Hidradenitis suppurativa (HS) is a chronic inflammatory entity characterized by the appearance of multiple nodules, abscesses, and fistulas, predominantly in apocrine regions. In addition to its dermatological involvement, it is associated with multiple systemic comorbidities. Its treatment is combined: topical pharmacological, systemic pharmacological and surgical. Regarding biologic or small molecule drugs, currently only adalimumab is approved. A narrative review of the literature on biological or small molecule drugs used in the treatment of hidradenitis suppurativa is presented. The arsenal we found is large, with multiple targets: inhibitors of tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-17, IL-23, IL-1, inhibitors of the janus kinase (JAK) pathway, and multiple other drugs in study. New prospective studies and comparative trials are needed to analyze the effectiveness and safety of these treatments, in an entity with a promising future.
La hidradenitis supurativa (HS) es una entidad inflamatoria crónica caracterizada por la aparición de múltiples nódulos, abscesos y fístulas, de predominio en regiones apocrinas. Además de su afectación dermatológica, se asocia a múltiples comorbilidades sistémicas. Su tratamiento es combinado: farmacológico tópico, farmacológico sistémico y quirúrgico. En cuanto a los medicamentos biológicos o de molécula pequeña, actualmente solo se encuentra aprobado adalimumab. En este artículo, se presenta una revisión narrativa de la literatura sobre fármacos biológicos o de molécula pequeña utilizados en el tratamiento de la hidradenitis supurativa. El arsenal que encontramos es numeroso, con múltiples dianas: inhibidores del factor de necrosis tumoral alfa (TNF-alfa), interleucina (IL)-17, IL-23, IL-1, inhibidores de la vía Janus kinasa (JAK) u otros múltiples fármacos en estudio. Son necesarios nuevos estudios prospectivos y ensayos comparativos que analicen la eficacia y seguridad de estos tratamientos, en una entidad con un futuro prometedor.
