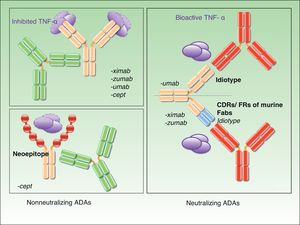
Four of the biologics currently available for the treatment of inflammatory diseases have been approved for the treatment of moderate to severe psoriasis: etanercept, infliximab, adalimumab, and ustekinumab. Although the desired clinical outcome is similar with all 4 biologics, the structure and mechanism of action are different in each case and their interaction with the therapeutic target varies considerably. Therefore, as has been suggested by several studies, it can be assumed that the targets of ADAs will vary according to the drug used, primarily because of structural differences. ADAs may target allotypes, idiotypes, or even epitopes generated by the formation of new structures, as occurs in the case of fusion proteins. The effects will be different in each case and will be discussed in the following sections. However, it is important to note that different types of ADAs, targeting different parts of the same drug, may coexist in certain patients.
Neutralizing and Nonneutralizing AntibodiesOne of the most important aspects of ADAs is the fact that their mechanism of action can be either neutralizing or nonneutralizing, and this is also the characteristic that has generated most debate. The specificity of a therapeutic antibody is determined by the conformation of the hypervariable portion of its light and heavy chains. The function of this portion, known as the paratope, is to bind directly to the epitope of the antigen. These hypervariable regions are the same in all antibodies produced by an individual B-cell clone. The epitopes present in these regions are called idiotopes and the set of these idiotopes constitutes an idiotype. Tumor necrosis factor-α and the p40 subunit can be immunogenic even in fully human antibodies, given that no specific idiotopes exist for them in humans.5 The presence of anti-idiotypic ADAs is common in patients on chimeric antibodies (infliximab) and those receiving treatment with humanized or human antibodies (adalimumab, ustekinumab, certolizumab, and golimumab). They are very probably almost always neutralizing because they interfere directly with the drug's therapeutic activity.6 The presence of neutralizing ADAs does not necessarily preclude a therapeutic effect. Clinical efficacy will depend on the balance between drug concentrations and antibody levels and whether the resulting drug levels are high enough to achieve the desired clinical outcome. As already mentioned, whether or not a satisfactory response is achieved depends on the maintenance of sufficiently high drug levels, and this level is not necessarily the same in each patient.
Nonneutralizing ADAs, by contrast, bind to a portion of the drug molecule that is not essential to its therapeutic activity (e.g., to the allotope). In such cases, the formation of antibodies is triggered, even when the structure of the molecule is fully human, by polymorphisms expressed in the constant portion of the light and heavy chains, which vary between individuals.
Finally, another potential target of nonneutralizing ADAs are the new epitopes found in fusion proteins such as etanercept.
Fig. 1 shows the mechanisms of action of neutralizing and nonneutralizing ADAs.
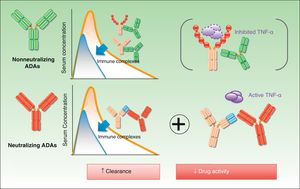
The biologic effect of nonneutralizing ADAs is less well understood than that of neutralizing ADAs, but this does not mean that it does not exist. The therapeutic molecule can be eliminated by mechanisms other than the direct blockage of the idiotype by antibodies. For instance, the formation of immune complexes, which are eliminated by the reticuloendothelial system, accelerates clearance of the drug7 (Fig. 2). This will occur whether or not the drug is present in sufficient levels to maintain its activity.
The presence of ADAs should therefore be considered a negative development caused by the interaction between the biologic agent and the patient's immune system. This interaction, through one or more different mechanisms, can reduce the likelihood of achieving a sufficient number of free drug molecules to bind to the therapeutic targets during treatment.8
Antibody Detection AssaysDue to the limitations of the assays currently available, it is not yet possible to measure antibodies against biologics in a simple, efficient manner in routine clinical practice. Methods used to detect ADAs include the enzyme linked immunosorbent assay (ELISA) (frequently associated with a lack of sensitivity) and newer techniques, such as the two-site (or bridging) assay and the radioimmune assay. The detection and measurement of ADAs is hindered by the presence of the drug in the patient's serum, an unavoidable situation in most patients receiving long-term treatment.9 For instance, when the number of drug molecules is equal to or higher than the number of ADAs in the sample, the ADAs will be bound to drug molecules and will not be detected. Accordingly, it is important to test for ADAs just before the drug is administered, when drug levels are presumably at their lowest. The logical procedure for measuring immunogenicity in patients who experience problems with safety or efficacy would seem to be to measure drug concentrations first and then test for ADAs if these levels are unusually low.
