Psoriasis is a chronic inflammatory skin disease with an estimated prevalence in Spain of 2.3% of the population. Approximately 30% of patients have moderate-to-severe forms. Treatment with biologic agents is proving to be a step forward in the management of the disease, although these treatments are very expensive. The objective of this study was to determine the efficiency, in terms of cost per number needed to treat (NNT), of the biologic drugs available in Spain for the treatment of moderate to severe plaque psoriasis.
MethodsNNT data were obtained from a network meta-analysis that included all randomized clinical trials of biologic drugs sold in Spain. The cost of each treatment was calculated based on the approved dosage for the first year of treatment, as indicated in the Summary of Product Characteristics. These data were used to calculate the cost per NNT of the drugs for various PASI scores (75, 90, and 100). A sensitivity analysis was performed taking into consideration only the PASI-response measurement time (after 10, 12, or 16 weeks, depending on the drug).
ResultsThe order of efficiency, from most to least efficient, in the case of a PASI 75 response was ixekizumab > ustekinumab 45mg > ustekinumab 90mg > secukinumab > infliximab > etanercept > adalimumab. The order for PASI 90 was ixekizumab >secukinumab >ustekinumab 45mg > ustekinumab 90mg > infliximab > adalimumab > etanercept. The order for PASI 100 was ixekizumab > secukinumab > infliximab > ustekinumab 90mg > ustekinumab 45mg > adalimumab > etanercept. The sensitivity analysis showed some changes in the order, depending on the response-assessment period.
ConclusionsThe findings show a link between the efficacy of the biologic therapies available in Spain for the treatment of moderate-to-severe plaque psoriasis and their efficiency. Ixekizumab had the lowest cost per NNT for all PASI-response scores (75, 90, and 100) during the first year of treatment.
La psoriasis es una enfermedad inflamatoria crónica de la piel cuya prevalencia en España se estima en el 2,3% de la población y en la que alrededor del 30% de los pacientes tienen formas moderadas a graves. El tratamiento con agentes biológicos está suponiendo un avance en el manejo de la enfermedad, aunque también representa un reto económico. El objetivo de este estudio es determinar la eficiencia, en términos de coste por número necesario a tratar (NNT), de las terapias biológicas disponibles en España para el tratamiento de la psoriasis en placas moderada a grave.
MétodosLos datos de NNT se obtuvieron de un metaanálisis en red que incluía todos los ensayos clínicos aleatorizados con medicamentos biológicos comercializados en España. Los costes de cada terapia se calcularon según las posologías aprobadas en las fichas técnicas para el primer año de tratamiento. A partir de estos datos se calculó el coste por NNT de los fármacos para los distintos niveles de PASI (75, 90 y 100). Se realizó un análisis de sensibilidad considerando solamente el periodo de medición de la respuesta PASI (de 10 a 16 semanas) según el tratamiento.
ResultadosPara la respuesta PASI 75, el orden de terapias de mayor a menor eficiencia es ixekizumab > ustekinumab 45mg > ustekinumab 90mg > secukinumab > infliximab > etanercept > adalimumab. Para la respuesta PASI 90, el orden es ixekizumab > secukinumab > ustekinumab 45mg > ustekinumab 90mg > infliximab > adalimumab > etanercept. Para la respuesta PASI 100 el orden es ixekizumab > secukinumab > infliximab > ustekinumab 90mg > ustekinumab 45mg > adalimumab > etanercept. El análisis de sensibilidad mostró algún cambio en el orden de las secuencias para el periodo de evaluación de la respuesta.
ConclusionesEste análisis muestra una relación entre la eficacia de los tratamientos biológicos disponibles en España para el tratamiento de la psoriasis en placas moderada a grave y su eficiencia, siendo ixekizumab el que mostró un menor coste por NNT en todos los niveles de respuesta PASI alcanzados (75, 90 y 100) para el primer año de tratamiento.
