To the Editor:
Basal cell carcinoma (BCC) is the most common skin cancer and represents 90% of malignant eyelid tumors.1 Management options are mainly surgical, including Mohs micrographic surgery and wide surgical excision.1 However, some of these lesions can progress to an advanced locally stage and represent a therapeutic challenge. In 2012 the US Food and Drug Administration (FDA) approved Vismodegib, an oral hedgehog pathway inhibitor, for the treatment of locally advanced and metastatic BCCs which are inappropriate for surgery or radiotherapy.2,3 Herein, we report a patient with an infraorbital locally advanced BCC who was offered oral vismodegib before surgery reducing surgery-associated morbidity and allowing the preservation of functional and aesthetic aspect of the affected area.
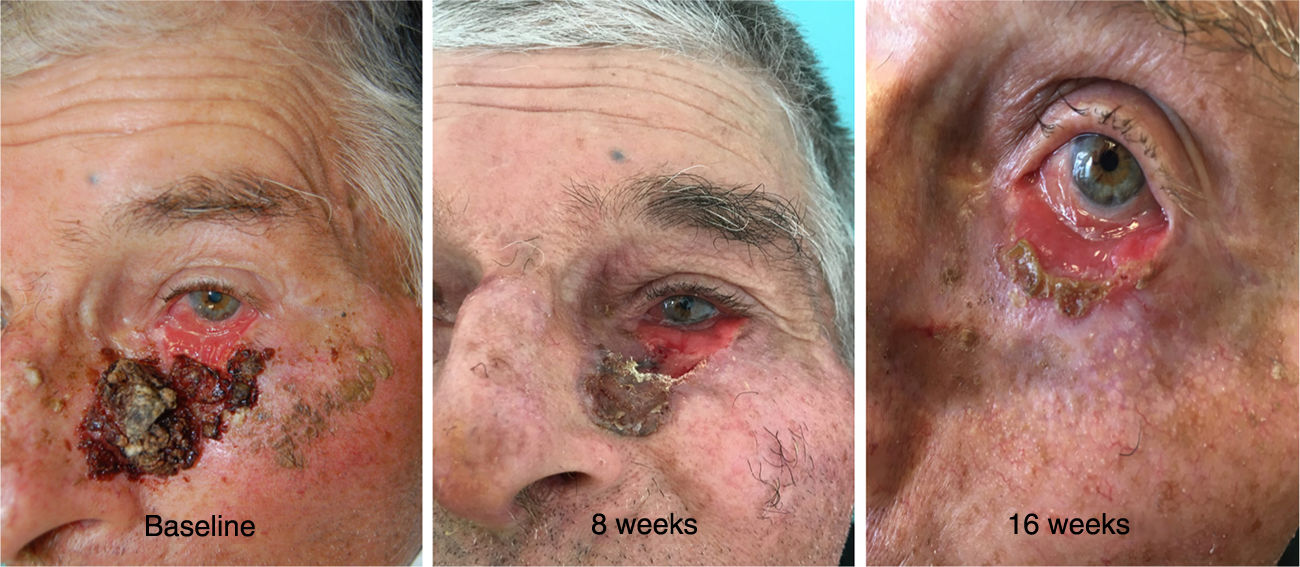
A 81-year-old man presented with a 6-year history of a progressively growing lesion in the left lower eyelid. Examination of the ocular adnexae showed an extensive, infiltrated and centrally erosive lesion, covered by a crust with 6cm of major axis and involving all the left lower eyelid with ectropion and extending onto the left malar cheek (Fig. 1). Biopsy showed a ulcerated BCC and computed tomography (CT) scan of the head did not identify alterations in the facial bones (left maxilla, inferior orbital rim, and base of the nasal bone). Due to the advanced nature of the lesion and involvement of more than two-thirds of the tarsal conjunctiva with a complex location for surgery and radiotherapy, the patient was offered oral vismodegib 150mg PO daily in order to shrink the tumor before surgery and reduce surgery-associated morbidity.