The Impact of Immunogenicity on Efficacy and SafetyImmunogenicity in biologic therapy is inevitable, although there is room for debate regarding the magnitude of its effect and, in particular, its therapeutic implications. ADAs have been detected in between 5% and 45% of samples analyzed in laboratory studies, with results varying according to the drug used, the type of assay, and the treatment duration and strategy.9–12 However, the importance of ADAs lies not in their presence or absence, or even in their relative numbers, but rather in their impact on efficacy and safety.
The impact of ADAs on efficacy can be linked either to their ability to inhibit the effect of the drug or to their role in accelerating its clearance. In either case, the result is the same: the therapeutic effect of the drug is diminished with a consequent negative effect on treatment.
It should be noted that most of the data available regarding the impact of immunogenicity on efficacy are indirect results derived from laboratory assays that are not always comparable between studies or drugs. Moreover, they refer to different diseases and are therefore not strictly applicable to the use of biologics in psoriasis.
In the case of infliximab and adalimumab, the presence of neutralizing ADAs has been shown to reduce drug serum levels, an effect attributable to accelerated clearance and direct interference with the function of the drug.5,13 Bendtzen et al.,14 for example, showed that serum from patients with anti-infliximab antibodies required more infliximab to block the same number of TNF-α molecules than serum from untreated individuals. ADAs are never an absolute determinant of efficacy but they do influence outcomes; for instance, these antibodies are more often detected among moderate responders and nonresponders than among responders.15 Another aspect of interest is the dynamic nature of immunogenicity, which can increase or decrease over time as the drug molecules are constantly interacting with an immune system designed to continuously adapt to new aggressions. This variation might help to explain, at least in part, why some patients who initially respond to treatment (i.e., who are responsive to the drug's mechanism of action) lose response as their levels of ADAs increase.16 In theory, continuous exposure to the drug might improve tolerance in certain cases.
Experience with biologics in psoriasis is limited. In the EXPRESS study, which assessed the efficacy of infliximab in patients with moderate to severe psoriasis, only 39% of patients who achieved a 75% improvement in the Psoriasis Area and Severity Index (PASI 75) after 10 weeks of treatment and developed anti-infliximab antibodies maintained this improvement at week 50, compared with 81% of those who did not develop antibodies.15 Poorer response to treatment in patients with ADAs has also been documented in other studies.11
In the landmark REVEAL study, 7 of the 240 patients who responded to adalimumab treatment (PASI 75) had ADAs at week 33. Of these, 3 (43%) failed to maintain this response at week 52 compared with 65 (28%) of the 233 patients who had not developed ADAs.17 In a prospective study of antibody formation against adalimumab, patients with moderate or high levels of ADAs had lower serum drug concentrations, poorer initial response, and greater loss of response over time.10
As psoriasis is the only indication for ustekinumab, cumulative experience with this drug is limited. In a study of a subgroup of patients from the landmark PHOENIX studies, the overall incidence of anti-ustekinumab antibody formation was low (4.9%); the titers were also generally low and the presence of ADAs did not appear to be associated with dose or treatment duration. The neutralizing nature of anti-ustekinumab antibodies has been demonstrated in vitro, with lower serum drug levels and increased drug clearance (35% higher) observed in antibody-positive patients. The presence of ADAs, however, was not identified as a predictor of poor response.18
Similarly, the implications of ADAs in etanercept treatment are not fully understood. Although anti-etanercept antibodies have been detected in up to 18.5% of patients after 96 weeks of treatment, no link has been detected between the presence of such antibodies and variations in treatment response. These findings are consistent with results from laboratory studies that demonstrate the apparently nonneutralizing effect of anti-etanercept antibodies and indicate that these ADAs primarily target the new epitopes in the fusion region between IgG and the TNF receptor, and have no direct impact on the portion of the biologic designed to interact with the target.19 While an association has been observed between clinical response and serum etanercept levels, this appears to be independent of the presence of ADAs. These findings underline the fact that immunogenicity is just one of the factors that can have an impact on drug concentrations and the achievement of the desired clinical outcome.20
The potential impact of immunogenicity on safety is another very important issue. Overall, it can be stated that the presence of ADAs in patients treated with anti-TNF agents has only been associated with a higher frequency of infusion-related reactions in the case of infliximab and that no such link has been observed with adalimumab, etanercept, or ustekinumab.21 Furthermore, while thromboembolic events due to platelet and complement activation have been documented in patients with a number of inflammatory diseases treated with adalimumab,22 no such adverse effects have been observed in patients with psoriasis.
