The use of fillers for esthetic purposes has increased in recent years. Complications secondary to the use of permanent and nonpermanent fillers have been widely described.1 However, the literature on complications in men is scarce. Here, we describe the clinical and epidemiological characteristics of men with adverse reactions to fillers.
We carried out a retrospective study of men with adverse reactions to fillers who visited the dermatology department of the Hospital Clínic de Barcelona between May 1, 2015, and October 31, 2018. Corresponding medical records, photographs, and additional tests were reviewed. Ultrasound evaluation was performed using an Esaote Mylab ClassC kit with 18-MHz and 22-MHz probes.
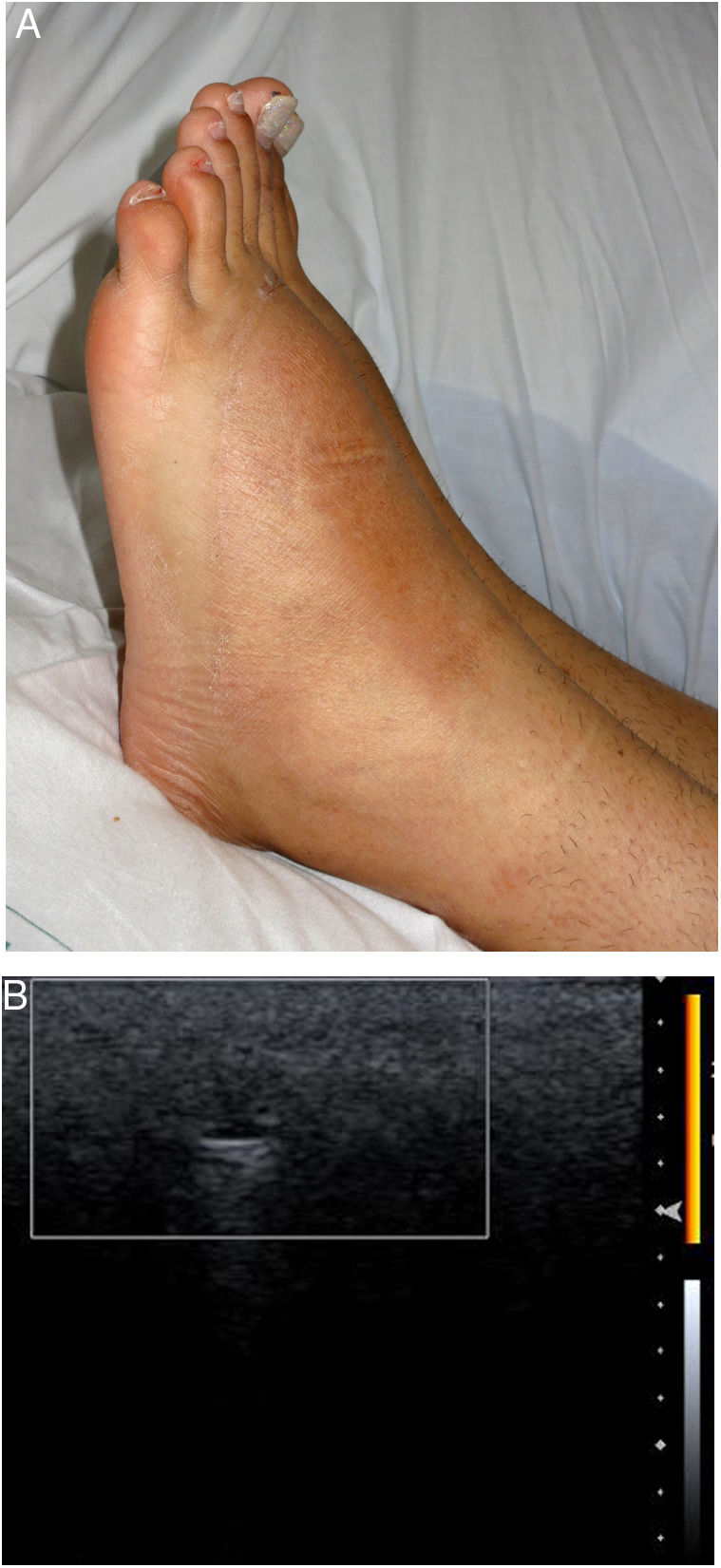
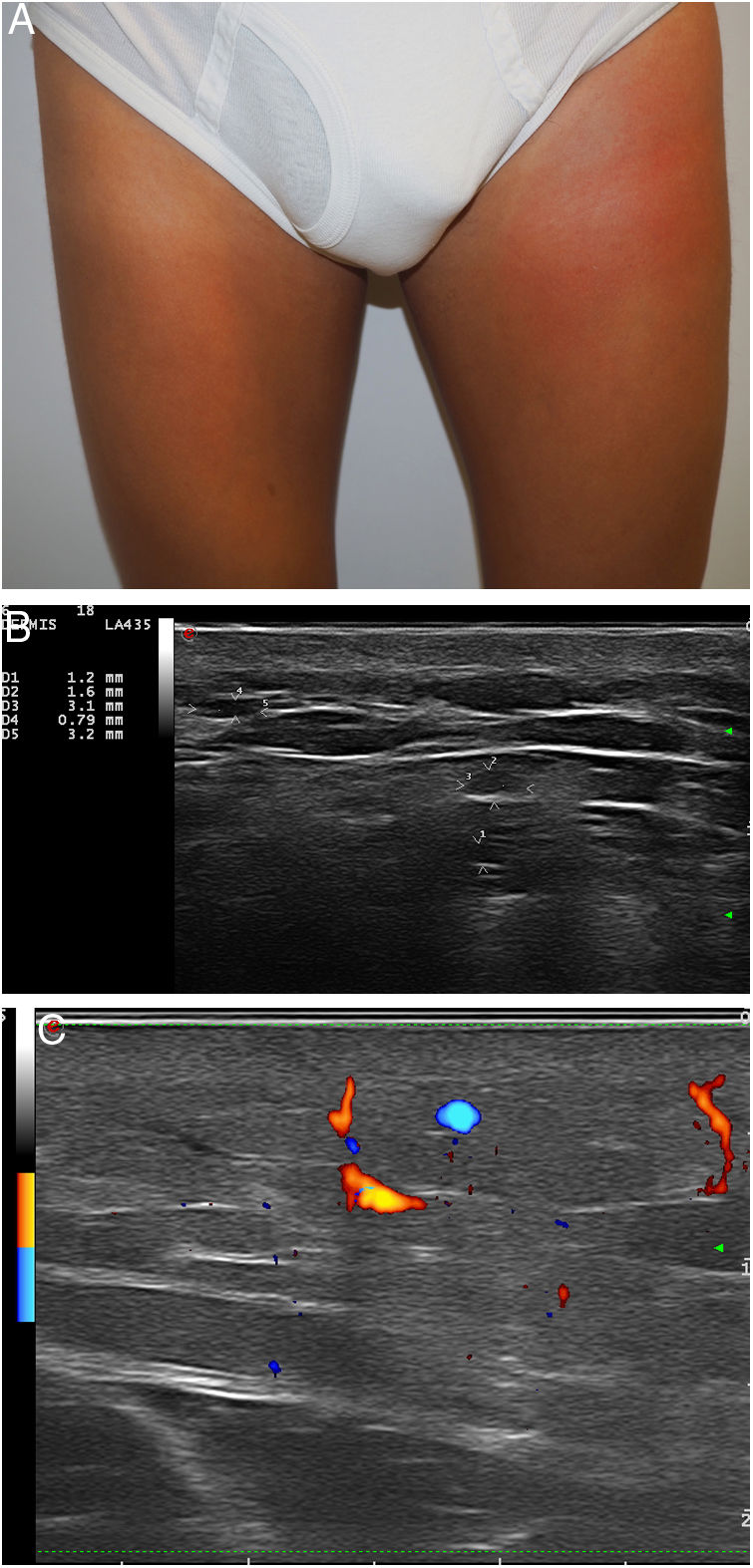
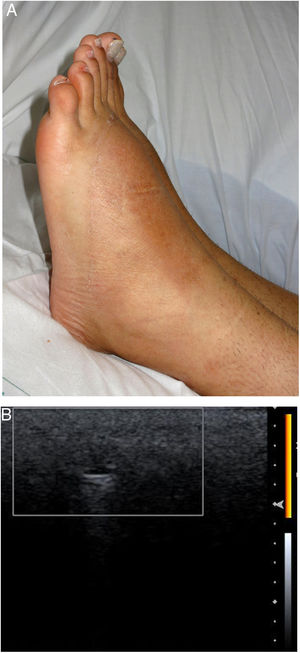
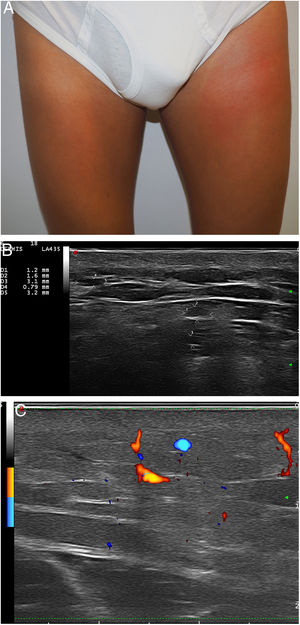
