Imatinib mesylate is a tyrosine kinase inhibitor that targets the BCR-ABL, c-kit, and PDGF (platelet-derived growth factor) receptors. Imatinib is mainly indicated for chronic myeloid leukemia and gastrointestinal stromal tumors but is also prescribed by dermatologists for dermatofibrosarcoma protuberans, systemic sclerosis, and systemic mastocytosis, among other conditions. Most adverse effects are mild or moderate and therapy is generally well tolerated. Adverse skin effects are very common and include nonspecific manifestations such as edema and maculopapular rashes or eruptions of diverse types (lichenoid or psoriasiform lesions, acute generalized exanthematic pustulosis, Stevens-Johnson syndrome, and more). Identifying and properly treating these reactions can help optimize adherence to treatment and improve the prognosis of the underlying disease.
El imatinib mesilato es un inhibidor de la tirosín cinasa de administración oral que inhibe la BCR-abl, c-KIT y el platelet-derived growth factor receptor (PDGFR). Sus indicaciones fundamentales son la leucemia mieloide crónica y los tumores del estroma gastrointestinal. En Dermatología se emplea en enfermedades como el dermatofibrosarcoma protuberans, esclerosis sistémica y mastocitosis sistémica, entre otras. Es un fármaco en general bien tolerado, con la mayoría de efectos adversos leves o moderados. Los efectos secundarios dermatológicos son muy frecuentes e incluyen erupciones cutáneas inespecíficas como edema o erupciones maculopapulosas o con características clínicas distintivas (liquenoides, psoriasiformes, pustulosis exantemática aguda generalizada, síndrome de Stevens- Johnson…). Identificar y tratar correctamente estas reacciones puede ayudar a optimizar la adherencia del paciente al tratamiento y mejorar el pronóstico de su enfermedad de base.
Imatinib mesylate (Glivec, Novartis; formerly known as STI 571) is an oral inhibitor of the tyrosine kinase BCR-Abl (a fusion protein from the Philadelphia chromosome, a cytogenetic abnormality of chronic myeloid leukemia [CML]), c-KIT, and platelet-derived growth factor receptor (PDGFR). The approval of this agent in 2001 by the US Food and Drug Administration revolutionized the treatment of patients with CML and significantly extended their overall survival.
Subsequently, the second generation of tyrosine kinase inhibitors nilotinib (Tasigna, Novartis) approved in 2007 and dasatinib (Sprycel; Bristol-Myers Squibb) approved in 2006, have extended the therapeutic options in patients intolerant of or resistant to imatinib.
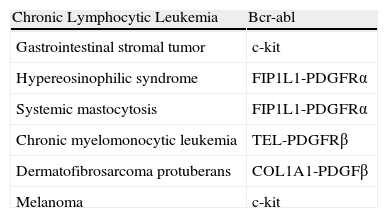
Imatinib is also approved for treating other diseases such as unresectable c-kit+ malignant gastrointestinal stromal tumors, acute Philadelphia chromosome+ lymphoblastic leukemia, myelodysplastic syndromes with PDGFR gene reordering, hypereosinophilic syndrome, and chronic eosinophilic leukemia. In dermatology, the agent has proved effective in certain diseases such as inoperable or metastatic dermatofibrosarcoma protuberans (DFSP),1 sclerodermous graft-versus-host disease,2 systemic sclerosis,3 systemic mastocytosis,4 and melanoma with c-kit+ mutations.5Table 1 shows the therapeutic targets in the different diseases in which imatinib has been used.
Therapeutic Targets in the Different Diseases in Which Imatinib Is Used.
| Chronic Lymphocytic Leukemia | Bcr-abl |
| Gastrointestinal stromal tumor | c-kit |
| Hypereosinophilic syndrome | FIP1L1-PDGFRα |
| Systemic mastocytosis | FIP1L1-PDGFRα |
| Chronic myelomonocytic leukemia | TEL-PDGFRβ |
| Dermatofibrosarcoma protuberans | COL1A1-PDGFβ |
| Melanoma | c-kit |
Doses of 400-600mg/d are used in chronic CML and GIST. In accelerated CML and DFSP, a dose of up to 800mg/d is indicated.