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic, recurrent, and debilitating inflammatory skin condition.1 It is characterized by the recurrent appearance of nodules, abscesses, and communicating fistulas predominantly in apocrine regions, typically in the axillae, inframammary folds, and groin.2 One of the main problems is the lack of knowledge about this entity, with delays of up to 12 years in its diagnosis being recorded in the literature. The delay in its diagnosis can lead to the appearance of established scarring lesions, which are very difficult to treat medically.3 In addition to its cutaneous implications, it is an entity that has been associated with numerous comorbidities, including increased risk of anemia, inflammatory bowel disease, psoriasis, spondyloarthropathy, metabolic syndrome, anxiety, depression, and increased suicide rates.4 Even though there are no large multicenter studies that address its epidemiology, most authors point to an estimated prevalence of 0.5–1%. The disease usually begins at the second decade of life, although there are cases of early onset in childhood and others older ages. HS predominantly affects women in a 3:1 ratio to men.5
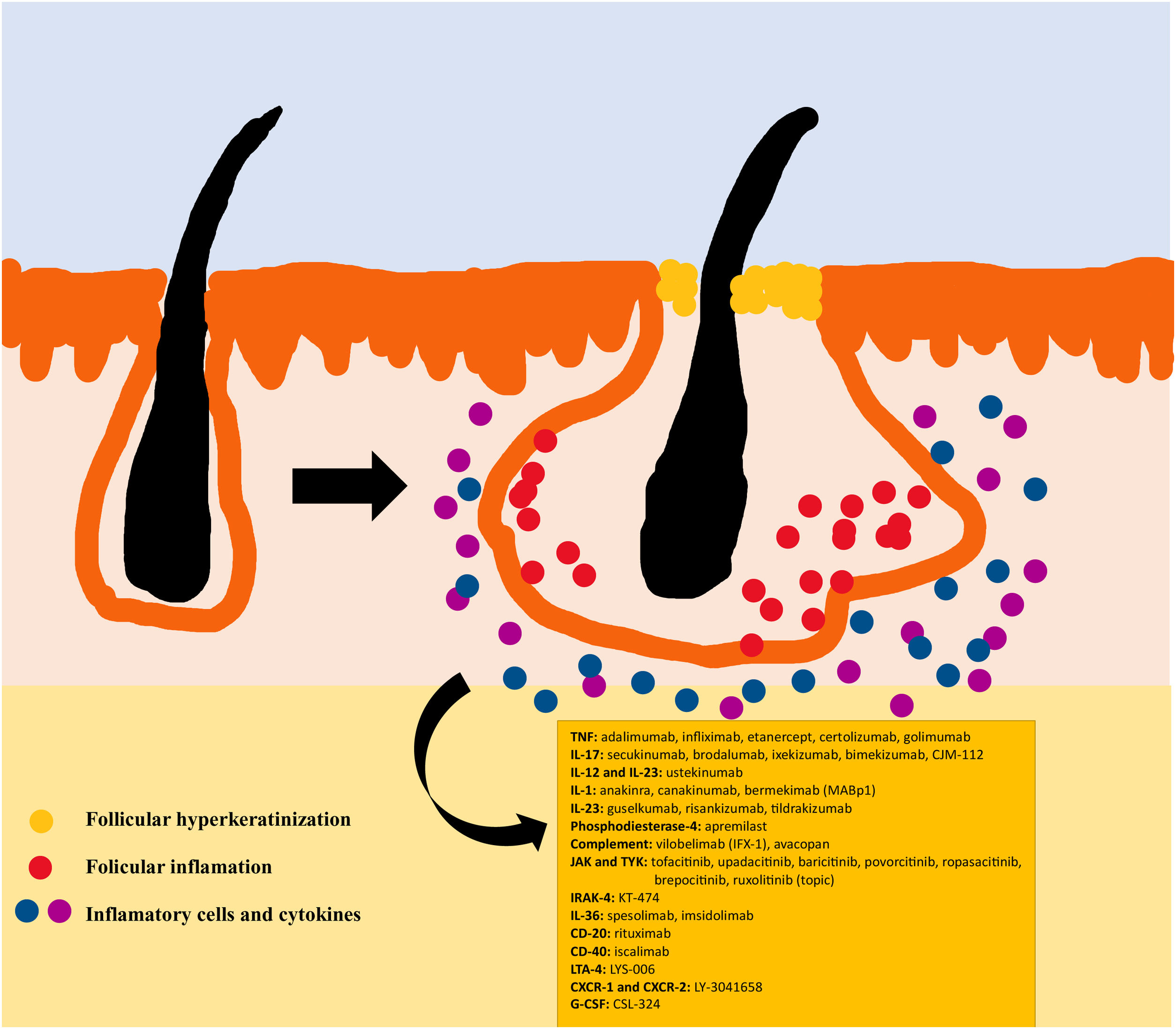
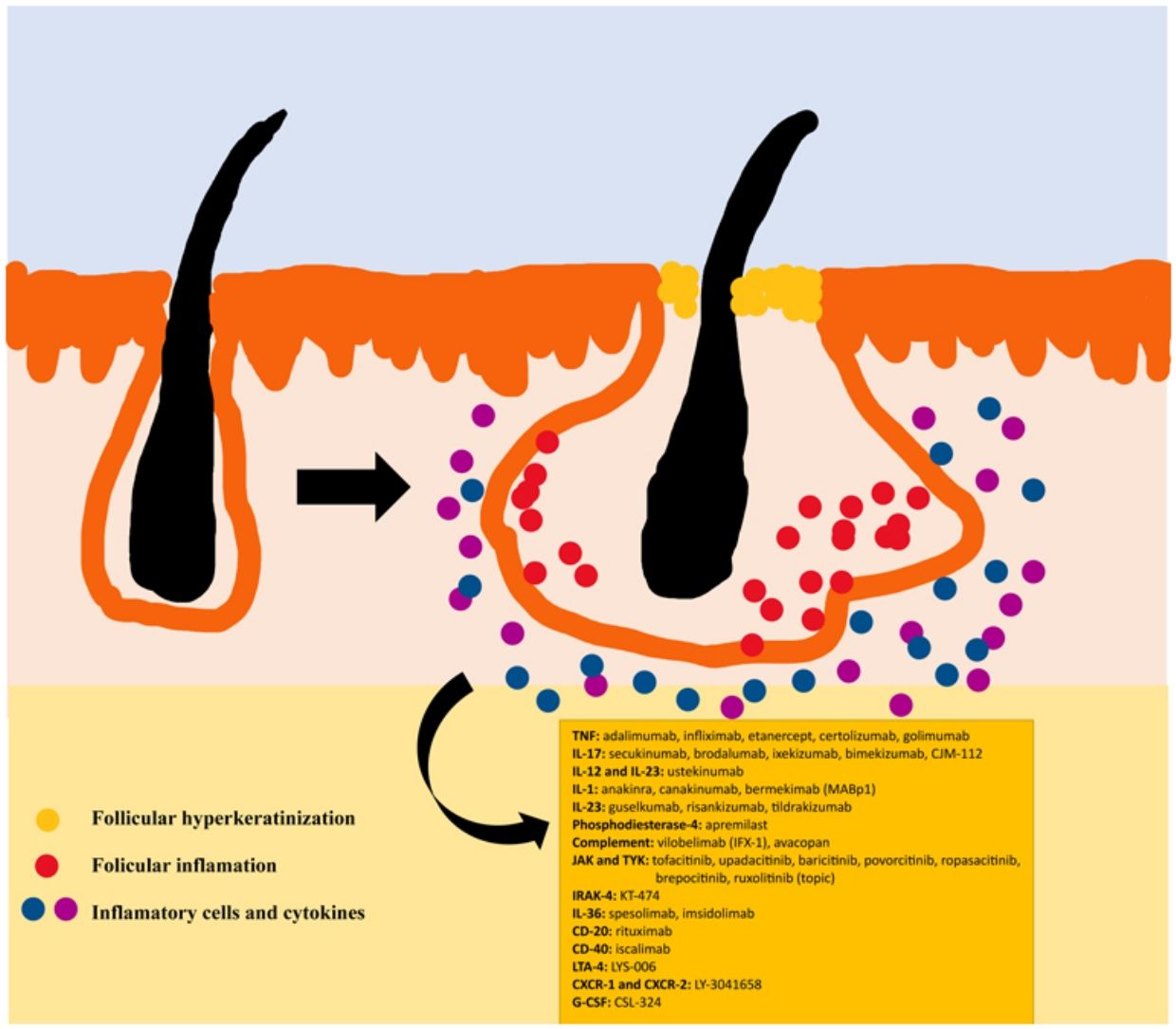
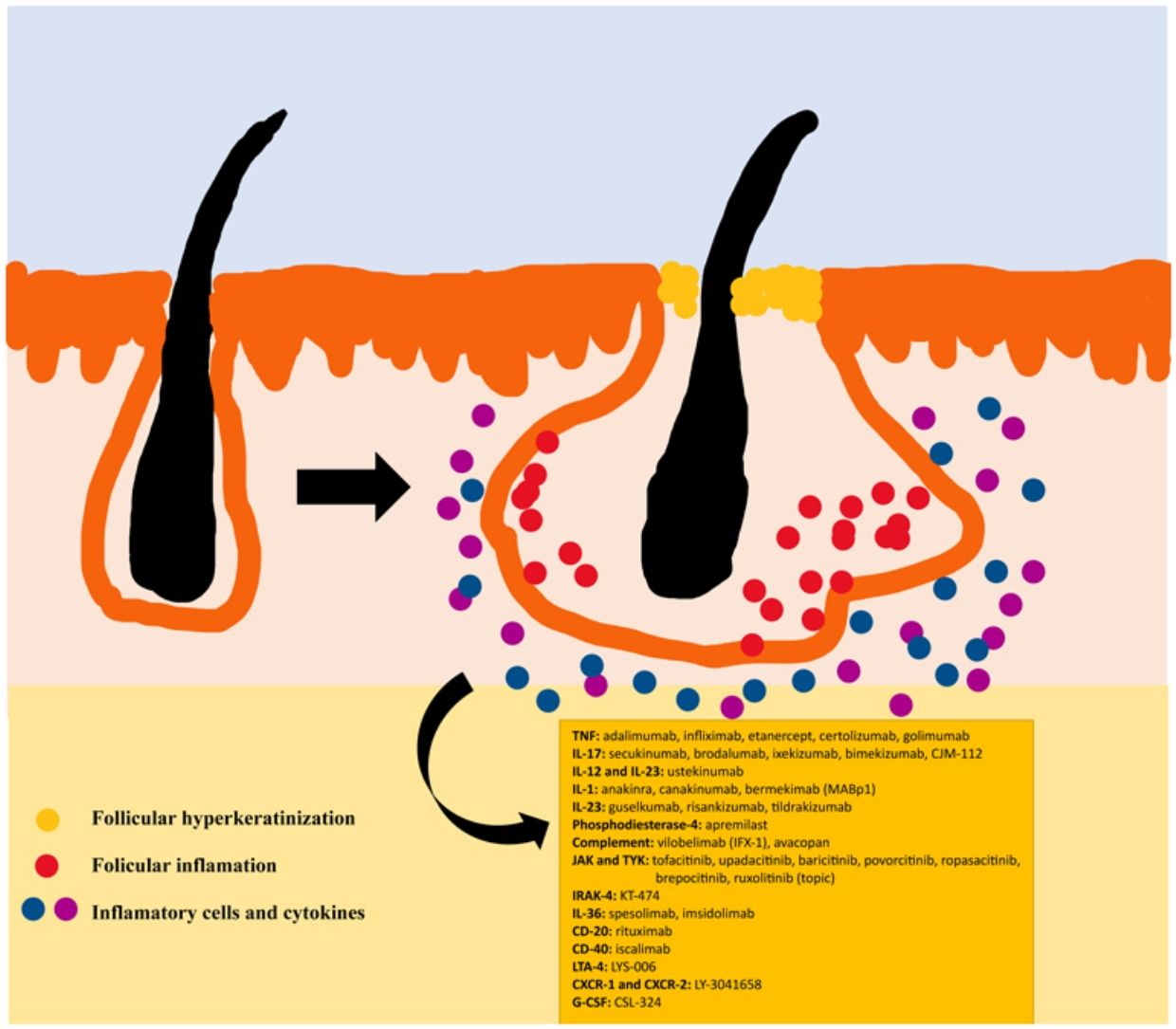
The pathogenesis of HS remains unclear. Although the genesis of HS has not been entirely elucidated, lesion formation is believed to center around follicular hyperkeratosis within the pilosebaceous-apocrine unit.6 Three main processes have been implicated in its origin: follicular hyperkeratosis and dilatation, follicular rupture and subsequent inflammatory response, and chronic inflammation with architectural tissue changes.7 In addition, recent research has provided new insight into the role of cytokines in the pathogenesis of HS, helping close some existing knowledge gaps in the development of this condition.6 The activation of lesional keratinocytes leads to increased