Psoriasis and hidradenitis suppurativa are often associated with obesity. Because chronic low-grade inflammation underlies these 2 diseases, they can progress to more severe forms in patients with obesity if weight-reduction measures are not taken. This review covers pharmacologic alternatives for treating obesity, with emphasis on the benefits associated with the novel use of glucagon-like peptide-1 (GLP-1) agonists that act on satiety receptors. These drugs have led to greater weight loss in clinical trials and real-world settings than orlistat, which until recently was the only drug approved for treating obesity in the European Union. Although experience with GLP-1 agonists in patients with obesity and inflammatory skin diseases is currently scarce, the promising results reported suggest they may offer a useful tool for managing obesity.
La psoriasis (PsO) y la hidradenitis supurativa (HS) se asocian frecuentemente con la obesidad. La inflamación crónica de bajo grado subyace a estas condiciones, por lo que si no se adoptan medidas para reducir el peso del paciente con obesidad y PsO o HS, estas podrían evolucionar hacia formas más graves. Este trabajo revisa las opciones farmacológicas para tratar la obesidad, profundizando en los beneficios asociados al uso novedoso de agonistas del receptor de GLP-1 (arGLP-1), que actúan sobre los centros de la saciedad. Los resultados de ensayos y vida real demuestran que esta medicación consigue mayores pérdidas de peso que orlistat, hasta recientemente el único fármaco específico para la obesidad comercializado en la Unión Europea. Aunque la experiencia con arGLP-1 en pacientes con obesidad y dermatosis inflamatorias es escasa, los resultados son alentadores, por lo que podrían constituir una herramienta útil para el manejo de su obesidad.
Obesity is a common comorbidity in chronic inflammatory conditions such as psoriasis (PsO) and hidradenitis suppurativa (HS), in which the common underlying pathogenic mechanisms are closely related to those found in obesity. It will be interesting to see whether the new and effective therapeutic alternatives under development for obesity enable a more effective decrease in body weight, which, in turn, could be beneficial for the outcome of immune-mediated dermatoses.
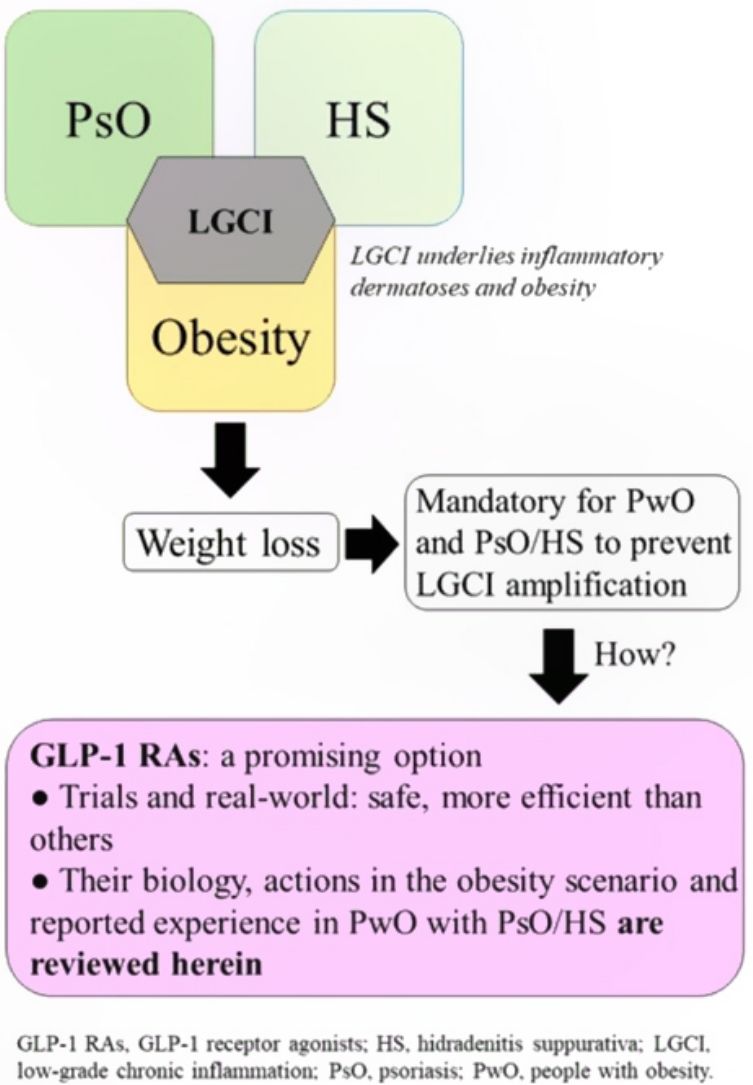
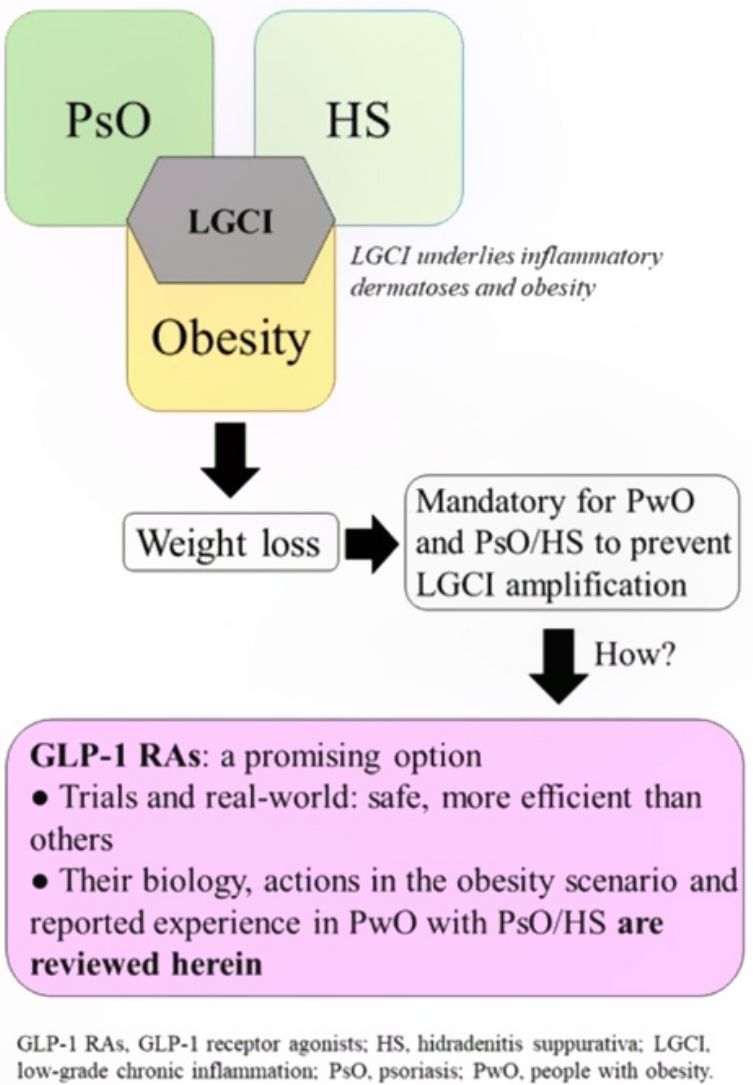
Low-grade chronic inflammation and immunological imbalance facilitate a bidirectional association between obesity and immune-mediated dermatosesAn excess of dysfunctional adiposity contributes to the inflammatory state that characterizes patients with obesity.1–4 As low-grade inflammation underlies obesity, PsO, and HS (Fig. S1 of additional material), any intervention that dampens the mechanisms that lead to this state will be positive for any of the 3 conditions.5,6 The mechanisms that underlie the bidirectional association between obesity and immune-mediated diseases7 have not been clearly established, although it is accepted that the skin plays a causative role in chronic inflammation and immune disorders (Fig. S2 additional material).7,8