Psoriasis is a chronic inflammatory skin disease. Its most common manifestation is the appearance of erythematous plaques on the skin. The condition has negative repercussions on the patients’ physical and emotional wellbeing and quality of life.1 Dermatologists generally assess the severity of psoriasis by measuring the percentage of total body surface area (BSA) affected or by using the Psoriasis Area Severity Index (PASI). It is generally accepted that patients with a greater than 10% affected BSA or a PASI score above 10 have moderate to severe psoriasis.2
In Spain, the prevalence of psoriasis is estimated to be 2.3% of the general population, evenly distributed between the sexes.3 Approximately 30% of these patients have moderate to severe forms of the disease.4 Moderate to severe psoriasis is currently managed with systemic therapies, both conventional and biologic. Clinical response and adverse effects are more variable with conventional systemic treatments and such therapy may not be acceptable to patients who demand more effective solutions. The advent of biologic therapies has brought about a qualitative leap in the management of psoriasis, marked by an improvement in response to treatment. The biologic agents with regulatory approval in Europe can be divided into 4 groups according to the mechanism of action in each case: tumor necrosis factor (TNF-α) inhibitors (etanercept, adalimumab, infliximab, and certolizumab pegol); interleukin (IL) 12/23 inhibitors (ustekinumab); IL-17 inhibitors (secukinumab, ixekizumab, and brodalumab); and IL-23 inhibitors (guselkumab and tildrakizumab). The last two groups are the most recent. At the time of writing, certolizumab pegol, brodalumab, guselkumab, and tildrakizumab have not obtained marketing approval in Spain. The increase in the number of biologic drugs has led to an increase in the use of these therapies. This, together with the need to contain health expenditure, has made it essential to consider the efficiency of these treatments.5
Data from randomized clinical trials that directly compare 2 or more alternatives is the highest quality of evidence for deciding on the best possible treatment option. When no direct comparisons are available, however, statistical methods, such as network meta-analysis (NMA) can prove very useful. The NMA technique allows us to synthesize the evidence available in studies in the literature (obtained from a systematic review) and use it to indirectly estimate the comparative efficacy of the different treatment options.6
One of the efficacy measures used to do this is the number of patients that must be treated to achieve a specific therapeutic objective. The number needed to treat (NNT) is the number of patients that must receive treatment with a specific therapy to achieve or avoid a result or clinical event, compared to the number needed to achieve the same result with an alternative treatment option or placebo. This statistical parameter is a measure of absolute risk developed in the context of evidence-based medicine as a useful tool for making clinical decisions. The range of values for NNT is from 1 to infinity. The ideal value is 1, since this indicates that every patient treated achieves the predefined clinical benefit; after that, the higher the NNT, the less effective the intervention.7
The advantage of NNT is that it provides clinicians who are taking therapeutic decisions with a more understandable concept of the effectiveness of a treatment.8 Its use is increasing in dermatology, and particularly psoriasis, because very similar efficacy variables are used in the different clinical trials and the populations studied are very homogeneous.7
The objective of this study was to determine the efficiency, in terms of cost per NNT stratified by PASI response levels, of the biologic therapies available in Spain for the treatment of moderate to severe plaque psoriasis.
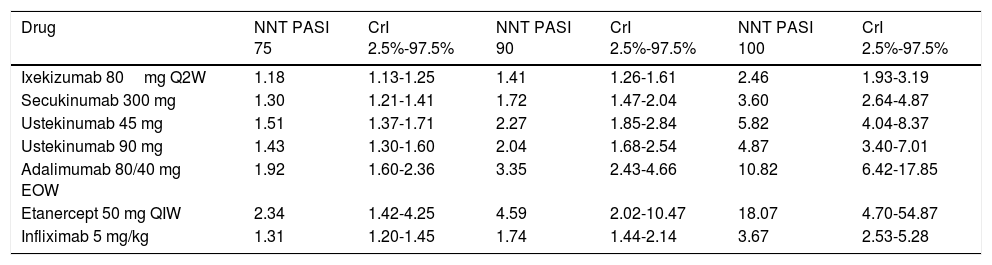
MethodsDetails of the methodology used for the original NMA and the systematic literature review have been published previously.9,10 This NMA provides indirect comparisons for all the biologic treatments currently used in the management of moderate to severe plaque psoriasis in Spain. The literature review included all the studies published in English between January 1990 and November 2015 and all the Phase II, III, and IV randomized clinical trials carried out with each biologic agent. The primary endpoint evaluated in the NMA was the PASI 75, 90, and 100 response (the number of patients with a 75%, 90% or 100% reduction in their baseline PASI score) at the end of the first 12 weeks for most biologic agents, at 10 weeks in the case of infliximab, and at 16 weeks for adalimumab. In this analysis, the NNT to achieve PASI 75, 90 and 100 responses was calculated as the difference in response between the biologic agent and placebo at the end of the interval considered. NNT was calculated as the inverse of the probability of response to biologic treatment minus the probability of response to placebo (Table 1).
NNT per PASI Response Level With Placebo as the Comparator (CrI 2.5-97.5%).
| Drug | NNT PASI 75 | CrI 2.5%-97.5% | NNT PASI 90 | CrI 2.5%-97.5% | NNT PASI 100 | CrI 2.5%-97.5% |
|---|---|---|---|---|---|---|
| Ixekizumab 80mg Q2W | 1.18 | 1.13-1.25 | 1.41 | 1.26-1.61 | 2.46 | 1.93-3.19 |
| Secukinumab 300 mg | 1.30 | 1.21-1.41 | 1.72 | 1.47-2.04 | 3.60 | 2.64-4.87 |
| Ustekinumab 45 mg | 1.51 | 1.37-1.71 | 2.27 | 1.85-2.84 | 5.82 | 4.04-8.37 |
| Ustekinumab 90 mg | 1.43 | 1.30-1.60 | 2.04 | 1.68-2.54 | 4.87 | 3.40-7.01 |
| Adalimumab 80/40 mg EOW | 1.92 | 1.60-2.36 | 3.35 | 2.43-4.66 | 10.82 | 6.42-17.85 |
| Etanercept 50 mg QIW | 2.34 | 1.42-4.25 | 4.59 | 2.02-10.47 | 18.07 | 4.70-54.87 |
| Infliximab 5 mg/kg | 1.31 | 1.20-1.45 | 1.74 | 1.44-2.14 | 3.67 | 2.53-5.28 |
Abbreviations: EOW, every other week; CrI, credible interval; NNT, number needed to treat; PASI, Psoriasis Area and Severity Index; QIW, weekly; Q2W, every 2 weeks.