secretion of antimicrobial peptides (e.g., psoriasin and calgranulin) and inflammatory cytokines (e.g., interleukin-1β (IL-1β), IL-18, IP-10, and RANTES).7 Follicular epithelial inflammation increases the production of cytokeratin 16 in the infundibulum and causes alteration and involution of follicle-associated sebaceous glands.8 These alterations cause follicular hyperkeratinization, with retrograde dilation and endogenous activation of host inflammation, especially focused on a Th1 and Th17 response, altering the follicular microenvironment.9,10 After the primary acute event, chronic inflammation is sustained by continuous infiltration of neutrophils via lipocalin-2, leading to permanent architectural changes typically in the form of organized fibrosis.11 Up to 30–42% of patients with HS have a family history of this disease, some of them present mutations in any of the components of γ-secretase complex (especially NCSTN, PSEN1, PSENEN and POGLUT1). This is an important complex implicated into the pathogenesis of HS, leading to impaired Notch 1 signaling, which alters the homeostasis of the infundibular unit.12,13 The role of microorganisms in the pathogenesis of HS is also controversial. A predominance of Staphylococcus species and anareobic bacteria has been found.14 Regarding modifiable factors involved in the genesis or worsening of HS, a relationship has been found with smoking, considered the most important exogenous factor and present in up to 90% of patients with HS.15 Others also contribute, such as overweight and obesity, tight clothing and excessive friction, certain drugs such as lithium, quetiapine or disulfiram. In addition, cases of aggravation with isotretinoin have been described, so its use is reserved for patients with HS where their acne predominates, since its efficacy is controversial in other HS lesions. HS also has been associated with the use of certain deodorants and traumatic hair removal.16 Due to its multifactorial pathogenesis and its chronic course, it