The incidence of PsO and HS has increased in western countries in recent decades.9,10 Obesity has increased in parallel, and could be responsible, at least partially, for the aforementioned increase.8
Psoriasis and obesityThe prevalence of obesity is higher among patients with PsO than in the general population.11–18 In addition, obesity increases the risk of PsO,6,19,20 the incidence of which could be twice as great in patients with grade II/III obesity compared with subjects with normal weight.21 Among the molecular mediators at the intersection between the 2 conditions are 3 adipokines—leptin, resistin, and adiponectin—whose levels impact the severity of PsO in patients with obesity.22–24
Many complications associated with obesity are also associated with PsO. There is a higher prevalence of type 2 diabetes mellitus (DT2) in patients with PsO than in the general population.25 In addition, insulin resistance induces dyslipidemia and contributes to the development of nonalcoholic hepatic steatosis (NAHS).26 Cardiovascular risk (CVR) is increased in patients with obesity and PsO who present psoriatic arthritis, and who often have other risk factors.27 The guidelines for the European Cardiology Society state that the body mass index (BMI) should not exceed 25kg/m2,28 and so early interventions to manage obesity or excess weight associated with PsO could contribute to preventing CVR. Finally, obesity is associated with worse response to drugs for PsO (Table S1 additional material).29,30
Hidradenitis suppurativa and obesityThere is a higher prevalence of obesity in subjects with HS than in the general population,31,32 and HS is detected more frequently in children with obesity.33 The association between HS and obesity, as is the case for PsO, is also bidirectional, with low-grade inflammation as the unifying thread. Inflammatory imbalance in obesity perpetuates inflammation and follicular occlusion. Multiple molecular mechanisms, activated by inflammatory mediators, contribute to the progression of HS.34,35
The prevalence of DT2 is higher in subjects with HS than in the general population.36 HS is also associated with other conditions traditionally linked with obesity, such as metabolic syndrome, polycystic ovarian syndrome, and inflammatory bowel disease,37–39 and patients with HS are exposed to higher CVR than the general population.40 Obesity negatively impacts the response of patients to the main treatments for HS (Table S1 additional information).15
Advantages associated with weight loss in patients with obesity and psoriasis or hidradenitis suppurativaIn addition to better response to treatments for inflammatory dermatoses, other arguments justify the desirability of patients with obesity and PsO or HS to lose weight.