NNT=1/(probability of response with biologic treatment – probability of response with placebo)
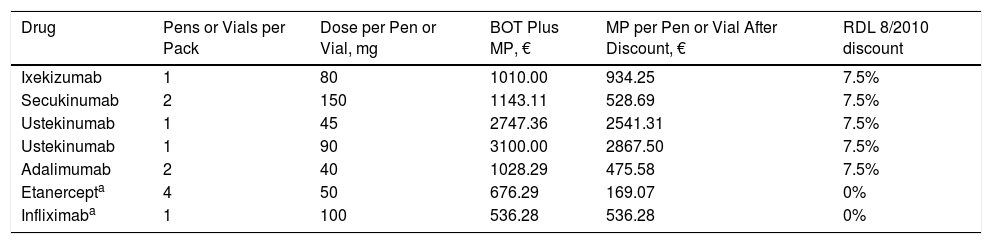
The cost in Spain of the biologic agents studied was based on the manufacturer's price (MP) for each pharmaceutical formulation as reported in the Bot Plus 2.0 database published by the General Council of Official Spanish Pharmacists Associations (CGCOF)11 applying the corresponding discount for each drug in accordance with RDL 8/201012 (Table 2).
Official Manufacturer's Price (MP) for the Biologic Agents, Showing Applicable Discounts, Number of Pens or Vials per Pack, and Dose per Pen or Vial.
| Drug | Pens or Vials per Pack | Dose per Pen or Vial, mg | BOT Plus MP, € | MP per Pen or Vial After Discount, € | RDL 8/2010 discount |
|---|---|---|---|---|---|
| Ixekizumab | 1 | 80 | 1010.00 | 934.25 | 7.5% |
| Secukinumab | 2 | 150 | 1143.11 | 528.69 | 7.5% |
| Ustekinumab | 1 | 45 | 2747.36 | 2541.31 | 7.5% |
| Ustekinumab | 1 | 90 | 3100.00 | 2867.50 | 7.5% |
| Adalimumab | 2 | 40 | 1028.29 | 475.58 | 7.5% |
| Etanercepta | 4 | 50 | 676.29 | 169.07 | 0% |
| Infliximaba | 1 | 100 | 536.28 | 536.28 | 0% |
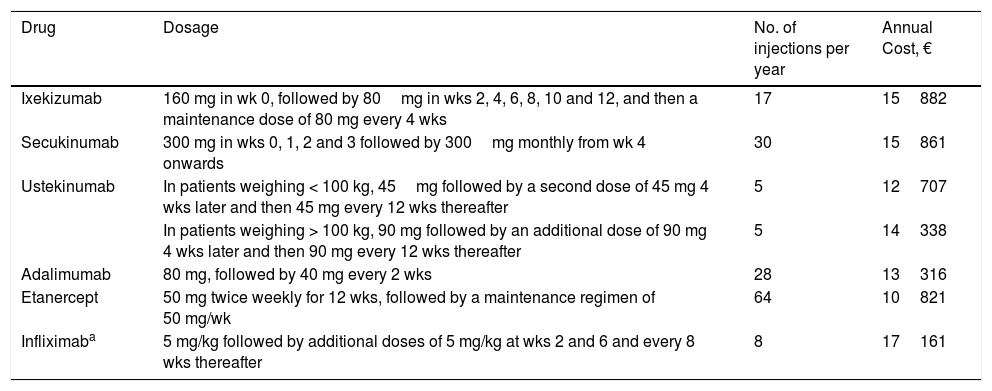
The resulting value was multiplied by the number of doses required for the first year of treatment as per the Summary of Product Characteristics for each drug,13–18 adjusted for the calculations at 52 weeks (Table 3). The cost of infliximab was calculated for an average patient weight of 80kg, since the dosage of this drug is dependent on the patient's weight.19
Dosage, Number of Injections, and Cost per Patient for the First Year of Treatment.
| Drug | Dosage | No. of injections per year | Annual Cost, € |
|---|---|---|---|
| Ixekizumab | 160 mg in wk 0, followed by 80mg in wks 2, 4, 6, 8, 10 and 12, and then a maintenance dose of 80 mg every 4 wks | 17 | 15882 |
| Secukinumab | 300 mg in wks 0, 1, 2 and 3 followed by 300mg monthly from wk 4 onwards | 30 | 15861 |
| Ustekinumab | In patients weighing < 100 kg, 45mg followed by a second dose of 45 mg 4 wks later and then 45 mg every 12 wks thereafter | 5 | 12707 |
| In patients weighing > 100 kg, 90 mg followed by an additional dose of 90 mg 4 wks later and then 90 mg every 12 wks thereafter | 5 | 14338 | |
| Adalimumab | 80 mg, followed by 40 mg every 2 wks | 28 | 13316 |
| Etanercept | 50 mg twice weekly for 12 wks, followed by a maintenance regimen of 50 mg/wk | 64 | 10821 |
| Infliximaba | 5 mg/kg followed by additional doses of 5 mg/kg at wks 2 and 6 and every 8 wks thereafter | 8 | 17161 |
Mean patient weight: 80kg.