is a complex disease, where treatment must be individualized and according to the needs of each moment according to the evolution of the disease. Treatment guidelines for HS vary among different countries. The management algorithm agreed by the European Hidradenitis Suppurativa Foundation (EHSF) establishes a treatment escalation according to its severity.17 Topical antibiotic therapy is used as the first line to limit bacterial overgrowth caused by an exaggerated inflammatory reaction. In more severe or refractory cases, the therapeutic range is broadened. Oral therapies include oral or iv antibiotics (clindamycin, rifampicin, metronidazole, moxifloxacin, ertapenem, etc.), systemic retinoids, systemic antiandrogens, hormonal contraceptives, metformin, zinc gluconate, dapsone, methotrexate or cyclosporine. Interventional treatments involve small infiltrations with corticosteroids, surgeries to release fistulas («deroofing») or large flaps or grafts for the treatment of larger defects.18–21 HS was deprived of biological or small molecule treatments until 2015, when the EMA and the FDA approved the use of adalimumab for the treatment of severe HS refractory to other treatments.22,23 With the involvement of tumor necrosis factor alpha (TNF-alpha) in its pathogenesis, new cytokines and molecular targets have been progressively studied for the treatment of severe HS, including IL-1, IL-17, IL-23, IL-36, complement 5a (C5a), C5a receptor, the Janus kinase pathway (JAK) or phosphodiesterase 4 (PDE4).24–29 A graphic summary of the pathophysiology of HS can be found in Fig. 1.28 However, despite the multiple targets studied, and the numerous prospective studies and functioning clinical trials, there is still no standardized therapeutic algorithm in those patients with failure or contraindication of adalimumab in severe HS. The objective of this study is to present an updated narrative review of the results obtained with biologic and small molecule drugs in the treatment of severe HS.