- •
Weight loss is associated with a decrease in PsO severity and improved quality of life (QoL).41,42
- •
Weight loss greater than 5% is associated with a higher rate of minimum disease activity in patients who are obese or overweight and in treatment for PsO.43,44
- •
The Mediterranean diet, which facilitates weight loss, reduces psoriatic lesions.45
- •
Weight loss in patients with HS leads to a notable decrease in the number of lesions and, in some cases, disease remission.46
- •
Dysfunctional adiposity has been associated with worse indices of CVR and Dermatology Quality of Life Index (DLQI) in patients with HS.47
- •
The decrease in fatty tissue is associated with a decrease in molecular mechanisms that characterize the systemic inflammatory state.48
Diet and exercise are the first therapeutic step but these interventions are, however, often insufficient to achieve favorable outcomes in skin diseases, good response to treatment for these conditions, and a decrease in CVR. Another limitation is the poor adherence and high drop-out rates.49 Therefore, pharmacological options should also be explored. The limited number of treatments able to significantly reduce weight, as well as the risk of renewed weight gain from 6 months after the end of intervention, have been an obstacle for considering pharmacological treatment for obesity in patients with PsO or HS.15
Approved and marketed drugs in SpainOrlistatOrlistat is an inhibitor of the gastric and pancreatic lipases that block hydrolysis of triacyl glycerides and absorption of fatty acids through the intestinal endothelium. The drug is often associated with gastrointestinal adverse events, thereby hindering adherence. Its effect is modest, with weight losses of not more than 3% after 12 months, after which time, the weight is partially regained, even with continuing therapy.50 There are no studies that have analyzed the effect of orlistat in patients with obesity and PsO or HS.
GLP-1 receptor agonistsGLP-1 receptor agonists have recently made a mark as a possible pharmacological alternative for weight loss.
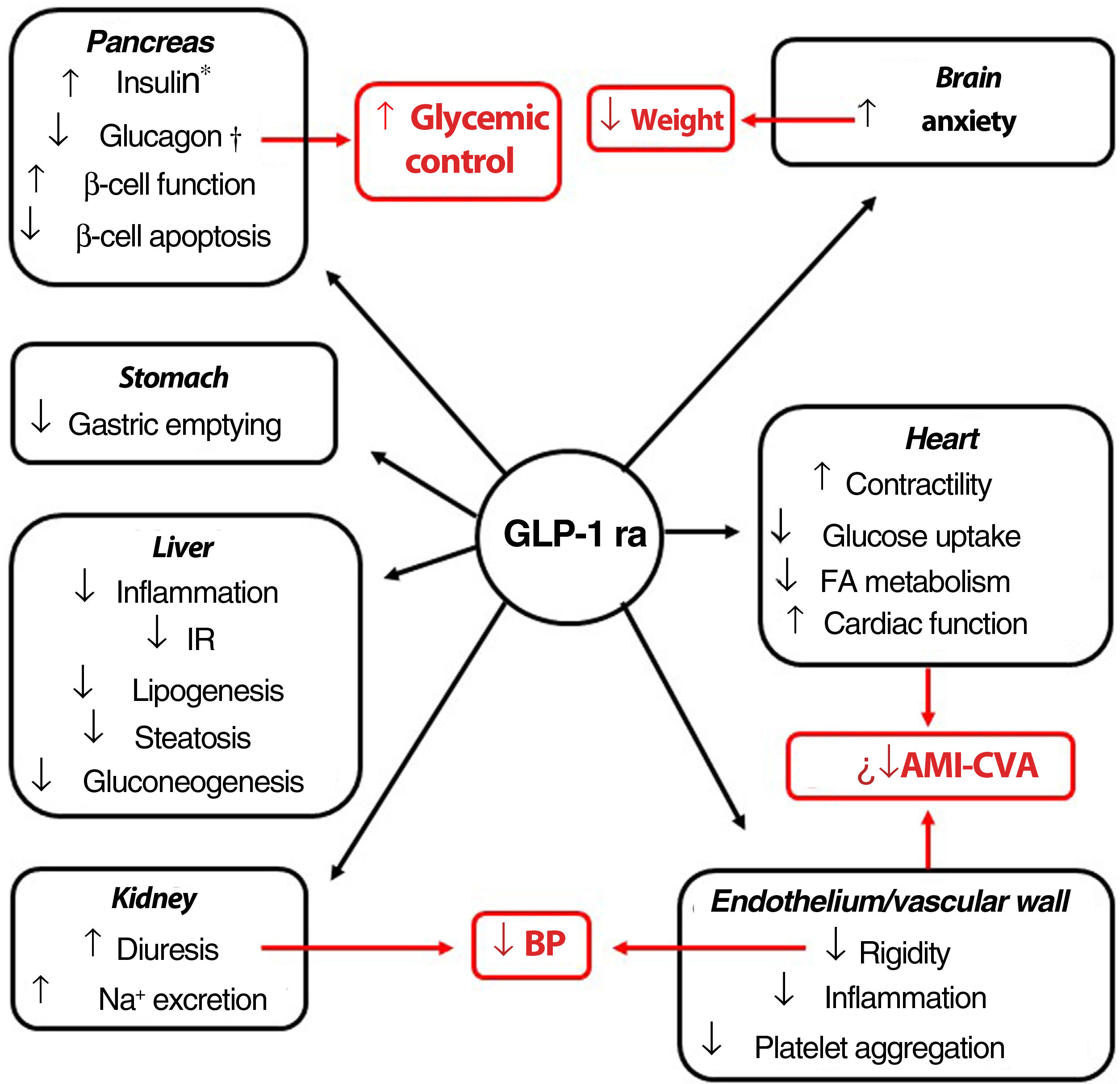
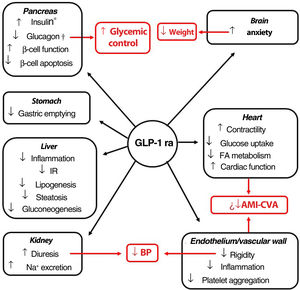
Indications, pleiotropic actionsGLP-1 receptor agonists are analogs of GLP-1, an intestinal peptide hormone of ∼3.3 kDa belonging to the incretin family. On binding to the GLP-1 cell receptor, these agents trigger the same molecular mechanisms as those induced by the endogenous peptide. The interaction occurs in different tissues (pancreas, brain, myocardium, endothelium, kidney, digestive system), which means that its actions, including glycemic control with minimum risk of hypoglycemia, have a pleiotropic character (Fig. 1).51 Moreover, a series of clinical trials suggest that the GLP-1 receptor agonists could induce cardiovascular protection.52–55
Pleiotropic effect of GLP-1 receptor agonists. The effects of GLP-1 receptor agonists on different organs and, in red, their consequences.
*Glucose-dependent biosynthesis and secretion. †Glucose-dependent secretion.
Abbreviations: AMI: acute myocardial infarction; BP: blood pressure; CVA: cerebrovascular accident; FA fatty acids; GLP-1 ra glucagon-like protein-1 receptor agonist; IR: insulin resistance.