Adverse Effects of ImatinibAlthough the drug is generally well tolerated, up to 5.4% of patients have to discontinue treatment due to adverse effects.6 Adverse effects are usually classified using the US National Cancer Institute scale, with 4 severity grades.7 Most of the effects observed after administration of imatinib are mild or moderate in severity (grade 1 or 2).8
The grade 1 and 2 side effects most frequently observed after 5 years of follow-up in patients diagnosed for the first time with CML and treated with imatinib were edema, muscle cramps, diarrhea, nausea, musculoskeletal pain, skin rash and other skin conditions, abdominal pain, fatigue, joint pain, and headache.8 Severe adverse effects, that is, grade 3 or 4 events, included neutropenia, thrombocytopenia, anemia, and liver enzyme elevation.9
Imatinib appears to cause a higher rate of headache, diarrhea, vomiting, muscle spasm, and edema compared to other tyrosine kinase inhibitors.10 However, imatinib is associated with a lower rate of skin rash, headache, pruritus, elevated transaminases, and alopecia. Hematologic adverse effects such as neutropenia were observed in 20% of patients compared to 12% with nilotinib. The 2 drugs caused similar rates of grade 3 and 4 thrombocytopenia and anemia.
Dasatinib appears to cause lower rates of nausea, vomiting, muscle inflammation, rash, fluid retention, and headaches compared to imatinib.11 Similar percentages of grade 3 and 4 neutropenia and a higher rate of thrombocytopenia were observed with dasatinib compared to imatinib.12Table 2 shows a comparison of the percentage of patients with drug-related adverse skin effects.13
Side Effects of the Different Tyrosine Kinase Inhibitors.
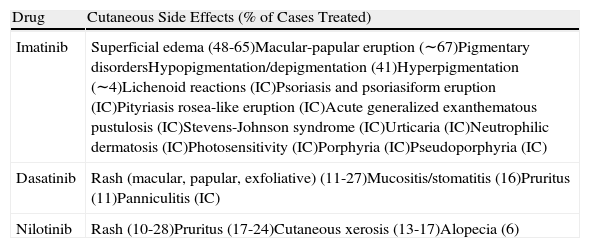
| Drug | Cutaneous Side Effects (% of Cases Treated) |
| Imatinib | Superficial edema (48-65)Macular-papular eruption (∼67)Pigmentary disordersHypopigmentation/depigmentation (41)Hyperpigmentation (∼4)Lichenoid reactions (IC)Psoriasis and psoriasiform eruption (IC)Pityriasis rosea-like eruption (IC)Acute generalized exanthematous pustulosis (IC)Stevens-Johnson syndrome (IC)Urticaria (IC)Neutrophilic dermatosis (IC)Photosensitivity (IC)Porphyria (IC)Pseudoporphyria (IC) |
| Dasatinib | Rash (macular, papular, exfoliative) (11-27)Mucositis/stomatitis (16)Pruritus (11)Panniculitis (IC) |
| Nilotinib | Rash (10-28)Pruritus (17-24)Cutaneous xerosis (13-17)Alopecia (6) |
Abbreviation: IC, isolated case. Source: Adapted from Amitay-Laish et al.13
In general, any grade 3 or 4 adverse effect is managed by suspending administration and resuming at a lower dose once the toxicity has resolved.
In these patients, a careful assessment of the adverse effects is important because of the impact on treatment adherence and therefore efficacy. Adherence to treatment can essentially be improved by helping the patient to identify and manage side effects.
Adverse Cutaneous Effects of ImatinibSkin rash is one of the most common adverse effects of imatinib, and estimates of incidence range from 7% to 88.9%.14,15 Most cases are mild and self-limiting, with onset shortly after starting administration of the drug.16Table 3 shows classification of the most common adverse cutaneous effects according to their severity.7 According to one study, 2.3% of patients treated at a dose of 400mg/d and 5.3% of those treated at a dose of 800mg/d17 have severe adverse cutaneous effects.