Material and methodsA narrative search of the literature was carried out in Medline and Google Scholar, from January 1, 2000 to April 1, 2023 with the keywords «hidradenitis», «hidradenitis suppurative», «systemic treatment», «treatment», «therapy», «biologics», «small molecules», «new treatment», «new perspectives», «adalimumab», «infliximab», «golimumab», «etanercept», «ustekinumab», «secukinumab», «brodalumab», «ixekizumab», «bimekizumab», «guselkumab», «risankizumab», «tildrakizumab», «upadacitinib», «tofacitinib», «baricitinib», «jak», «jak inhibitor», «janus kinase». Likewise, a search was carried out in https://clinicaltrials.gov/, through the «Other terms» tab and including the term «hidradenitis suppurativa». All studies were included, with no limitation on the number of patients or study design. The search was limited to articles in English and Spanish. A selection of the articles was made according to their summary (abstract), and they were selected for their relevance after reading the studies. In relation to clinical trials, the most relevant ones were collected, emphasizing those with phase ≥2, except for some phase 1 trials with a very novel mechanism of action. The main investigator (MMP) oversaw the search and selection of the studies. This search was later confirmed by another researcher (RBE).
ResultsThe most relevant studies carried out or being carried out with various systemic and small-molecule drugs for severe HS are presented below and outlined in Table 1.
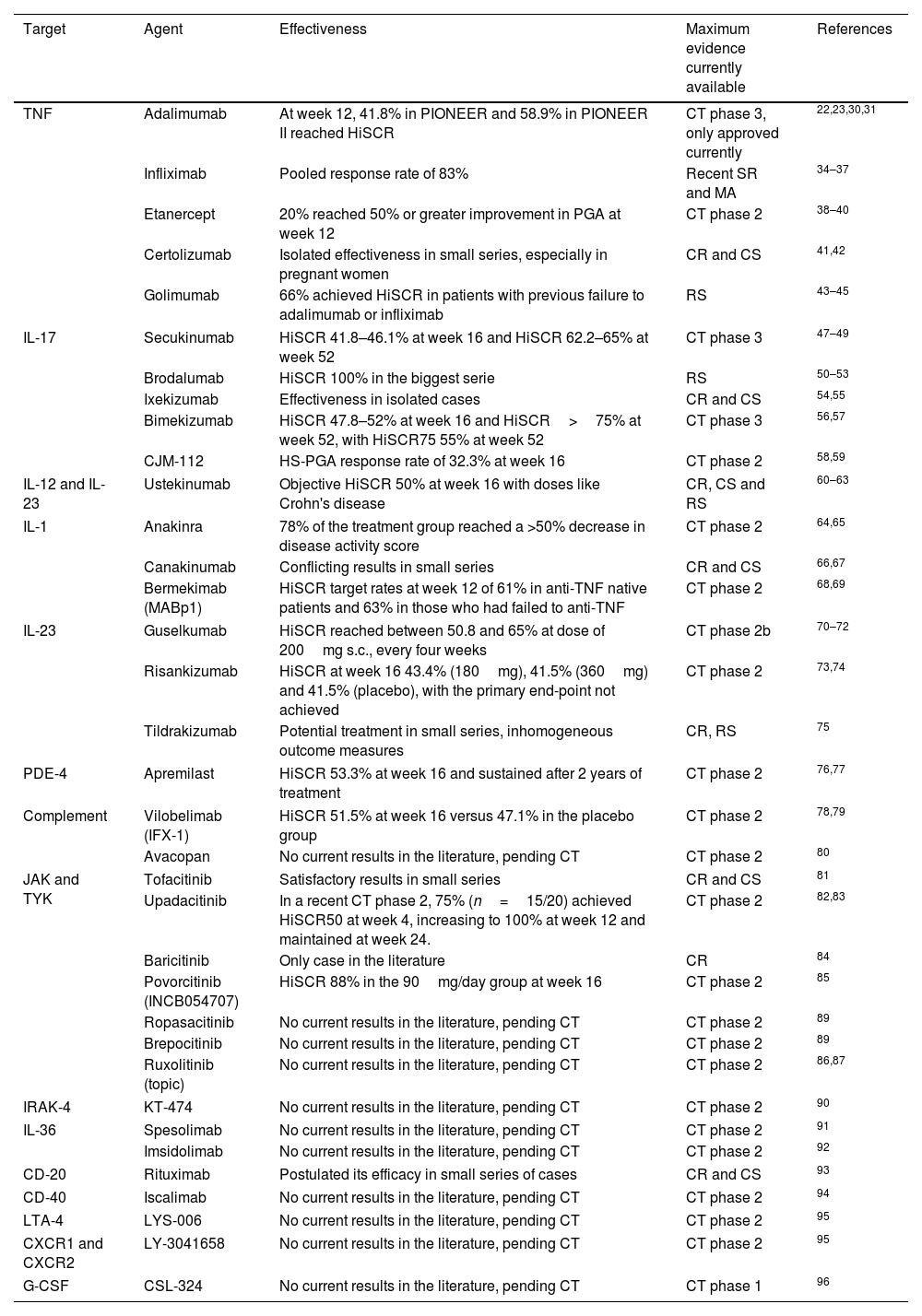
Biologics and small molecule drugs currently used or under investigation for the treatment of HS.