The GLP-1 receptor agonists currently on the market have a molecule weight (MW) less than 5 kDa (except for dulaglutide), and a circulating half-life greater than the 5minutes of the native GLP-1 molecule.56 Brain nuclei such as the paraventricular nucleus of the thalamus and arcuate nucleus express GLP-1 receptors, although it is the former that appears to play the most important part in control of food intake.57,58 The GLP-1 receptor agonists for treatment of obesity in the European Union (EU), liraglutide (3mg/day, subcutaneous) and semaglutide (2.4mg in a weekly subcutaneous dose), are >90% homologous with the structure of human GLP-1. Liraglutide has a half-life of 13h, and reaches brain tissue on crossing the blood brain barrier. It is debated whether this action occurs by passive diffusion or, in contrast, requires interaction with the GLP-1 receptor.59–70 The semaglutide molecule has a longer aliphatic chain, conferring greater hydrophobicity. The incorporation of polyethylene glycol not only modifies its hydrolysis target by dipeptidyl peptidase-4, but also increases its affinity for albumin. These characteristics prolong its circulating half-life to up to 165h, and, when conjugated with albumin, the molecule is able to penetrate the spinal chord, septal nucleus, and hypothalamus via the circumventricular organs.60 Once in the satiety centers, liraglutide and semaglutide act on the neuronal populations of the pro-opiomelanocortin and NPY/AgRP neurons, implicated in metabolic activity, and, through as yet little known mechanisms, are able to reduce the hunger sensation. This action makes these agents a therapeutic option for weight loss.56 Although to a lesser extent, delay in gastric emptying contributes to the anorexigenic effect.61
Clinical trials conducted with GLP-1 receptor agonists in patients with obesity (or excess weight [BMI ≥ 27] with comorbidities), or with DT2 and excess weight or obesity, have achieved weight reductions greater than those reported for orlistat, as well as those observed with oral antidiabetic agents such as the sodium glucose type 2 cotransporter inhibitors (SGLT2i) or metformin.62 In a meta-analysis of more than 10 000 patients with obesity, those treated with GLP-1 receptor agonists lost, on average, 7kg more than those in the control group.63 Weight loss of up to 15% has been observed after 12 months of treatment,64,65 a level that had only been achieved through surgical methods.
Dosing, safety profileGLP-1 receptor antagonists with an indication for obesity (or excess weight [BMI ≥ 27kg/m2] with complications) are administered subcutaneously every week or daily. The use of GLP-1 receptor agonists is frequently associated with gastrointestinal adverse effects, particularly nausea, vomiting, diarrhea or constipation; these are transient and of mild to moderate intensity. Their onset can be avoided or the severity reduced to a large extent if dose escalation is performed as indicated in the label, and if, while treatment lasts, a series of well-defined dietary and behavioral recommendations are followed.66 In patients who observe these recommendations, the adverse effects may disappear in a few days or weeks.67
Arguments in favor of GLP-1 receptor agonists for weight control in patients with obesity and inflammatory dermatosesAs mentioned earlier, weight loss has a positive effect on the outcome and response to treatments for PsO and HS. It is plausible to speculate that GLP-1 receptor agonists can induce greater weight loss than those obtained with other therapeutic interventions. This can also improve adherence. Furthermore, GLP-1 receptor agonists induce anti-inflammatory effects that could attenuate the low-grade inflammation that underlies obesity, PsO, and HS (reviewed recently in the context of PsO and psoriatic arthritis).68 Finally, improved metabolic control and cardio-renal protection could be beneficial for reducing CVR and managing comorbidities such as DT2 or metabolic syndrome.