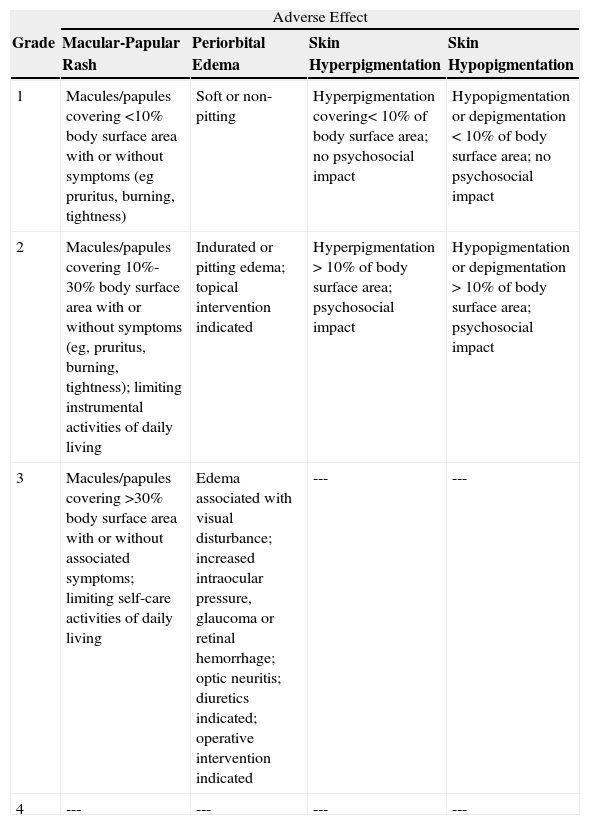
Classification of Severity of Adverse Cutaneous Reactions Most Frequently Associated With Imatinib Use: United States National Cancer Institute Scale Version 4, Which Classifies Severity According to 4 Grades.
| Adverse Effect | ||||
| Grade | Macular-Papular Rash | Periorbital Edema | Skin Hyperpigmentation | Skin Hypopigmentation |
| 1 | Macules/papules covering <10% body surface area with or without symptoms (eg pruritus, burning, tightness) | Soft or non-pitting | Hyperpigmentation covering<10% of body surface area; no psychosocial impact | Hypopigmentation or depigmentation <10% of body surface area; no psychosocial impact |
| 2 | Macules/papules covering 10%-30% body surface area with or without symptoms (eg, pruritus, burning, tightness); limiting instrumental activities of daily living | Indurated or pitting edema; topical intervention indicated | Hyperpigmentation >10% of body surface area; psychosocial impact | Hypopigmentation or depigmentation >10% of body surface area; psychosocial impact |
| 3 | Macules/papules covering >30% body surface area with or without associated symptoms; limiting self-care activities of daily living | Edema associated with visual disturbance; increased intraocular pressure, glaucoma or retinal hemorrhage; optic neuritis; diuretics indicated; operative intervention indicated | --- | --- |
| 4 | --- | --- | --- | --- |
The effects are thought to be independent of dose. A recent study reported such events in 7% of patients treated at a subtherapeutic dose of 25-140mg/d and in 21% to 88% of patients treated with a dose of 400-800mg/d.18 This fact, along with the relatively low molecular weight of the drug, suggests that the cutaneous toxicity of imatinib is mediated by a toxic pharmacologic effect rather than an immunogenic effect.
Different types of skin reactions have been reported after using imatinib. These can be nonspecific, such as macular-papular rash, superficial edema, or pruritus, or less frequently, rash with clinically distinctive characteristics (lichenoid, psoriasiform, acute generalized exanthematous pustulosis, Stevens-Johnson syndrome [SJS], etc).
The most frequent adverse cutaneous effects associated with imatinib are described in the following sections.