Strategies for Reducing ImmunogenicityIf we consider that immunogenicity, given its potential to interfere with treatment, is an important factor that should be taken into account in the overall treatment strategy, our goal as dermatologists should be to take actions to reduce the likelihood of ADA formation. There are essentially 3 ways of doing this: we can modify the administration or management of the drug, increase the dose (and therefore the number of available molecules), or interfere with the ability of the immune system to produce ADAs, generally by adding immunosuppressive agents to the regimen.
We know that immunogenicity is influenced by the route of administration. Infusion and oral administration, for example, are associated with lower immunogenicity than subcutaneous administration. Likewise, high-dose regimens are linked with lower immunogenicity than low-dose or interrupted regimens, as has been clearly shown in studies that have tested alternative administration routes for infliximab, a drug usually administered by infusion. In the RESTORE study, while satisfactory response to infliximab treatment (PASI 75) was maintained throughout the study period in the majority of patients (78%) who received continuous treatment, PASI scores fell considerably (from PASI 75 to PASI 20) in patients in whom treatment was discontinued and restarted upon relapse of the disease.23 A higher rate of adverse events (mostly infusion-related reactions) was also observed in the interrupted treatment group, showing how the management of biologic treatment can influence clinical course. The results of the RESTORE study, combined with experience gained in clinical practice, have led to infliximab being administered in continuous regimens in an attempt to maximize its therapeutic potential.
If we consider that ADAs may cause a loss of treatment response by reducing the number of drug molecules with therapeutic activity, clinical efficacy could in theory be restored by increasing the dose or by modifying the dosing interval (shortening the time between doses) to produce a situation in which the drug molecules would once again outnumber the ADAs. It should be noted, however, that actions based on this theory—which is supported by the results of various clinical trials (in particular those that are still in the open-label phase) for all the biologics indicated for psoriasis—would lead to increased costs and exposure to higher doses, at least initially.24
Finally, the addition of methotrexate to biologic regimens—a common practice in rheumatology but not so usual in dermatology—is an example of how adjunctive therapy can be used to interfere with the immune system and reduce its ability to recognize and eliminate foreign molecules by forming ADAs.25 Although the mechanism of action is not yet fully understood, the effectiveness of low (subtherapeutic) doses of methotrexate in this setting would seem to tip the balance in favor of an immunosuppressive effect rather than a synergistic action between methotrexate and the biologic agent.
Immunogenicity, Clinical Application, and Therapeutic OptimizationTo what extent is immunogenicity already a consideration in the routine management of biologics in psoriasis? The general recommendation that highly immunogenic drugs, such as infliximab, should be administered continuously and generally in association with methotrexate, would suggest that it is indeed a factor that is taken into account. Likewise, it appears logical that in the case of etanercept (the least immunogenic of the biologics) considerable experience has been gained with interrupted treatment regimens, including, in some cases, several withdrawal and retreatment cycles.26–28
It is not so clear that immunogenicity has been taken into account in the case of other drugs, such as adalimumab and ustekinumab. While experience with interrupted therapy has shown that a substantial proportion of patients treated with adalimumab and ustekinumab are capable of regaining response after interrupted treatment, response is generally better in patients treated with a continuous regimen. Whatever the case, it is difficult to draw any definitive conclusions since experience with interrupted regimens is scarce and the evidence comes primarily from results from comparator arms in clinical trials.29 From a theoretical perspective, at least, the possibility that the patient will develop neutralizing antibodies should be taken into account when contemplating the use of interrupted treatment regimens for adalimumab and ustekinumab.
After this short revision of the basic concepts underlying the immunogenicity of the biologics used in psoriasis, the next step is to determine how useful our understanding and management of this phenomenon could be for optimizing biologic therapy.