GLP-1 receptor agonists with indication for obesityAs mentioned earlier, there are 2 GLT-1 receptor agonists indicated for the treatment of obesity in the EU in subjects with BMI ≥ 30kg/m2 or BMI ≥ 27kg/m2 and at least one weight-related comorbidity, always in combination with appropriate diet and physical exercise: liraglutide 3mg and semaglutide 2.4 mg. The second, although approved by European Medicines Agency (EMA) and the Spanish Agency for Medicines and Health Products (AEMPS), is still not marketed in Spain.
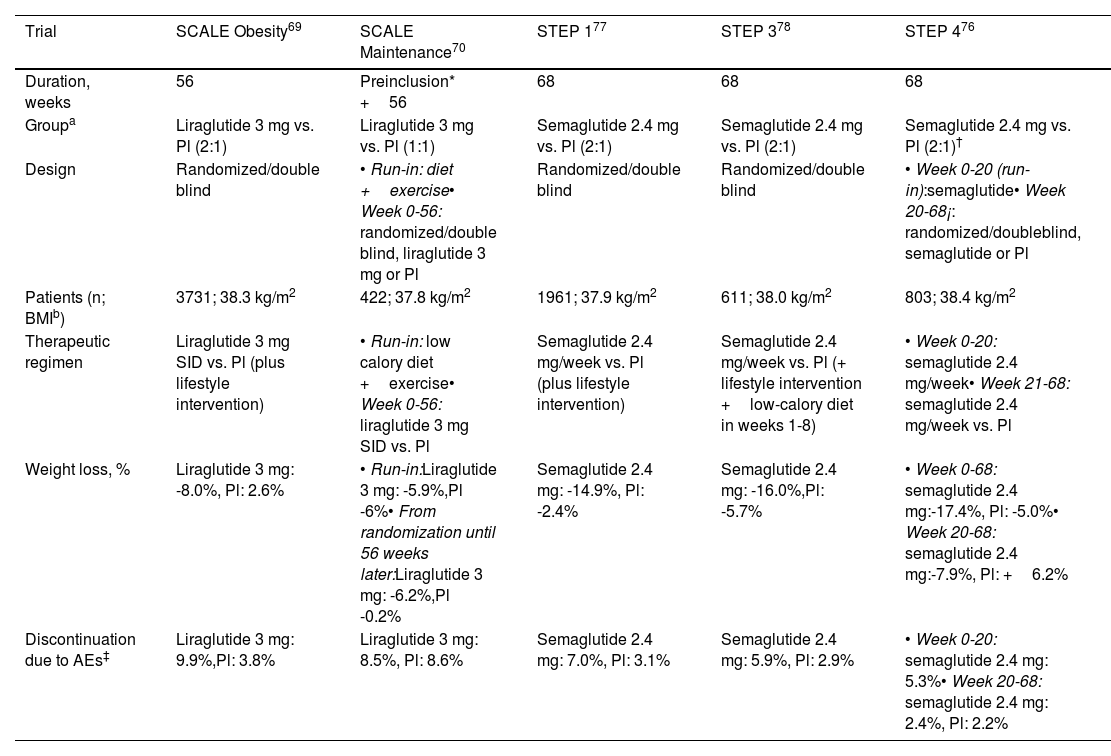
Liraglutide 3mgThis agent is administered subcutaneously every day. The SCALE clinical trials were designed to assess the effectiveness and safety of this agent for weight loss in patients with obesity or overweight (BMI ≥ 27kg/m2) with comorbidities. Table 1 shows the most relevant findings. In a cohort of 3731 patients, weight loss of 5%, 10%, and 15% was observed in 63%, 33%, and 14%, respectively, after 56 weeks of treatment (27%, 11%, and 4% with placebo),69 or of an additional 6% at 56 weeks in patients who had already lost 6% through hypocaloric diet and exercise.70 Positive results were also observed in adolescent patients with obesity,71 and in real-life studies substantial weight loss has also been reported.72–75
Phase 3 trials with GLP-1 receptor agonists indicated specifically for obesity and overweight.
