Granuloma annulare (GA) is a benign and typically self-limiting granulomatous disease of unknown etiology; it tends to resolve spontaneously over a period of months or years. It usually presents with annular lesions on the hands, upper limbs, trunk, or lower limbs; facial involvement is rare. It is associated with diabetes mellitus, paraneoplastic disorders, thyroid disturbances, and some drugs.1
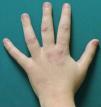
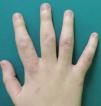
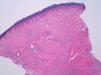
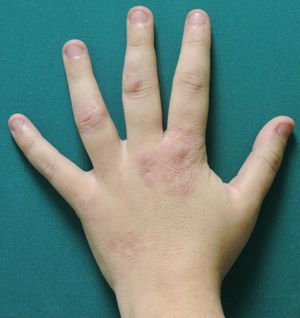
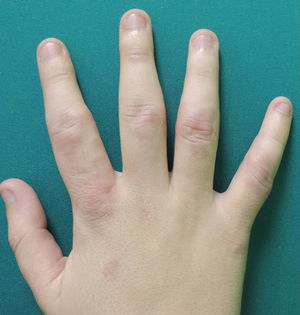
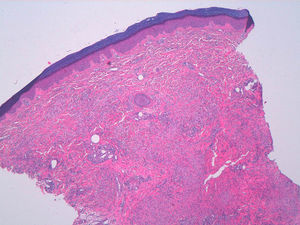
A woman aged 38 years, with a personal history of an eating disorder for which she had been on treatment with topiramate for several months, was seen in dermatology outpatients for slightly pruritic lesions that had arisen on the dorsum of both her hands some months earlier. On physical examination, confluent papules with an annular morphology were observed on the dorsum of the fingers of both hands and over the metacarpophalangeal joints of the left hand (Figs. 1 and 2). Histology revealed focal degeneration of collagen and elastic fibers, mucin deposits, and a perivascular and interstitial lymphohistiocytic infiltrate in the upper and mid dermis, confirming the diagnosis of GA (Fig. 3). There were no significant findings in the blood tests requested. The patient was initially treated with topical tacrolimus and corticosteroids, and subsequently with oral corticosteroid therapy, with no improvement. On reviewing her medical history, we observed a chronological relationship between the introduction of topiramate and the appearance of the GA lesions. With a suspicion of GA secondary to topiramate, we decided, with the consent of the psychiatry department, to withdraw the drug, and this led to complete resolution of the lesions within a month.
Four types of drug-induced granulomatous dermatitis have been identified: interstitial granulomatous dermatitis, exacerbation of rheumatoid nodules secondary to methotrexate, drug-induced sarcoidosis, and drug-induced GA.
The first case of drug-induced GA, published in 1980, was associated with gold salts for the treatment of juvenile arthritis.4 Other cases have been reported since that time, mainly involving allopurinol, calcitonin, diclofenac, anti-tumor necrosis factor drugs, calcium channel blockers, and various chemotherapeutic agents.2–7 However, we have only found 3 cases involving topiramate (Table 1),1–3 all with lesions affecting the lower limbs, in contrast to our patient, in whom the lesions were on her hands. As in the 3 cases described in the literature, we detected a chronological relationship between treatment with topiramate and the appearance of GA in our patient, as the lesions appeared very soon after the introduction of topiramate and disappeared within 2 to 3 weeks of withdrawal of the drug, with no subsequent recurrence.1,3 Additionally, although it is widely known that GA lesions sometimes resolve after biopsy,1–3 we observed no improvement in our patient after performing biopsy. However, confirmation of the reappearance of GA lesions after rechallenge with topiramate has not been performed in our patient.
Cases of Topiramate-Induced Granuloma Annulare.
| Age | Sex | Latency Period Until the Appearance of GA | Latency Period Until the Disappearance of GA | Site | |
|---|---|---|---|---|---|
| Cassone G et al.1 | 50 | Female | 1 mo | 2 wk | Left lower limb |
| Lagier L et al.2 | 44 | Female | 3 mo | 2 wk | Dorsum of the feet Lower limbs |
| Ruzzetti F et al.3 | 29 | Female | 6 y | 2 wk | Dorsum of the hands Lower limbs Dorsum of the feet |
| Heras S et al. | 38 | Female | 2 mo | 1 mo | Dorsum of the hands |
Topiramate is an antiepileptic drug that has a structure based on D-fructose, a monosaccharide, and differs considerably from other antiepileptic drugs. It is indicated in the treatment of partial or generalized seizures, migraine prophylaxis, and in impulse control.2,3 It has a multiple mechanism of action, including inactivation of the voltage-gated sodium channels and potentiation of amino-butyric acid-mediated neurotransmission, and it is a glutamate receptor antagonist.3 Rare cutaneous side effects reported with topiramate include alopecia, oligohydrosis, pemphigus, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis2; the appearance of GA is very rare. The pathogenesis of GA secondary to topiramate is unknown, but, given the extensive use of this drug, the disorder is probably underdiagnosed. In patients with GA refractory to the usual treatments, a detailed medical history should therefore be taken to exclude the possible implication of any drug in its onset.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Heras-González S, Piqueres-Zubiaurre T, Salinas-Quintana AMd, González-Pérez R. Granuloma anular posiblemente secundario a la ingesta de topiramato. Actas Dermosifiliogr. 2017;108:952–954.