| Target | Agent | Effectiveness | Maximum evidence currently available | References |
|---|---|---|---|---|
| TNF | Adalimumab | At week 12, 41.8% in PIONEER and 58.9% in PIONEER II reached HiSCR | CT phase 3, only approved currently | 22,23,30,31 |
| Infliximab | Pooled response rate of 83% | Recent SR and MA | 34–37 | |
| Etanercept | 20% reached 50% or greater improvement in PGA at week 12 | CT phase 2 | 38–40 | |
| Certolizumab | Isolated effectiveness in small series, especially in pregnant women | CR and CS | 41,42 | |
| Golimumab | 66% achieved HiSCR in patients with previous failure to adalimumab or infliximab | RS | 43–45 | |
| IL-17 | Secukinumab | HiSCR 41.8–46.1% at week 16 and HiSCR 62.2–65% at week 52 | CT phase 3 | 47–49 |
| Brodalumab | HiSCR 100% in the biggest serie | RS | 50–53 | |
| Ixekizumab | Effectiveness in isolated cases | CR and CS | 54,55 | |
| Bimekizumab | HiSCR 47.8–52% at week 16 and HiSCR>75% at week 52, with HiSCR75 55% at week 52 | CT phase 3 | 56,57 | |
| CJM-112 | HS-PGA response rate of 32.3% at week 16 | CT phase 2 | 58,59 | |
| IL-12 and IL-23 | Ustekinumab | Objective HiSCR 50% at week 16 with doses like Crohn's disease | CR, CS and RS | 60–63 |
| IL-1 | Anakinra | 78% of the treatment group reached a >50% decrease in disease activity score | CT phase 2 | 64,65 |
| Canakinumab | Conflicting results in small series | CR and CS | 66,67 | |
| Bermekimab (MABp1) | HiSCR target rates at week 12 of 61% in anti-TNF native patients and 63% in those who had failed to anti-TNF | CT phase 2 | 68,69 | |
| IL-23 | Guselkumab | HiSCR reached between 50.8 and 65% at dose of 200mg s.c., every four weeks | CT phase 2b | 70–72 |
| Risankizumab | HiSCR at week 16 43.4% (180mg), 41.5% (360mg) and 41.5% (placebo), with the primary end-point not achieved | CT phase 2 | 73,74 | |
| Tildrakizumab | Potential treatment in small series, inhomogeneous outcome measures | CR, RS | 75 | |
| PDE-4 | Apremilast | HiSCR 53.3% at week 16 and sustained after 2 years of treatment | CT phase 2 | 76,77 |
| Complement | Vilobelimab (IFX-1) | HiSCR 51.5% at week 16 versus 47.1% in the placebo group | CT phase 2 | 78,79 |
| Avacopan | No current results in the literature, pending CT | CT phase 2 | 80 | |
| JAK and TYK | Tofacitinib | Satisfactory results in small series | CR and CS | 81 |
| Upadacitinib | In a recent CT phase 2, 75% (n=15/20) achieved HiSCR50 at week 4, increasing to 100% at week 12 and maintained at week 24. | CT phase 2 | 82,83 | |
| Baricitinib | Only case in the literature | CR | 84 | |
| Povorcitinib (INCB054707) | HiSCR 88% in the 90mg/day group at week 16 | CT phase 2 | 85 | |
| Ropasacitinib | No current results in the literature, pending CT | CT phase 2 | 89 | |
| Brepocitinib | No current results in the literature, pending CT | CT phase 2 | 89 | |
| Ruxolitinib (topic) | No current results in the literature, pending CT | CT phase 2 | 86,87 | |
| IRAK-4 | KT-474 | No current results in the literature, pending CT | CT phase 2 | 90 |
| IL-36 | Spesolimab | No current results in the literature, pending CT | CT phase 2 | 91 |
| Imsidolimab | No current results in the literature, pending CT | CT phase 2 | 92 | |
| CD-20 | Rituximab | Postulated its efficacy in small series of cases | CR and CS | 93 |
| CD-40 | Iscalimab | No current results in the literature, pending CT | CT phase 2 | 94 |
| LTA-4 | LYS-006 | No current results in the literature, pending CT | CT phase 2 | 95 |
| CXCR1 and CXCR2 | LY-3041658 | No current results in the literature, pending CT | CT phase 2 | 95 |
| G-CSF | CSL-324 | No current results in the literature, pending CT | CT phase 1 | 96 |
HiSCR: hidradenitis suppurativa clinical response, defined as a ≥50% reduction in the inflammatory lesion count (sum of abscesses and inflammatory nodules), with no increase in the number of abscesses or draining fistulas from baseline; CT: clinical trial; SR: systematic review; MA: meta-analysis; RS: retrospective studies; CS: case series; CR: case report; TNF: tumor necrosis factor; IL: interleukin; PDE: phosphodiesterase; JAK: janus kinase; TYK: tyrosine kinase; CD: cluster of differentiation; LTA: leukotriene antagonist drug; CXCR 1 and 2: chemokine receptor type 1 and 2; G-CSF: granulocyte colony stimulating factor.