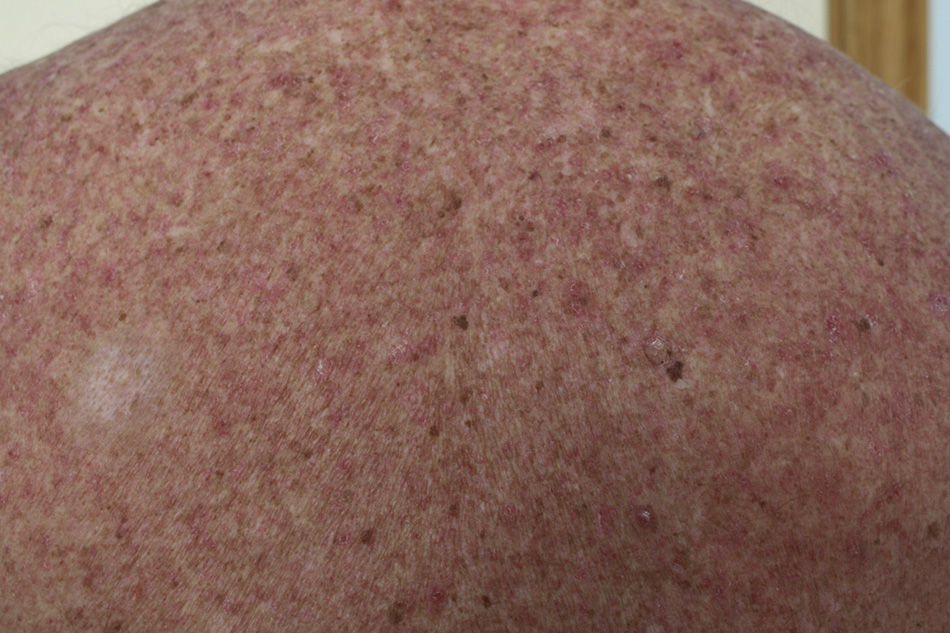
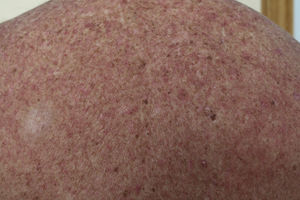
Macular-Papular RashOnset of rash caused by imatinib usually occurs a few days after starting treatment, although it can present after several months of treatment. According to some studies, rash is a frequent event and may present in up to 66.7% of patients within 2 months of starting the drug.19
The eruption is usually erythematous, macular-papular, sometimes desquamative, and pruritic (Fig. 1). The lesions are located on the forearms, trunk, and legs, and less often on the face.20 They also present more frequently in patients who take high doses (>600mg/d) of the drug, and so the rash is thought of as a pharmacological toxicity rather than a hypersensitivity reaction.
Most cases of rash are self-limiting and readily treated with emollients, topical corticosteroids, and antihistamines. Dose reductions are therefore not needed. When the rash progresses to erythroderma, it is considered grade 4 toxicity, and suspension of the drug is indicated. In general, severe cases usually require treatment with oral corticosteroids and suspension of imatinib until the rash has improved to grade 1. Imatinib can then be reintroduced at a lower dose (50-100mg/d) along with use of concomitant oral corticosteroids; the dose of imatinib can then be gradually increased.20
The initial recommended oral corticosteroid dose is usually 0.5-1mg/kg of prednisone or equivalent. In certain patients, desensitization has been successful, suggesting that hypersensitivity mechanisms could also be present.21
In very severe cases, reintroduction of the drug is not recommended and replacement with nilotinib or dasatinib is recommended.