| Trial | SCALE Obesity69 | SCALE Maintenance70 | STEP 177 | STEP 378 | STEP 476 |
|---|---|---|---|---|---|
| Duration, weeks | 56 | Preinclusion* +56 | 68 | 68 | 68 |
| Groupa | Liraglutide 3 mg vs. Pl (2:1) | Liraglutide 3 mg vs. Pl (1:1) | Semaglutide 2.4 mg vs. Pl (2:1) | Semaglutide 2.4 mg vs. Pl (2:1) | Semaglutide 2.4 mg vs. Pl (2:1)† |
| Design | Randomized/double blind | • Run-in: diet +exercise• Week 0-56: randomized/double blind, liraglutide 3 mg or Pl | Randomized/double blind | Randomized/double blind | • Week 0-20 (run-in):semaglutide• Week 20-68¡: randomized/doubleblind, semaglutide or Pl |
| Patients (n; BMIb) | 3731; 38.3 kg/m2 | 422; 37.8 kg/m2 | 1961; 37.9 kg/m2 | 611; 38.0 kg/m2 | 803; 38.4 kg/m2 |
| Therapeutic regimen | Liraglutide 3 mg SID vs. Pl (plus lifestyle intervention) | • Run-in: low calory diet +exercise• Week 0-56: liraglutide 3 mg SID vs. Pl | Semaglutide 2.4 mg/week vs. Pl (plus lifestyle intervention) | Semaglutide 2.4 mg/week vs. Pl (+ lifestyle intervention +low-calory diet in weeks 1-8) | • Week 0-20: semaglutide 2.4 mg/week• Week 21-68: semaglutide 2.4 mg/week vs. Pl |
| Weight loss, % | Liraglutide 3 mg: -8.0%, Pl: 2.6% | • Run-in:Liraglutide 3 mg: -5.9%,Pl -6%• From randomization until 56 weeks later:Liraglutide 3 mg: -6.2%,Pl -0.2% | Semaglutide 2.4 mg: -14.9%, Pl: -2.4% | Semaglutide 2.4 mg: -16.0%,Pl: -5.7% | • Week 0-68: semaglutide 2.4 mg:-17.4%, Pl: -5.0%• Week 20-68: semaglutide 2.4 mg:-7.9%, Pl: +6.2% |
| Discontinuation due to AEs‡ | Liraglutide 3 mg: 9.9%,Pl: 3.8% | Liraglutide 3 mg: 8.5%, Pl: 8.6% | Semaglutide 2.4 mg: 7.0%, Pl: 3.1% | Semaglutide 2.4 mg: 5.9%, Pl: 2.9% | • Week 0-20: semaglutide 2.4 mg: 5.3%• Week 20-68: semaglutide 2.4 mg: 2.4%, Pl: 2.2% |
Abbreviations: AEs, adverse events; BMI, body mass index at baseline; Pl: placebo; SID, once daily.
In the run-in period, all patients, regardless of whether we are randomized to receive lira or placebo later, were subject to a hypocaloric diet and exercise regime, without GLP-1 receptor agonist, with the duration of the period for each patient being the time required to lose ≥5% of their baseline body weight
In the STEP trials (Table 1), designed to evaluate the efficacy and safety of semaglutide 2.4mg (subcutaneous, weekly) in patients with obesity, or excess weight (BMI ≥ 27kg/m2) with complications, weight loss of between 15% and 17% was achieved 68 weeks after starting treatment.76–78 In one of the trials, weight loss of 10% was reported after 20 weeks of therapy. The subjects who, at that time, switched from semaglutide 2.4mg to placebo had regained half of that weight after 48 weeks, whereas those who continued on active treatment achieved an additional weight loss of around 8% in the same time period. After 68 weeks with semaglutide 2.4mg, losses of 5%, 10%, and 15% were reported in 88%, 79%, and 63% of patients, respectively.76 A recent meta-analysis concluded that semaglutide 2.4mg and liraglutide 3mg are more efficacious than naltrexone-bupropion (not marketed in Spain) and orlistat.79
Experience with liraglutide and semaglutide in psoriasis and hidradenitis suppurativaLiraglutide and psoriasisIn studies with liraglutide, patients with PsO and obesity had a diagnosis of DT2. and so the doses did not exceed 1.8mg, which is the indicated dose for such patients. Although the series had limited sample sizes, the findings are interesting. In a cohort of 7 patients with DT2 and obesity, daily use of liraglutide 1.2mg achieved, after 10 weeks, a weight reduction of 5% and a decrease in BMI of 2kg/m2.80 In another study, 3 patients with DT2 and obesity achieved a decrease in BMI of between 0.8 and 3.8kg/m2 after 18 weeks with this same dose of liraglutide.81 Another 2 patients with obesity and DT2 achieved weight losses of more than 5kg and a decrease in BMI of 1.5 and 2kg/m2 after 6 weeks of treatment.82 Finally, in a series of 20 subjects with obesity and DT2, an average weight loss of 4.7kg was reported after 8 weeks of treatment with liraglutide 1.8mg.83 In most of these patients, improvements in the psoriasis area severity index (PASI) was observed, and on occasions, a decrease in psoriatic plaques. This observation could encourage investigation into whether this treatment is able to induce additional benefit.
Semaglutide and psoriasisExperience in this case is limited, and no studies have been performed with the 2.4mg dose. There is a case report of a 73-year-old man with PsO, obesity, and DT2 who achieved a decrease in BMI from 40.3 to 38.3kg/m2 after 10 months treatment with 1mg weekly of semaglutide.84
Liraglutide and hidradenitis suppurativaExperience with this agent is limited in HS. There is a case report of a patient aged 19 years with HS and grade 2 obesity who started combination therapy with liraglutide 1.8mg, metformin, levonorgestrel/ethinylestradiol, dapsone, and finasteride. She lost 16kg in the first 6 months, although she then progressively regained weight.85 There have been no reports of treatment of obesity with semaglutide in patients with HS.