The cost per NNT for each agent was obtained by multiplying the cost of the drug in the first year of treatment by the NNT for each of the levels of PASI response considered.
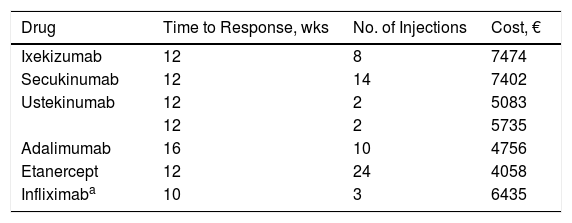
We also performed a sensitivity analysis that took into account only the number of injections during the response assessment period (from 10 to 16 weeks depending on the drug). The number of injections and the cost of treatment during this period, are shown in Table 4.
Data on Actual Response Used in Sensitivity Analysis, With Number of Injections During the Assessment Period and Cost per Patient.
| Drug | Time to Response, wks | No. of Injections | Cost, € |
|---|---|---|---|
| Ixekizumab | 12 | 8 | 7474 |
| Secukinumab | 12 | 14 | 7402 |
| Ustekinumab | 12 | 2 | 5083 |
| 12 | 2 | 5735 | |
| Adalimumab | 16 | 10 | 4756 |
| Etanercept | 12 | 24 | 4058 |
| Infliximaba | 10 | 3 | 6435 |
Mean patient weight: 80kg.
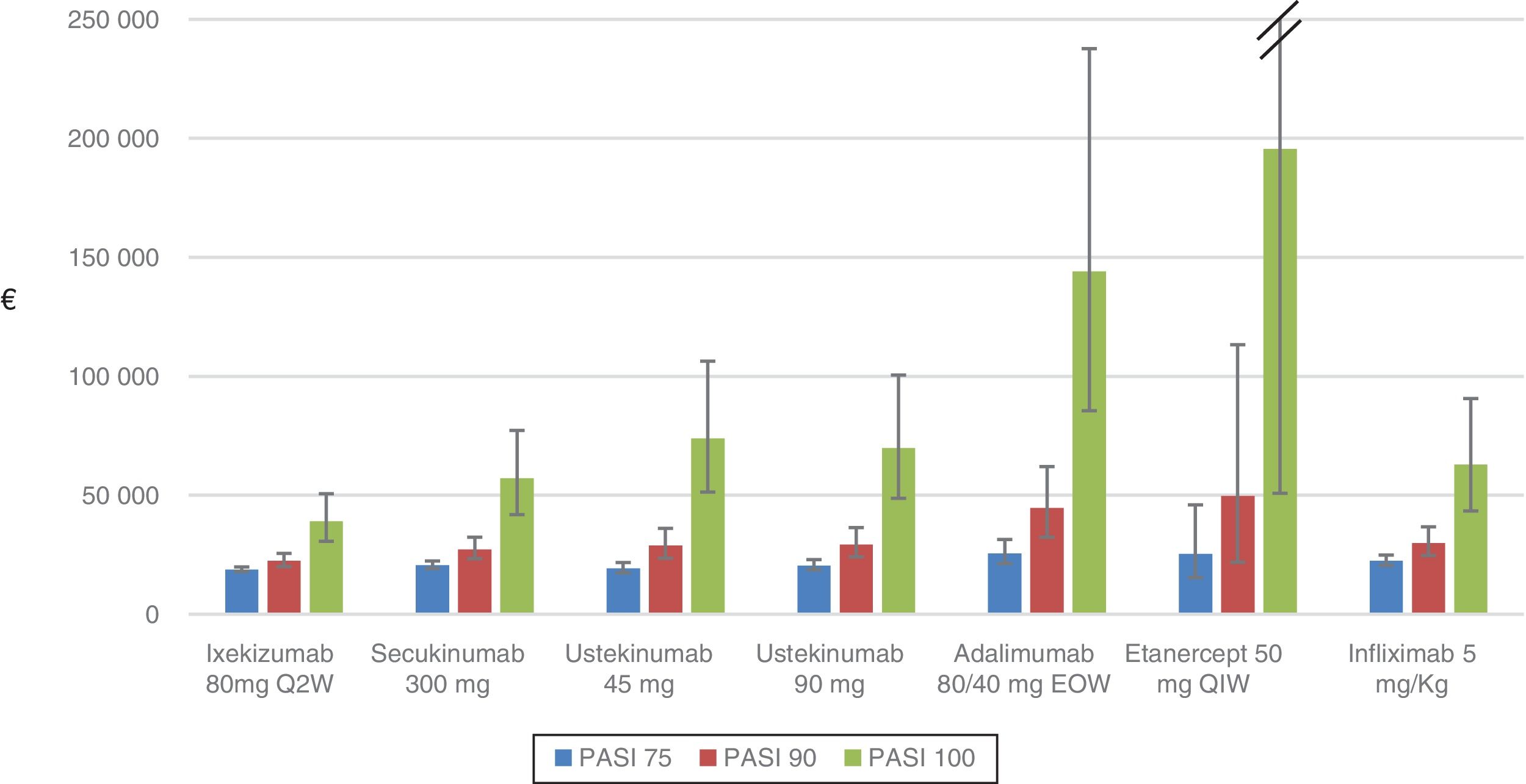
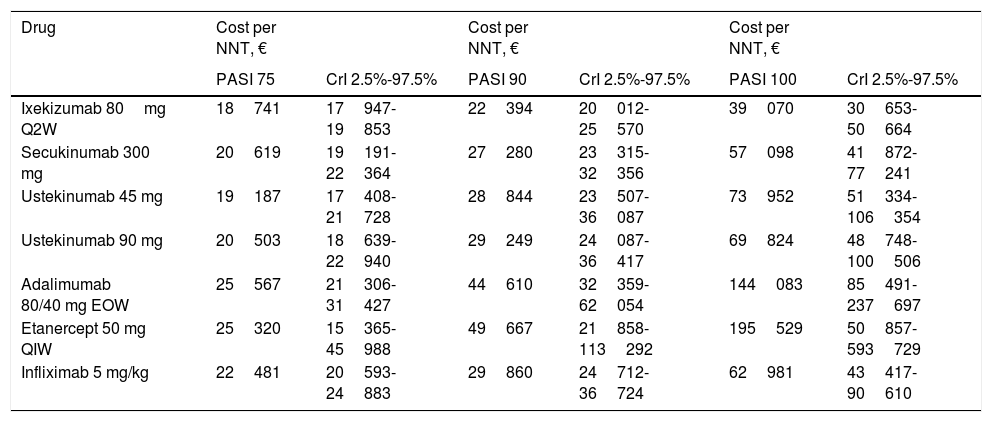
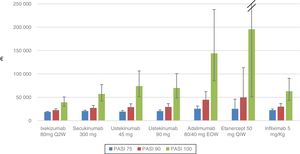
Table 5 and Figure 1 show the cost per NNT to achieve a PASI 75, 90, or 100 response during the first year of treatment for each of the biologic therapies available in Spain that are used to treat moderate to severe plaque psoriasis.
Cost per NNT in the First Year of Treatment.
| Drug | Cost per NNT, € | Cost per NNT, € | Cost per NNT, € | |||
|---|---|---|---|---|---|---|
| PASI 75 | CrI 2.5%-97.5% | PASI 90 | CrI 2.5%-97.5% | PASI 100 | CrI 2.5%-97.5% | |