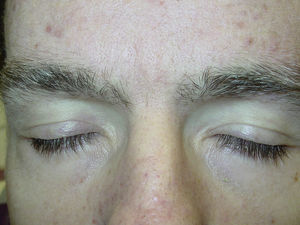
Superficial EdemaOne of the most frequent adverse cutaneous effects is superficial edema. According to several studies, between 48% and 65% of patients treated with this drug develop this complication within 6 weeks of starting treatment, often in association with weight gain.15 Edema is normally mild to moderate in intensity, and presents on the face, and particularly the eyelids. It may be more severe in the mornings.22 Edema of the limbs is much rarer. Central water retention (manifest as events such as pleural effusion and congestive heart failure) has also been reported in 1% to 3% of patients treated with imatinib.23
Superficial edema is thought to be dose independent and arises as a result of increased pressure of interstitial dermal fluid caused by inhibition of PDGFR, which is responsible for interstitial fluid homeostasis.24
In most cases, management of superficial edema does not require the drug to be suspended or indeed any specific treatment. In some cases, a restriction of dietary salt and topical application of phenylephrine 0.25% may be beneficial.25 In patients with central edema, diuretics may be indicated.25
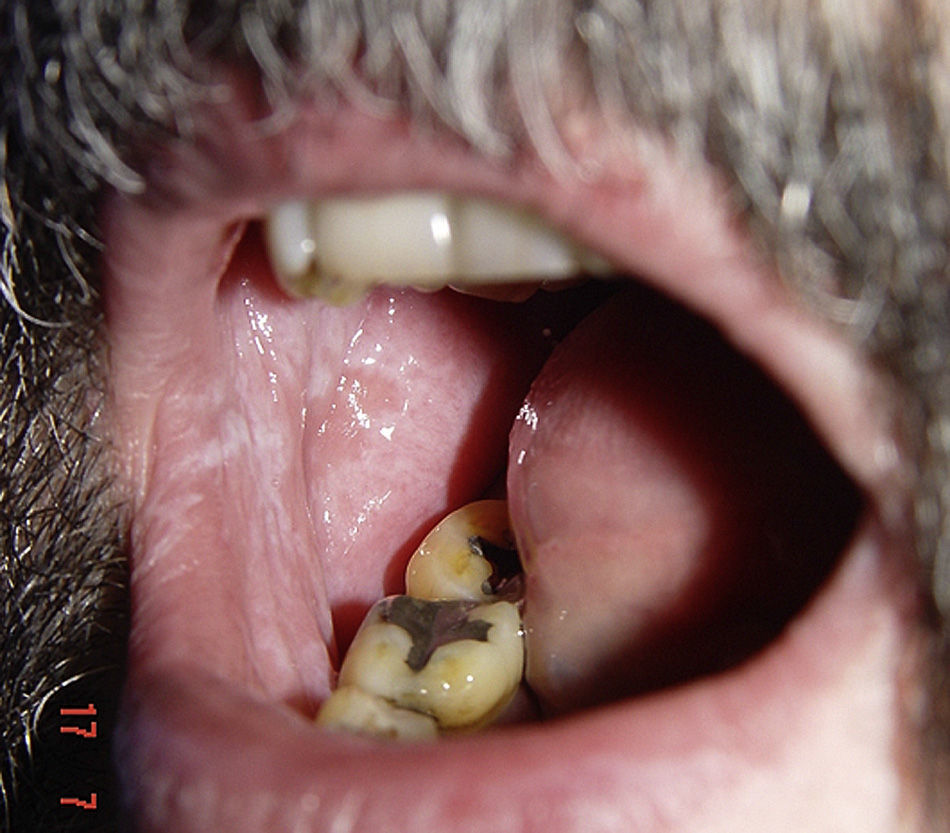
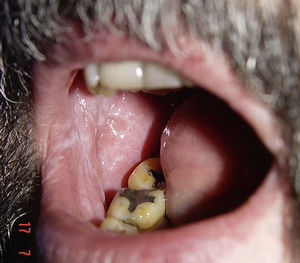
Lichenoid ReactionsFifteen cases of lichenoid reactions have been reported in patients treated with imatinib.26–29 The lesions occurred on the skin and/or mucosa (Fig. 2). These reactions appear to be dose dependent given that all reports were in patients who were receiving high doses of the drug (>400mg/d). In most cases, onset occurred from 1 to 3 months after starting treatment, although some patients did not develop the lesions until 1 year after starting imatinib. Suspension of the drug was not necessary in most patients, and the reactions were managed satisfactorily with the use of oral corticosteroids or acitretin.30