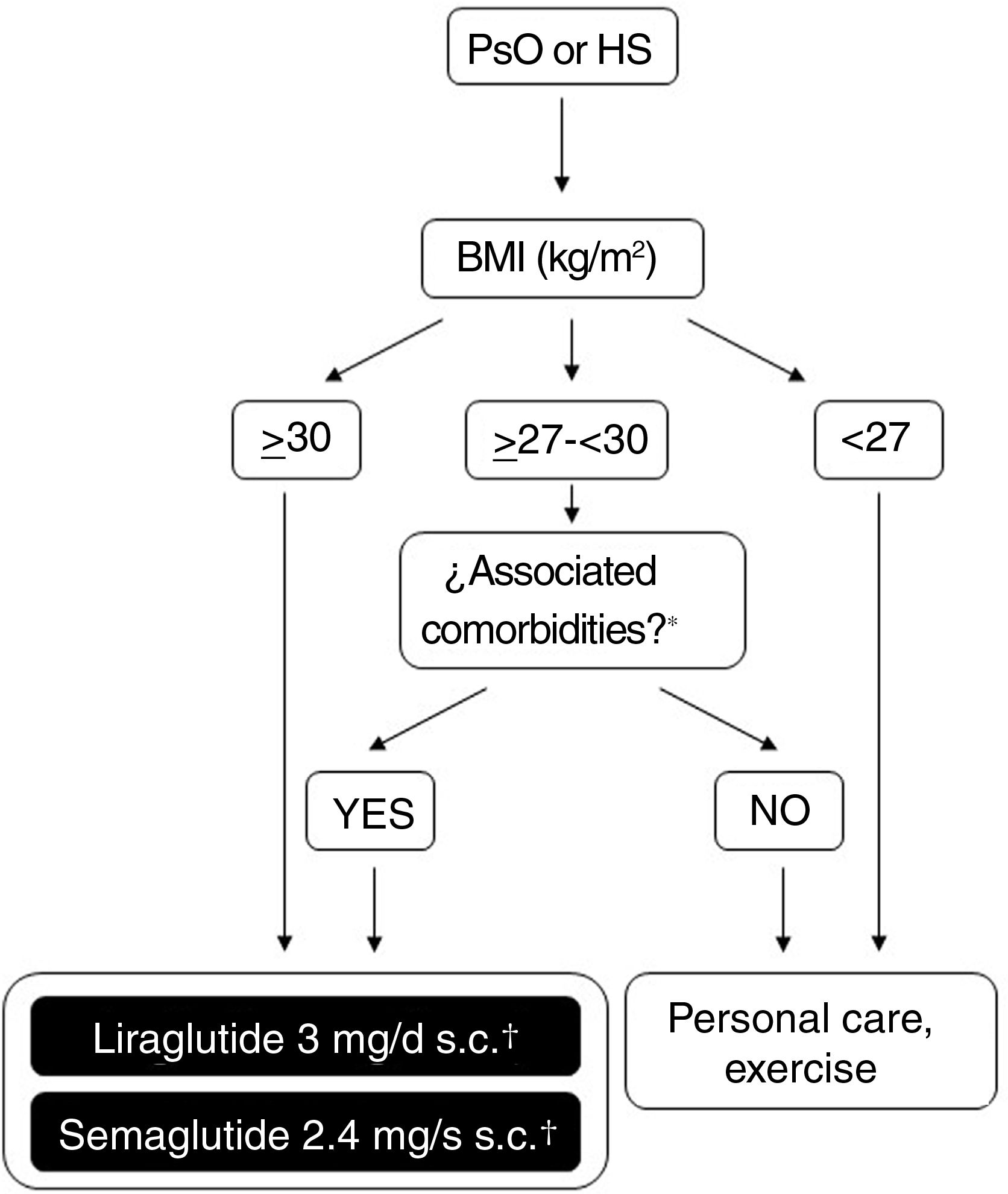
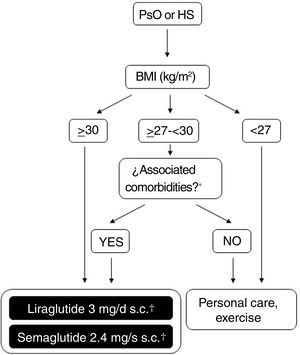
Possibilities for use of GLP-1 receptor agonists in Spain for treatment of obesityFigure 2 shows a schematic overview of the treatment options which are currently available for weight control in Spain and which are applicable to patients who also have PsO or HS. The doses of liraglutide or semaglutide are different depending on whether the patient has DT2, is overweight (BMI ≥ 27kg/m2) with comorbidities or has obesity (with or without DT2). In addition, in patients with DT2, there is the possibility of using an oral presentation of semaglutide. The effects of this presentation on weight and glycemia in clinical trials and real-world settings are comparable to those induced by the subcutaneous presentation, although a specific indication for weight control without DT2 is still lacking.86 In order to avoid confusion in daily clinical practice, it is important to note that liraglutide 3mg and semaglutide 2.4mg can be used in patients with DT2, provided these are obese or overweight with comorbidities. Patients with DT2 without obesity or excess weight with comorbidities can use liraglutide 1.8mg (daily, subcutaneous) and semaglutide 1mg (weekly, subcutaneous), or 14mg (daily, oral) for glycemic control. However, they should not be treated with liraglutide 3mg or semaglutide 2.4mg, because these doses are not indicated for this condition. The underlying reason is that, in the case of parenteral presentations, the effective doses of GLT-1 receptor agonists necessary for weight control are greater than those needed for glycemic control.