Our analysis included 6 men (one of whom was the subject of a previous publication2): 1 was transsexual; 3 were men who have sex with men (MSM); and 2 were heterosexual (Table 1). Three of the patients had human immunodeficiency virus (HIV). All had been referred from another service with different diagnoses. Adverse reactions to fillers were never suspected. The most frequent filler injection site was the buttocks (4 patients), followed by the face (2 patients). The patients reported receiving injections of hyaluronic acid (1 patient), liquid silicone (2 patients), petrolatum (1 patient), polyalkylimide (1 patient), and an unknown agent (1 patient). Two of the 6 patients initially denied having undergone dermal filling. The time from infiltration to adverse reaction ranged from 2 to 15 years. The most frequent clinical presentations were recurrent pseudocellulitis (4 patients), filler migration (3 patients), nodule formation (2 patients), and knee pain (1 patient). Two of the patients had undergone computed axial tomography (CAT), which revealed signs of myositis and cellulitis (patients 2 and 5), but not the presence of filling agent. High frequency ultrasound (HIFU) revealed findings compatible with adverse reaction to filler in all cases. In 1 patient who was unaware of the type of filler used, HIFU revealed a snowstorm-like pattern characteristic of liquid silicone (Fig. 1). In another individual who reported having received hyaluronic acid infiltrations, HIFU revealed characteristics compatible with petrolatum (anechoic pseudocysts) (Fig. 2). In 1 case, treatment consisted of removal of the filler. Three patients were treated with oral corticosteroids, 2 with minocycline, and 2 with nonsteroidal anti-inflammatory drugs. One patient was lost to follow-up. The clinical response was variable (Table 1).