Pigmentary DisordersSeveral cases of pigmentary disorders due to the use of imatinib have been reported. Normally, hypopigmentation is manifest as hypochromic areas or diffuse or localized achromic areas, with remission after a dose reduction or discontinuation.31,32
Patients with dark phototypes are more likely to develop this adverse effect. Some studies have shown an incidence of hypopigmentation of 41% in patients treated for CML.32
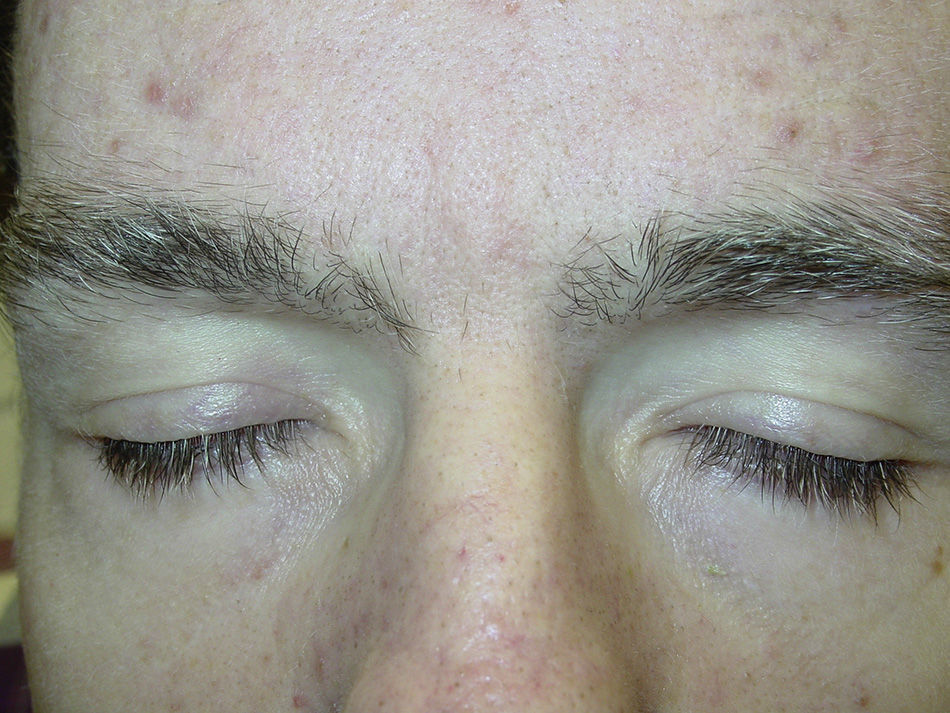
Likewise, cases of hair depigmentation have been reported (Fig. 3).33
In addition, there have been case reports of patients treated with imatinib who develop skin, mucosal (hard palate), and nail hyperpigmentation.34–37 In a study of 118 patients with CML treated with imatinib, only 4% developed hyperpigmentation.32
These reactions are likely to occur because imatinib inhibits c-KIT in the melanocytes, thereby reducing the activity of these cells and leading to hypopigmentation.37 Moreover, imatinib can also cause hyperpigmentation by chelating one of its metabolites with iron and melanin in a mechanism similar to minocycline and antimalarial-associated hyperpigmentation.38
Psoriasis and Psoriasiform RashIn a study of 54 patients treated with imatinib, 4 developed psoriasiform rash between 1 and 7 months after starting treatment.15 Half of these patients had a history of psoriasis. There have also been reports of imatinib-related exacerbation of psoriasis.39,40 Deguchi et al.41 reported psoriasiform palmoplantar hyperkeratosis and nail dystrophy after treatment with imatinib in 3 patients with no history of psoriasis. All patients improved after suspending or reducing the dose of the drug.
However, a case was published in which psoriasis improved in a patient with a GIST who started treatment with 400mg/d of imatinib.42
T lymphocytes are known to play an important pathogenic role in psoriasis and imatinib can modulate T lymphocyte signalling.43 The exact circumstances of the modulation are thought to influence whether a T-lymphocyte stimulatory or inhibitory effect occurs, leading to exacerbations or remissions, respectively, of the disease.