| Ixekizumab 80mg Q2W | 18741 | 17947-19853 | 22394 | 20012-25570 | 39070 | 30653-50664 |
| Secukinumab 300 mg | 20619 | 19191-22364 | 27280 | 23315-32356 | 57098 | 41872-77241 |
| Ustekinumab 45 mg | 19187 | 17408-21728 | 28844 | 23507-36087 | 73952 | 51334-106354 |
| Ustekinumab 90 mg | 20503 | 18639-22940 | 29249 | 24087-36417 | 69824 | 48748-100506 |
| Adalimumab 80/40 mg EOW | 25567 | 21306-31427 | 44610 | 32359-62054 | 144083 | 85491-237697 |
| Etanercept 50 mg QIW | 25320 | 15365-45988 | 49667 | 21858-113292 | 195529 | 50857-593729 |
| Infliximab 5 mg/kg | 22481 | 20593-24883 | 29860 | 24712-36724 | 62981 | 43417-90610 |
Abbreviations: EOW, every other week; CrI, credible interval; NNT, number needed to treat; PASI, Psoriasis Area and Severity Index; QIW, weekly; Q2W, every 2 weeks.
The cost per NNT of all the biologic therapies increased with each increment in PASI response (75/90/100), taking into account the first year of treatment.
For the PASI 75 response, the order from lowest to highest cost per NNT was as follows: ixekizumab (€18741) <ustekinumab 45mg (€19187) <ustekinumab 90mg (€20503) <secukinumab (€20619) <infliximab (€22481) <etanercept (€25320) <adalimumab (€25567).
For the PASI 90 response, the order from lowest to highest cost per NNT was as follows: ixekizumab (€22394) <secukinumab (€ 27280) <ustekinumab 45mg (€ 28844) <ustekinumab 90mg (€ 29249) <infliximab (€29860)) <adalimumab (€ 44610) <etanercept (€49667).