Jamnitski et al30 provide a good example of how our knowledge of immunogenicity can be used to optimize treatment. In a study of 292 patients who initiated etanercept treatment (some of whom were receiving their first anti-TNF agent and others who had been switched from infliximab or adalimumab), anti-TNF-naïve patients and switchers with ADAs had a similar response, which was better that that of the switchers without ADAs. These findings suggest that the absence of ADAs might reflect a lack of responsiveness to the mechanism of action shared by all anti-TNF agents and indicate the need to switch to a drug with a different mechanism of action. By contrast, the presence of such antibodies could be an additional (acquired) factor specific to the use of a given drug in a given patient, which could cause treatment failure for that drug even though the mechanism of action is potentially effective for the patient in question. In this second case, the option of using a drug with a similar mechanism of action but a different molecular structure (in this case etanercept) would be justifiable. Access to such analytical information, which is currently not available, would allow dermatologists to choose the best treatment option (either an alternative anti-TNF agent or a drug with a different mechanism of action) for each patient, thereby increasing the chances of a successful outcome.
Measuring Immunogenicity (Not Just ADAs) in Clinical PracticeDespite the sensitivity and specificity problems of currently available ADA detection assays, detection kits will probably soon be available for use in routine clinical practice.
Where possible, the priority should be to measure drug concentrations, as serum drug levels are the most reliable sign that immunogenicity or other factors are not interfering with the treatment strategy. This test should be complemented by the measurement of ADAs.31
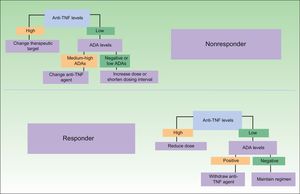
One difficulty in this respect is the lack of data from clinical trials on how long a treatment must be administered to obtain a satisfactory response, assuming that the patient is responsive to treatment. However, evidence from smaller-scale studies should accumulate over time,32 opening the way for new treatment algorithms that will guide decision-taking and favor a more rational use of biologics. Some of the proposed treatment strategies based on immunogenicity data are shown in Fig. 3. As can be seen, as well as leading to a more appropriate treatment strategy in the case of nonresponders, analysis of immunogenicity could also lead to a dose reduction or even the withdrawal of treatment in responders, with a very favorable economic impact.33 The dose reduction or withdrawal of treatment in apparent responders would be justified by the fact that the good clinical outcome could not be attributed to the therapeutic action of the drug in a patient with very low drug levels and high immunogenicity.
Proposed clinical practice algorithm for biologic therapy based on immunogenicity. TNF indicates tumor necrosis factor; ADA, antidrug antibodies. Adapted from Martín-Mola et al.33
The term drug survival refers to the length of time a particular drug remains an appropriate option for a given patient. While the definition is not very academic, the concept is very relevant to clinical practice. What can be considered “appropriate” varies from patient to patient and is determined by an asymmetric equation that includes efficacy, safety, tolerance, and convenience, alongside other factors such as the patient's treatment history, response to other treatments, and alternative treatments available.
In light of the issues addressed in this article, there would appear to be little doubt that immunogenicity is an important factor in the interaction between a drug and an individual's immune system that should be taken into account in treatment strategies. However, its overall impact on the potential therapeutic effect of a drug will not be the same in all patients, not even among those who develop ADAs. The impact of ADAs is only relevant in patients in whom these antibodies actually influence the final effects of the drug, an outcome dependent on the mechanism of action and/or titers of the ADAs in each case. Immunogenicity is particularly relevant in patients with secondary loss of efficacy, that is, in patients who initially respond satisfactorily but then exhibit a gradual loss of response over the treatment period. Most individuals who maintain a satisfactory response do not have ADAs and assessment of immunogenicity is not required in these patients.34
Immunogenicity should therefore be routinely taken into account when designing a treatment strategy for a specific patient, together with other factors, such as the intrinsic efficacy of the drug and its short-term and long-term safety profile, the patient's weight, and the presence of comorbidities.35
Immunogenic reactions are an inevitable corollary of the treatment of psoriasis with biologic agents since the purpose of the immune system is to interfere with the presence and action of any foreign molecule; such interference is much more likely to reduce efficacy when neutralizing ADAs are formed.
Now that the importance of immunogenicity in the overall treatment strategy has been recognized, we can apply this knowledge to help us design treatment strategies and manage and limit the impact of the immune response in biologic therapy.
A better understanding of immunogenicity may also be a useful ally in the design of predictive models and, probably in the near future, treatment regimens tailored for individual patients.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestDr. José Manuel Carrascosa has received speaking and consultancy fees and/or research grants from Abbott, Pfizer, Janssen-Cilag, MSD, and Amgen.
I thank Professor Denis Julien for providing Figs. 1 and 2 showing the mechanisms of action of antidrug antibodies in biologic therapy.
Please cite this article as: Carrascosa JM. Inmunogenicidad en terapia biológica. Implicaciones en Dermatología. Actas Dermosifiliogr. 2013;104:471–9.