Decision tree for use of GLP-1 receptor agonists indicated for weight control in patients with obesity or excess weight with comorbidities. Different possibilities are considered depending on whether the patient is obese (BMI≥ 30kg/m2) or overweight (BMI≥ 27- <30kg/m2) with ≥ 1 weight-related comorbidity. These therapeutic options can be used for weight control in patients with immune-mediated dermatosis who meet the above criteria. Semaglutide 2.4mg, although approved by the EMA and AEMPS, has still not been marketed in Spain.
* At least 1 weight-related comorbidity: in the case of liraglutide 3mg, poor glycemic control (prediabetes or DT2), hypertension, dyslipidemia or sleep obstructive apnea; in the case of semaglutide 2.4mg, glucose disorder (prediabetes or DT2), hypertension, dyslipidemia, sleep obstructive apnea, or cardiovascular disease.
† 3mg, daily, subcutaneous administration, in monotherapy or in combination with other medications.
‡ 1.8mg, daily, subcutaneous administration, in monotherapy or in combination with other medications.
Abbreviations: AEMPS: Spanish Agency for Drugs and Medicinal Products; BMI: body mass index; DT2: type 2 diabetes; EMA: European Medicines Agency; GLP-1: glucagon-like peptide 1 receptor; HS: hidradenitis suppurativa; MET: metformin; PsO: psoriasis.
Tirzepatide, still not approved in the EU for specific use in patients with obesity, is a dual agonist of the GLP-1 receptors and another incretin, gastric inhibitor polypeptide (GIP). In a phase 3 trial in subjects with obesity or overweight (BMI ≥ 27kg/m2), its use for 18 months achieved weight loss of up to 20.9%.87
Conclusions- •
Low-grade inflammation underlies obesity and PsO/HS, and this inflammation has a negative impact on both conditions.
- •
Weight loss is beneficial for PsO and HS outcomes, through a reduction in the inflammatory component of CVR.
- •
GLP-1 receptor agonists, by achieving greater weight loss than obtained with other medications, may represent a therapeutic opportunity for improving obesity in patients with PsO and HS, with resulting decrease in CVR and, in general, improvement in the medium to long term.
- •
The formation of multidisciplinary teams comprised of dermatologists and endocrinologists may contribute to better management of patients with obesity/overweight and immune-mediated dermatosis.
Laboratorios Novonordisk collaborated through funding for Medical Writing Services.
Conflicts of InterestEva Vilarrasa has received fees for consulting and/or as a speader and/or for travel and/or has participated in clinical trials sponsored by Abbvie, Almirall, Amgen, Boehringer Ingelheim, Bristol-Meyers Squibb, Celgene, Gebro, Isdin, Janssen, LEO Pharma, Lilly, Merck Serono, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, and UCB.
Joana Nicolau has received fees for presentations and consulting on behalf of Sanofi, Novo Nordisk, Lilly, Boehringer-Ingelheim, Fresenius, AstraZeneca, Fresenius, Dexcom, Amgen, and Senseonics.
Pablo de la Cueva has participated as consultant and/or investigator and/or speaker for the following pharmaceutical companies: Abbvie, Almirall, BMS, Boehringer-Ingelheim, Celgene, Janssen Cilag, LEO Pharma, Lilly, MSD, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, and UCB.
Albert Goday has received fees for investigation projects, consulting, and postgraduate training, and has participated in clinical trials sponsored by Almirall, Ascensia, Colegio de Farmacéuticos de Barcelona, Esteve, Federación Farmacéutica, Fundación Catalana Síndrome de Down, Fundació Gol i Gorina, Instituto de Salud Carlos III, Jansen, Lilly, Menarini, MSD, Mutuam, Novo Nordisk, Pronokal, RedGDPS, Rovi, Sanofi Aventis.
Fernando Gallardo has received fees as presenter, consultant, and support for attendance of course and congresses and/or investigation projects in the following companies: Janssen, Abbvie, UCB, Amgen, Bristol, Lilly, Novartis, Almirall, Leo Pharma.
Antonio Martorell has received fees and/or travel grants and/or acted as a member of the steering committees from Novartis, Abbvie, Janssen Cilag, UCB, Lilly, LEO Pharma, L’Oreal, Sanofi, Boehringer Ingelheim, Almirall, Bristol Myers Squibb, and Amgen. He has also worked as the principal investigator in clinical trials sponsored by Abbvie, UCB, Janssen, Bristol Myers Squibb, Lilly, Galderma, Sanofi, and Novartis.
José Manuel Carrascosa has participated as PI/SI and/or guest presenter and/or advisor for Almirall, Janssen, Abbvie, UCB, Boehringer-Ingelheim, Lilly, Novartis, Amgen, BMS, and Sandoz.