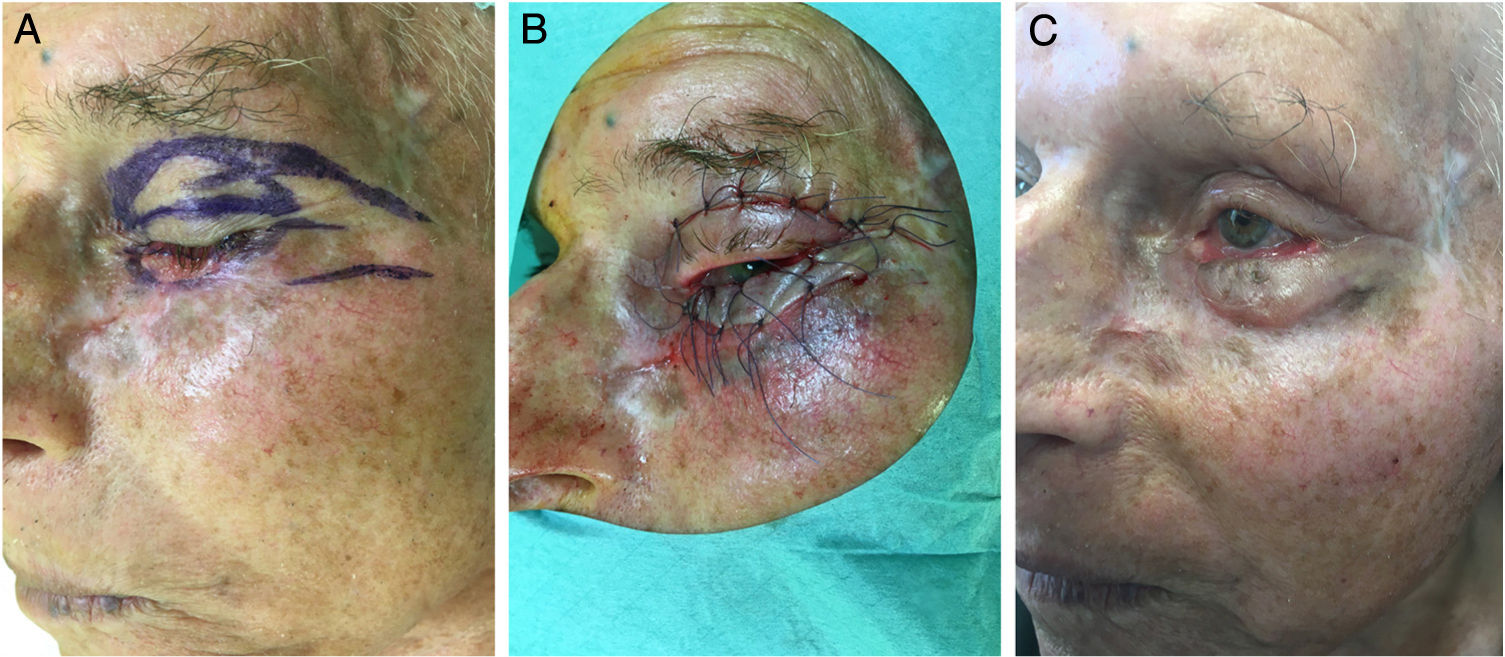
The reassessment was performed at 4, 8 and 16 weeks after initiation of oral therapy (Fig. 1). At 16 weeks the lesion showed a significant clinical improvement with substantial regression and a biopsy of the tarsal conjunctiva was performed. The histopathologic examination of the tarsal conjunctiva was compatible with “absence of neoplasia”. Vismodegib treatment was discontinued at 23 weeks and wide excision of the residual lesion with reconstruction was performed. The pre-surgical clinically visible lesion size was 2,5×0,8cm and the final surgical defect measured 3,2×1,5cm. For the reconstruction of the defect, it was suggested to perform a modified Tripier flap (Fig. 2A). A single pedicle, randomized and lateral, was drawn with a relatively wide base (Fig. 2B). The surgical solution was consistent with a good functional result. Histological evaluation of the surgical specimen revealed “absence of neoplasia”. The follow-up 6 months after surgery revealed no recurrences and an excellent functional and aesthetic result (Fig. 2C). The patient tolerated vismodegib very well just reporting a slight muscles spasm, dysgeusia and alopecia of the eyebrows and eyelids. All the side effects were graded as 1 using the Common Terminology Criteria for Adverse Events (CTCAE). No severe adverse events were observed.
It is established that neoadjuvant treatment with vismodegib for an average of 4 months before surgery reduces tumor area and surgical defect size.4,5 In literature, a clinical trial with short-term vismodegib showed a reduction of the surgical defect area by 31% if used for at least 3 months in non-recurrent BCCs of functionally sensitive locations.5 Furthermore, previous studies revealed that the clinical appearance of tumors after vismodegib was variable and did not predict histologic cure.3,5 This achievement enhances the importance of performing biopsies or diagnostic techniques such as confocal laser scanning microscopy in order to identify residual tumor nests. The margins performed in this case had the intention to excise the clinical residual lesion with 10mm margin and the reconstruction of the inferior eyelid ectropion. Some studies support the idea that vismodegib “cure” is temporary, as BCCs recur at the same site within a few months of drug discontinuation.3,5 We consider that probably the best surgical option after vismodegib would be micrographic Mohs surgery (MMS) as this surgical technique allows to localize residual nests of BCC that can remain during the reduction of the tumor.6
In our clinical case, a modified Tripier flap was performed as eyelid wound repair must be carefully designed so that eyelid function is maintained and the globe is protected. The original Tripier flap is considered a myocutaneous bipedicular flap originating from the upper eyelid and recommended for the reconstruction of lower lateral eyelid defects.7 Usually this technique is time-consuming and requires a second surgical stage for sectioning the lateral pedicles.8 Its modification consists in planning a single pedicle, lateral, randomized in one-stage surgery which does not rely on the inclusion of orbicularis muscle or innervation. The surgical solution of our case demonstrates an option of the lower eyelid reconstruction, simple and versatile without ectropion or eyelid distortion.
This case highlights the potential of short-term vismodegib as neoadjuvant to surgery for high-risk locally advanced basal cell carcinomas, especially in functionally sensitive locations as the periocular and orbital areas.
Conflicts of interestDr. César Martins is a member of the Roche Pharmaceutics Advisory board. The other authors declare no conflicts of interest.
Funding sourcesNone.
Please cite this article as: Monteiro AF, Rato M, Trigo M, Martins C. Carcinoma basocelular agresivo del párpado inferior: ventaja del vismodegib neoadyuvante. Actas Dermo-Sifiliográficas. 2019;110:863–865.