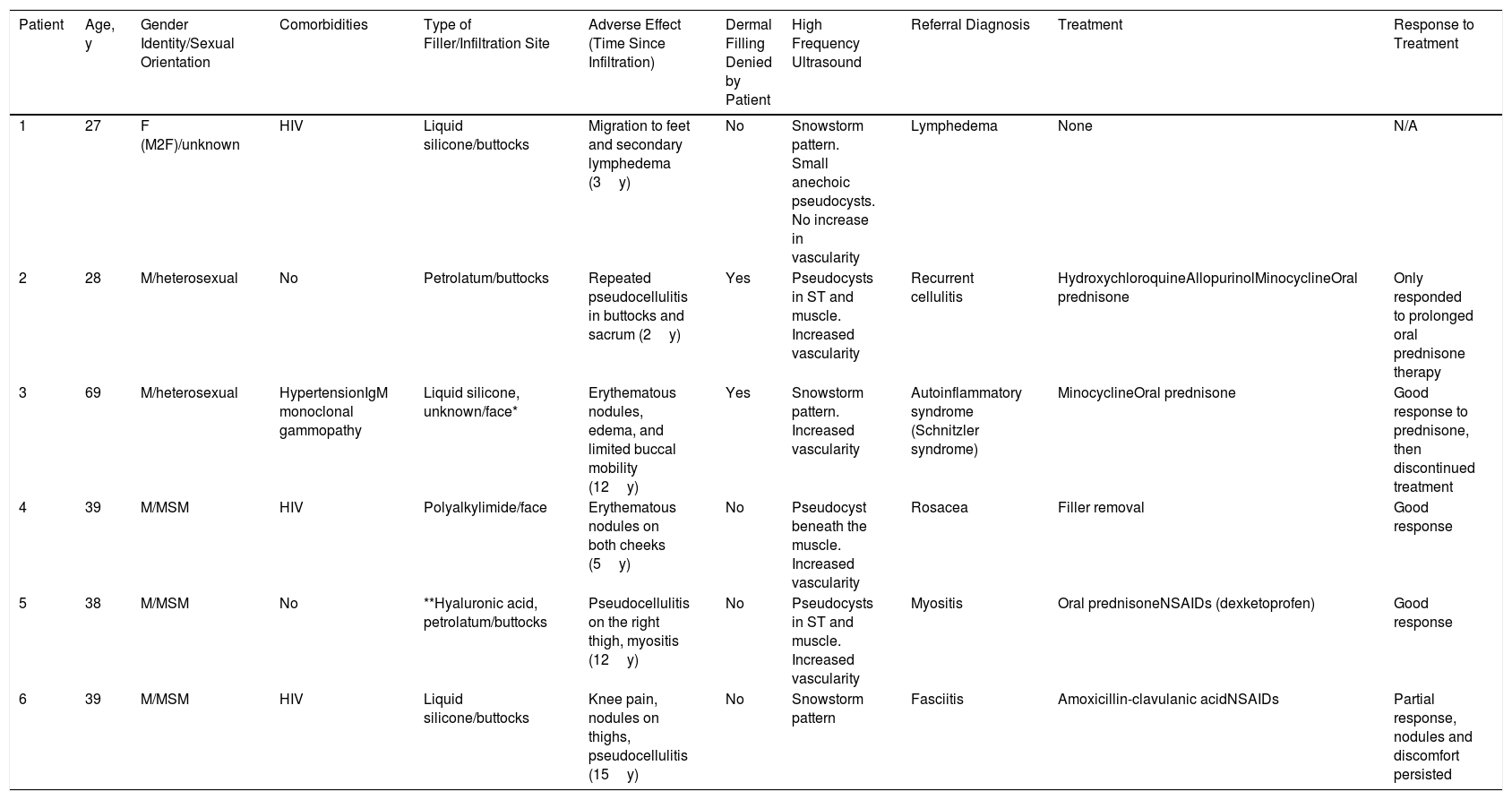
Epidemiological and Clinical Characteristics, Additional Tests, and Treatments of Men with Adverse Reactions to Fillers
| Patient | Age, y | Gender Identity/Sexual Orientation | Comorbidities | Type of Filler/Infiltration Site | Adverse Effect (Time Since Infiltration) | Dermal Filling Denied by Patient | High Frequency Ultrasound | Referral Diagnosis | Treatment | Response to Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | F (M2F)/unknown | HIV | Liquid silicone/buttocks | Migration to feet and secondary lymphedema (3y) | No | Snowstorm pattern. Small anechoic pseudocysts. No increase in vascularity | Lymphedema | None | N/A |
| 2 | 28 | M/heterosexual | No | Petrolatum/buttocks | Repeated pseudocellulitis in buttocks and sacrum (2y) | Yes | Pseudocysts in ST and muscle. Increased vascularity | Recurrent cellulitis | HydroxychloroquineAllopurinolMinocyclineOral prednisone | Only responded to prolonged oral prednisone therapy |
| 3 | 69 | M/heterosexual | HypertensionIgM monoclonal gammopathy | Liquid silicone, unknown/face* | Erythematous nodules, edema, and limited buccal mobility (12y) | Yes | Snowstorm pattern. Increased vascularity | Autoinflammatory syndrome (Schnitzler syndrome) | MinocyclineOral prednisone | Good response to prednisone, then discontinued treatment |
| 4 | 39 | M/MSM | HIV | Polyalkylimide/face | Erythematous nodules on both cheeks (5y) | No | Pseudocyst beneath the muscle. Increased vascularity | Rosacea | Filler removal | Good response |
| 5 | 38 | M/MSM | No | **Hyaluronic acid, petrolatum/buttocks | Pseudocellulitis on the right thigh, myositis (12y) | No | Pseudocysts in ST and muscle. Increased vascularity | Myositis | Oral prednisoneNSAIDs (dexketoprofen) | Good response |
| 6 | 39 | M/MSM | HIV | Liquid silicone/buttocks | Knee pain, nodules on thighs, pseudocellulitis (15y) | No | Snowstorm pattern | Fasciitis | Amoxicillin-clavulanic acidNSAIDs | Partial response, nodules and discomfort persisted |
Abbreviations: HIV, human immunodeficiency virus; F, female; IgM, immunoglobulin M; M, man; M2F, male-to-female transition; MSM, men who have sex with men; N/A, not applicable; NSAIDs, nonsteroidal anti-inflammatory drugs; ST, subcutaneous tissue.
Adverse reactions to fillers can cause severe morbidity with foreign body granulomatous reactions, pseudocellulitis, skin necrosis, embolization and migration of filler material, and even death.1,3 In our series, the most common complications were pseudocellulitis and filler migration.