The only drug approved to date for HS is adalimumab, a fact that was accomplished after the results of the trials PIONEER I and PIONEER II, with response rates at week 12 were significantly higher for the groups receiving adalimumab weekly than for the placebo groups: 41.8% versus 26.0% in PIONEER I (P=0.003) and 58.9% versus 27.6% in PIONEER II (P<0.001) and good safety profile.22,23,30,31 In non-responders or with a contraindication to adalimumab, the therapeutic range is wide, and there is no clear algorithm for its management. The good initial response in some patients, followed by secondary failure, prompted studies looking at adalimumab intensification from its standard dose 80mg/14 days to 80mg/7 days, with a proven rate of improvement.32,33 In addition to adalimumab, other anti-TNF agents have been studied for the treatment of HS. Infliximab has demonstrated its effectiveness in various studies.34,35 A recent systematic review and meta-analysis showed that the pooled response rate of HS patients to infliximab was 83% (95% CI, 0.71–0.91), with a favorable side effect profile.36 Parallel to the dose intensification that some authors suggest for adalimumab, there are other authors who have shown greater efficacy of infliximab when it is given at doses similar to those for inflammatory bowel disease: 7.5mg/kg every 4 weeks or even 10mg/kg every 4 weeks.37 Regarding etanercept, in the trial by Giamarellos-Bourboulis et al.,38 a reduction in disease burden was found at week 12, but the values were not specified. Subsequently, in the trial by Lee et al.,39 only 3 of 15 patients achieved the primary outcome, defined as 50% or greater improvement in PGA at week 12. Therefore, insufficient evidence has been reached for the recommendation of etanercept as a treatment of severe HS.40 Concerning certolizumab, we found few articles on the treatment of HS, most of them small case series, generally with favorable results, but with the possibility of publication bias characteristic of the small sample size. Due to its pharmacodynamics, it avoids the placental passage, which makes it an attractive option in pregnant patients with severe HS.41,42 Finally, in relation to anti-TNF agents, golimumab initially demonstrated its usefulness in small case reports and case series,43,44 a fact that was strengthened with the publication of a retrospective series with 13 patients who failed adalimumab and had good results with golimumab, therefore it could be an option in patients with failure to adalimumab.45
Anti-IL-17 agentsIL-17 has been implicated in numerous studies in the pathogenesis of HS.24 In the phase 3 clinical trials SUNSHINE and SUNRISE, secukinumab passed the primary end-point at week 16, with sustained responses at week 52. At 16 weeks, in the SUNSHINE trial, 45% of patients on secukinumab 300mg/14 days achieving HiSCR, compared with 41.8% of patients on secukinumab 300mg/28 days or 33.7% in the placebo group. In the SUNRISE trial, slightly better results at 16 weeks were found for secukinumab 300mg/14 days, with a HiSCR responder of 46.1%, compared with 42.3% in patients with secukinumab 300mg/28 days or 31.2% in the placebo group. The results at week 52 were recently published, with a percentage of patients achieving HiSCR of 56.4% with secukinumab 300mg/14 days and 56.3% with secukinumab 300mg/28 days in the SUNSHINE study, while the percentage of patients achieving HiSCR in the SUNRISE study was 65% with secukinumab 300mg/14 days and 62.2% with secukinumab 300mg/28 days. In both trials, the rate of side effects was mild, the most frequent being headache.46 A recent multicenter retrospective study of forty-seven patients with severe HS demonstrated a 48.9% (n=23/47) of patients achieved objective HiSCR, with adverse events in 6.4% (n=3/47) of the patients.47 This efficacy has been supported in some recent studies with longer observation periods.48 With the demonstration of the decrease in the disease burden of HS by blocking the IL-17A receptor, brodalumab began to be used in its treatment.49,50 Only small case series have been reported, the largest of them with 10 patients to whom 210mg was administered at weeks 0, 1 and 2 and subsequently every 2 weeks. In this study, all patients (100%) achieved target HiSCR, and 80% achieved IHS4 category change at week 12. HiSCR achievement occurred as early as week 2, with a favorable safety profile.51 Recently, therapeutic