For the PASI 100 response, the order from lowest to highest cost per NNT was as follows: ixekizumab (€39070) <secukinumab (€57098) <infliximab (€62981) <ustekinumab 90mg (€69824) <ustekinumab 45mg (€73952) <adalimumab (€144083) <etanercept (€195529).
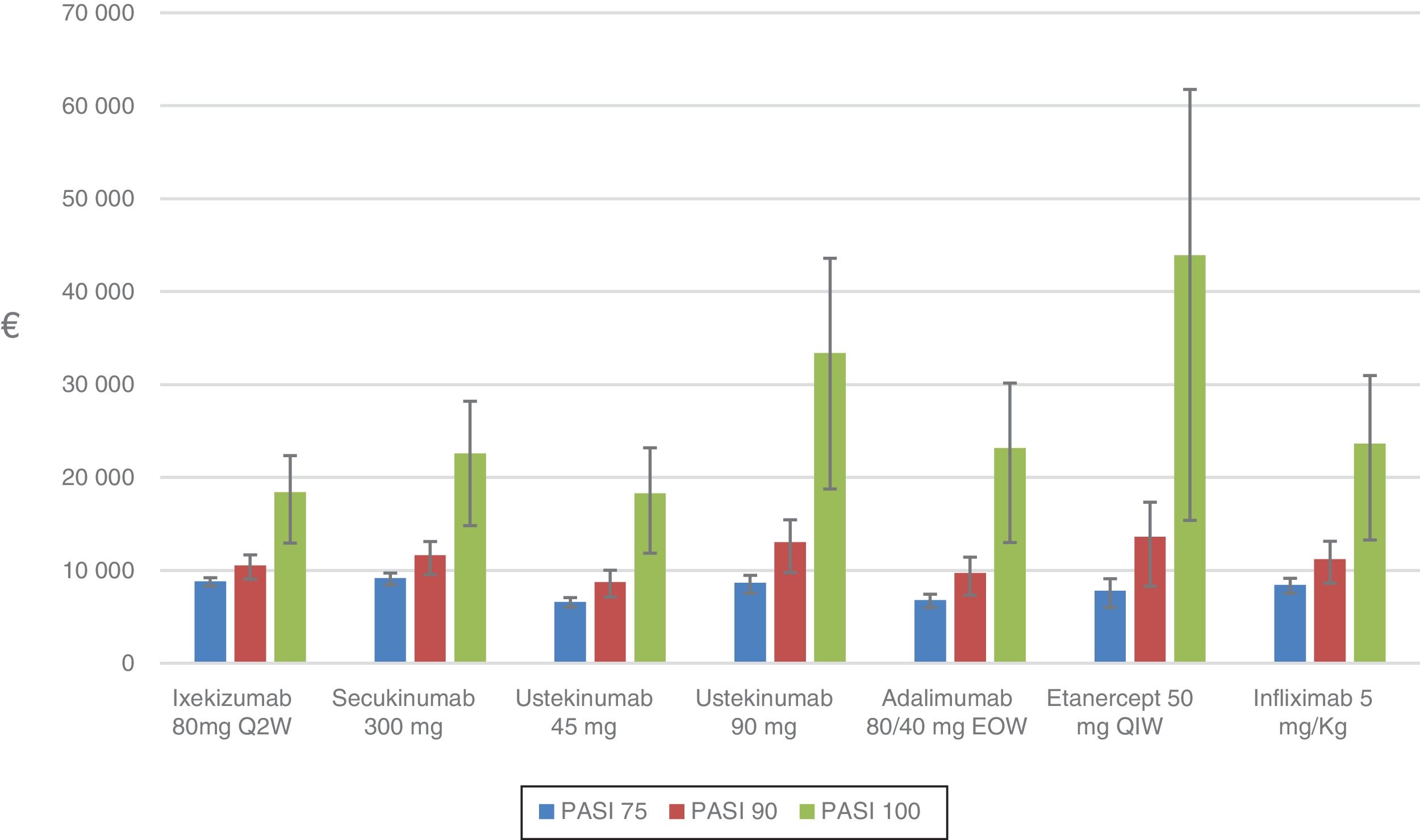
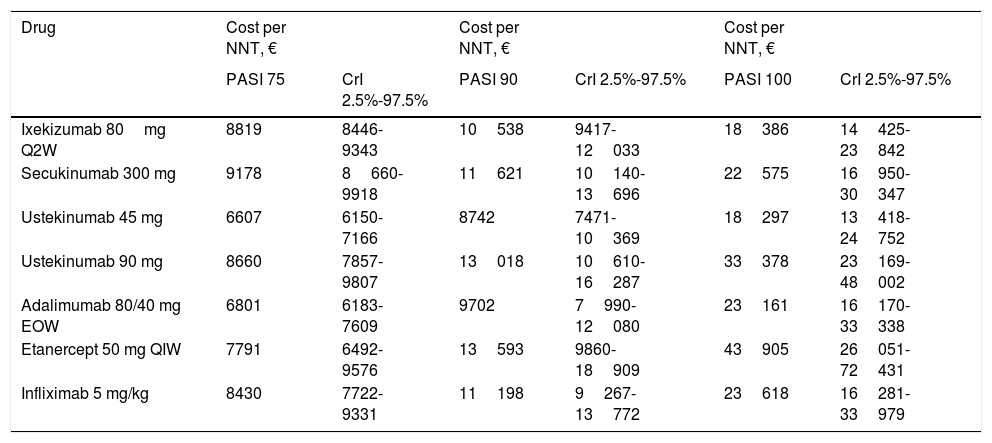
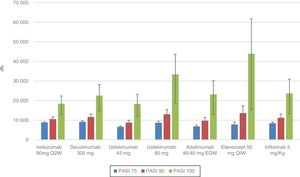
The sensitivity analysis shows the cost per NNT for each drug for the period during which the PASI was actually measured: week 10, 12, or 16 depending on the drug (Table 6, Fig. 2).