It should be noted that all patients were referred from other services and in no case was adverse reaction to filler suspected, likely due to the great variability in clinical presentation, the long latency to symptom onset (up to 15 years in our series), a reluctance to admit having undergone the procedure (or failure to recall the procedure in cases in which many years had elapsed), and a lack of suspicion of this condition in men. The misdiagnosis of recurrent cellulitis or myositis led in several cases to costly imaging tests and multiple cycles of broad-spectrum antibiotic therapy, with potential associated adverse effects.
Some authors have suggested that the use of unauthorized fillers such as liquid silicone and petrolatum is increasing among transsexuals and bodybuilders.3,4 In our series, most of the fillers used corresponded to this group (silicone and petrolatum in 3 and 2 cases, respectively). However, only 1 of the individuals was transsexual, none were bodybuilders, and 2 were heterosexual, underscoring the need for a high index of clinical suspicion when evaluating men with inflammatory dermatoses, especially on the face, buttocks, and thighs.
HIFU is a fast, inexpensive, and safe test. It is useful in the diagnosis of adverse reaction to filler to assess the magnitude of the inflammatory reaction, identify the type of filling agent used, monitor the response to treatment, and guide procedures.5–7 In our series, HIFU revealed the presence of fillers in all cases, even in 2 patients in whom CAT failed to do so.
The only curative treatment for adverse reaction to filler is extraction of the filling agent,4 which can be difficult; this was achieved in only 1 patient in our series. Variable responses have been described in patients treated with oral and intralesional corticosteroid therapy, tacrolimus, allopurinol, etanercept, and antibiotics such as clarithromycin and tetracyclines.1,3 Most patients in our series received oral corticosteroids, and 1 patient (patient 2) required low doses of prednisone for more than 12 months.
Adverse reaction to filler in men can occur more than a decade after the procedure, can have varied clinical presentations, and is not limited to MSM or transsexuals. It should be suspected in individuals with inflammatory conditions affecting the face, thighs, or buttocks, even in those who deny having undergone dermal filling.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Morgado-Carrasco D, Bosch-Amate X, Fustà-Novell X, Giavedoni P. Complicaciones de rellenos (fillers) en hombres. Estudio retrospectivo de las características epidemiológicas y clínicas. Utilidad de la ecografía en el diagnóstico. Actas Dermosifiliogr. 2020;111:429–432.