Pityriasis Rosea-Like RashSeveral cases of rashes resembling pityriasis rosea have been reported in patients treated with imatinib. In most cases, rash onset was 1 to 2 months after starting treatment.44
In some cases, the relationship with imatinib seems fairly probable, as the skin lesions (clinically and pathologically consistent with pityriasis rosea) resolved after withdrawal of the drug and reappeared on rechallenge.45,46
The pathogenesis of this adverse effect is unknown.
Acute Generalized Exanthematous PustulosisA case typical of acute generalized exanthematous pustulosis caused by imatinib has been reported.47
Subsequently, there were 3 reports of another 3 atypical cases in which an eruption with an atypical localization occurred between 1 and 4 years after starting the drug.48–50
Cases of acute generalized exanthematous pustulosis have not been reported in patients who received less than 600mg/d of imatinib, and so this adverse effect is thought to be dose-dependent. The pathogenesis is not entirely understood.
Stevens-Johnson SyndromeSeveral cases of SJS have been reported in patients treated with imatinib.51–55 Hsiao et al.54 described a patient with CML who developed signs and symptoms reminiscent of SJS one week after starting the drug. These resolved after withdrawal of the drug and reappeared on rechallenge, strongly suggesting that the drug played a role in the eruption.
In other cases, however, the eruption did not appear after rechallenge at lower doses.52
In another case report of SJS, a patient treated with 400mg/d of imatinib underwent rechallenge at a dose of 200mg/d and the lesions reappeared.55 At a second rechallenge at a dose of 100mg/d and 1mg/kg/d of prednisone, the lesions did not reappear. On suspending prednisone after 6 weeks, the imatinib dose was increased to 300mg/d with no adverse effects.
Some authors suggest that desensitization should be attempted to manage these severe mucocutaneous eruptions when the lesions reappear even with concomitant use of prednisone.21 Currently, with availability of alternatives such as dasatinib and nilotinib, we believe that switching to another drug is more appropriate in these cases.
Neutrophilic DermatosesTwo cases of Sweet syndrome have been reported in patients treated with imatinib, and in one case the temporal association was clear.56,57 There has also been a report of a case of a patient with imatinib-induced neutrophilic eccrine hidradenitis,47 2 cases of erythema nodosum,58 3 cases of recurrent neutrophilic paniculitis,59–61 and a case of neutrophilic foliculitis.62
Toxic Epidermal NecrolysisA case report has been published of a patient with CML who developed a very severe blistering skin and mucosal eruption after allogeneic bone marrow transplantation (with fludarabine and busulphan conditioning) and treatment with imatinib.63 A causal relationship with imatinib is doubtful, as the patient was taking other drugs and the eruption appeared after 3 months of treatment with imatinib.
PhotosensitivityTwo studies have been published of several cases of photosensitivity in patients in long-term treatment with imatinib,15,64 as well as a case of photo-induced dermatitis.65
Other Skin ReactionsThere have been case reports of fungoid-like mycosis,66 cutaneous porphyria cutanea tarda,67 pseudoporphyria,68 and skin fragility and blistering69,70 in patients treated with imatinib.
ConclusionImatinib is a well-tolerated drug, although it is not devoid of adverse effects, some of which are serious. Cutaneous adverse reactions are among the most frequent side effects. In this article, we have exhaustively reviewed the side effects of this drug, paying particular attention to cutaneous effects, and we have provided guidance for their management. As this is a drug with many indications, including skin conditions, dermatologists are increasingly seeing patients in the clinic with adverse effects resulting from its use. Appropriate knowledge of these reactions and their management is of great importance, as adherence to treatment is essential to ensure efficacy in potentially fatal diseases such as CML.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that patient data do not appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pretel-Irazabal M, Tuneu-Valls A, Ormaechea-Pérez N. Efectos adversos cutáneos del imatinib (inhibidor de la tirosín cinasa). Actas Dermosifiliogr. 2014;105:655–662.