Cost per NNT During the Treatment Response Assessment Period.
| Drug | Cost per NNT, € | Cost per NNT, € | Cost per NNT, € | |||
|---|---|---|---|---|---|---|
| PASI 75 | CrI 2.5%-97.5% | PASI 90 | CrI 2.5%-97.5% | PASI 100 | CrI 2.5%-97.5% | |
| Ixekizumab 80mg Q2W | 8819 | 8446-9343 | 10538 | 9417-12033 | 18386 | 14425-23842 |
| Secukinumab 300 mg | 9178 | 8660-9918 | 11621 | 10140-13696 | 22575 | 16950-30347 |
| Ustekinumab 45 mg | 6607 | 6150-7166 | 8742 | 7471-10369 | 18297 | 13418-24752 |
| Ustekinumab 90 mg | 8660 | 7857-9807 | 13018 | 10610-16287 | 33378 | 23169-48002 |
| Adalimumab 80/40 mg EOW | 6801 | 6183-7609 | 9702 | 7990-12080 | 23161 | 16170-33338 |
| Etanercept 50 mg QIW | 7791 | 6492-9576 | 13593 | 9860-18909 | 43905 | 26051-72431 |
| Infliximab 5 mg/kg | 8430 | 7722-9331 | 11198 | 9267-13772 | 23618 | 16281-33979 |
Abbreviations: EOW, Every other week; CrI, credible interval; QIW, weekly; Q2W, every 2 weeks.
For the PASI 75 response, the order from lowest to highest cost per NNT was as follows: ustekinumab 45mg (€6607) <adalimumab (€6801) <etanercept (€7791) <infliximab (€8430) <ustekinumab 90mg (€8663) <ixekizumab (€8819) <secukinumab (€9178).
For the PASI 90 response, the order from lowest to highest cost per NNT was as follows: ustekinumab 45mg (€8742) <adalimumab (€9702) <ixekizumab (€10538) <infliximab (€11198) <secukinumab (€11 621) <ustekinumab 90mg (€13018) <etanercept (€13593)).
For the PASI 100 response, the order from lowest to highest cost per NNT was as follows: ustekinumab 45mg (€18297) <ixekizumab (€18396) <secukinumab (€22575) <adalimumab (€23161) <infliximab (€23618) <ustekinumab 90mg (€33378) <etanercept (€43905).
DiscussionIn recent years, the advent of biologic therapies has led to improved clinical outcomes in patients with moderate to severe plaque psoriasis. However, the increasing expenditure on these drugs makes it necessary to analyze the efficiency of the available treatments. Our findings show that the more effective drugs are also the most efficient. The order from lowest to highest cost per NNT of the biologic agents studied evidences the greater efficiency of the therapies with more recent mechanisms of action, such as the IL-17 and IL-12/23 inhibitors, which obtained higher response levels than TNF-α inhibitors in clinical trials. Moreover, the cost per NNT increases when the treatment objective requires more complete skin clearance (a higher PASI response level). However, given the overlapping credible intervals for most of the biologic agents and PASI response levels studied, these sequences are only indicative and the differences observed are not statistically significant.
This is the first study to compare the cost per NNT of all the biologic treatments currently available in Spain for the treatment of moderate to severe plaque psoriasis. The more recently developed biologic agents (certolizumab pegol, brodalumab, guselkumab, and tildrakizumab) were not included in this study for 2 reasons: first, because the results of clinical trials were not available when the NMA was performed; and, second, because the official price had not been established. However NNT values for these drugs have since been published.20–22 Our findings are consistent with those of earlier studies, which reported very similar NNT values for biologic therapies, likewise obtained through indirect comparison methods, such as NMA,22,23 and similar sequences from lowest to highest cost per NNT.10,19,24–26
While a PASI 75 response has traditionally been considered the treatment objective of reference in this setting,27,28 patients who achieve a PASI 90 response or higher have a score of 0 to 1 on the Dermatology Quality of Life Index (DLQI) in a significantly higher percentage than those whose response is PASI 75. This score of 0 to 1 on the DLQI scale signifies that the disease has a zero or minimal impact on the patient's quality of life.29–31 This improvement in patient quality of life arising from an improved response to treatment, together with the availability of new therapies that can achieve a PASI 90 response in a sizable percentage of patients, has led to a PASI 90 or higher response being considered the most important treatment objective.32–34 It is interesting to note that, for most of the biologic therapies, the cost increase per NNT required to achieve a PASI 90 as compared to a PASI 75 response is less than the cost increase per NNT to achieve a PASI 100 compared to a PASI 90 response. This is a consequence of the skin clearance results achieved by the biologic agents during clinical development: the newer biologics achieved PASI 90 responses in a higher proportion of patients and also achieved higher PASI 100 response rates than those achieved by earlier biologic treatments, such as the TNF-α inhibitors. These differences are reflected in the cost per NNT results reported in this study.
Analysis of cost per NNT differs from complete economic evaluations, such as cost-effectiveness analysis.35 That methodology, endorsed by scientific societies and commonly used to assess health technologies, models outcome and cost variables over a considerable period, taking into account not only the pharmacological cost of therapies but also the consumption of other types of resources involved in the management of the disease, an important consideration when making decisions on how to allocate the health care budget. However, calculating the cost per unit of clinical efficacy (NNT) may be another approach that can provide very comprehensible information for clinicians and others involved in making health care decisions. Cost per NNT is not, however, a useful measure for establishing a threshold to inform decisions on how much should reasonably be invested in achieving a health outcome, unlike cost-effectiveness analysis, which does provide information that can inform such decisions. It may, therefore, be useful to have both methods in order to provide information to inform different types of decisions.
One of the limitations of this study is the lack of data from clinical trials that directly compare the different drugs. This lack of data led us to combine the evidence available and to carry out indirect comparisons by means of an NMA of all the studies on the biologic therapies available in Spain published in the current literature.9,10 Our NMA was based on a systematic literature review of clinical trials published before the end of November 2015 and, given the high level of activity in this area, other studies using a similar methodology (NMA) and including clinical trials after that date have been carried out. However, the findings reported by the later NMA corroborate the results of our study.20,26 The NNT obtained in our NMA were used to calculate the cost of the first year of treatment. In the methodology used, it was assumed that all the drugs studied maintained the efficacy observed in the short term (during the induction period) through to the end of the first year of treatment. This approach provides the best possible approximation of the actual situation as we found no clinical trials with homogeneous designs providing data on the maintenance of response over a longer period to facilitate indirect comparisons (and the calculation of cost per NNT). Basing the analysis on the assumption that the response was maintained over a longer period than the induction phase is not a new approach: the same assumption has been made by the authors of most studies that compare the cost of biologic therapies over longer periods.19,24,25 We also performed a sensitivity analysis including only the data from the period during which the PASI response was assessed in the clinical trials included in the NMA. In that analysis, ustekinumab was the most efficient treatment for all levels of PASI response, both because of the clinical response obtained and the dosage regimen used. The cost per NNT of the new IL-17 inhibitors decreases with each increment in PASI response (75/90/100). Another limitation of this study is the use of the manufacturer's catalogue price (MP) to calculate the cost of treatment. In the current Spanish system, the official MP may be discounted by the vendor to ensure that the treatment is covered by the National Health System (giving rise to a subsidized price). This reduced price more closely reflects the actual cost of the drug. It is, however, extremely difficult to obtain precise data on the actual prices paid. Moreover, the final price of biologic agents in Spain is also subject to additional discounts in hospitals, giving rise to even greater variability. For all these reasons, we opted to base our analysis on the MP. This study provides information on the efficiency, in terms of cost per NNT, of the biologic treatments currently available in Spain for the treatment of moderate to severe plaque psoriasis, stratified by level of treatment response (PASI 75, 90 and 100). The results show that the more effective biologic treatments are also more efficient. Of the therapies studied, ixekizumab was the biologic agent with the lowest cost per NNT and, therefore, the most efficient treatment for moderate to severe plaque psoriasis at all PASI response levels over a 1-year treatment period. Ustekinumab was the most efficient treatment when only the response assessment period was taken into account (10 to 16 weeks depending on the drug).
FundingThis study was funded by Eli Lilly and Company.
Conflicts of InterestP. de la Cueva has participated in clinical trials or received consultancy or lecture fees from Abbvie, Almirall, Biogen, Boehringer-Ingelhaim, Celgene, Gebro, Janssen, Leo Pharma, MSD, Novartis, Pfizer, Lilly, Sanofi, and UDC Pharma.
M. Nuñez, T. Huete, JA Sacristán, S. Hartz and T. Dilla are employees of Eli Lilly and Company.
Please cite this article as: Núñez M, Huete T, de la Cueva P, Sacristán JA, Hartz S, Dilla T. Evaluación de la eficiencia de los tratamientos biológicos en la psoriasis moderada a grave en España: análisis de coste por número necesario a tratar (NNT). Actas Dermosifiliogr. 2019;110:546–553.