intensification with brodalumab has been proposed, with a weekly regimen instead of a biweekly regimen.52 Regarding ixekizumab, there are no studies with sufficient evidence to recommend its use in the treatment of severe HS, limiting the literature to small case reports and case series.53,54 Finally, in relation to this pathway, it is worth highlighting the results of bimekizumab, a dual inhibitor of IL 17-A and 17-F, were presented at the 2023 American Academy of Dermatology Annual Meeting, in its two phases 3 (BE HEARD I and BE HEARD II), achieving statistically significant and consistent clinically meaningful improvements over placebo in the signs and symptoms of HS at week 16, which were maintained to week 48, which positions it as a promising therapeutic weapon. At Week 16 in the trials, 47.8% of patients in BE HEARD I and 52% in BE HEARD II experienced the 50% reduction skin abscesses and inflammatory nodules. That compared with 28.7% and 32% for placebo, respectively. At week 48, clinical responses were maintained with continuous bimekizumab treatment, since over 75 percent of patients achieved HiSCR50, and over 55 percent achieved HiSCR75, at week 48. The safety profile of bimekizumab across BE HEARD I and BE HEARD II was consistent with previous studies with no new safety signals observed.55,56 CJM112, another monoclonal antibody that neutralizes soluble IL-17 and IL-17A/F has demonstrated in a phase II randomized controlled study HS-PGA response rate of 32.3% at week 16, compared to 12.5% in patients treated with placebo.57,58
Anti-IL-12 and IL-23 agentsUstekinumab, a dual inhibitor of IL-12 and IL-23 by blocking their common p40 subunit, has demonstrated its efficacy in several HS.59 In addition, an increase in its efficacy has been postulated with intravenous administration with 90mg every 8 or even 6 or 4 weeks, doses like Crohn's disease,60–62 reaching in a retrospective study 50% (n=7/14) objective HiSCR and with a reduction in pain in 71.4% of patients at week 16.62
Anti-IL-1 agentsThe interleukin-1 (IL-1) family is a complex involved in the correct functioning and regulation of the innate immune system, linking innate and adaptative immune responses and its failure is related with psoriasis, HS, and atopic dermatitis. Anakinra, an IL-1 receptor inhibitor, has been shown to be effective in some patient series, especially at doses of 200mg/day.63 In a placebo-controlled clinical trial, at week 12, a >50% decrease in disease activity score was used as the primary end-point, which was achieved by 78% of the treatment group and 30% of the placebo group. However, these results have not been subsequently verified in larger trials and, in addition, early and frequent relapses have been reported.64 Canakinumab, an anti-IL-1β monoclonal antibody, provides controversial data in HS, with small series with positive65 and other negative results.66 Bermekimab (also called MABp1), a specific antibody inhibitor of IL-1α, has demonstrated in a recent phase II clinical trial HiSCR target rates at week 12 of 61% in anti-TNF native patients and 63% in those who had failed to anti-TNF.67 Currently, a new randomized phase II trial (NCT04988308) with this drug is being recruited.68
Anti-IL-23 agentsAs far as anti-IL-23 agents are concerned, guselkumab has been shown to be effective in some case series.69 In a phase IIb placebo-controlled, double-blind study (NOVA-trial, NCT03628924) comparing guselkumab at a dose of 200mg/4 weeks subcutaneously at weeks 0, 4, 8 and 12 versus 1200mg intravenously at weeks 0, 4, 8 followed by 200mg subcutaneously versus placebo, it was observed that HiSCR was achieved at week 16 in 50.8% of the patients treated with subcutaneous administration versus 45% of intravenous ones.70 A recent multicenter, phase IIa trial with guselkumab at 200mg s.c., every four weeks for 16 weeks shows that 65% (n=13/20) achieved HiSCR with also a significant decrease in the median IHS4-score (8.5–5.0, P=0.002) and the median AN-count (6.5–4.0, P=0.002).71 Risankizumab had demonstrated its possible therapeutic use in HS in small case series.72 However, the recently published randomized trial with 243 patients (risankizumab 180mg, n=80; risankizumab 360mg, n=81; placebo, n=82) demonstrated that HiSCR was achieved by 46.8% of patients with risankizumab 180mg, 43.4% with risankizumab 360mg, and 41.5% with placebo at week 16, with the primary end-point not achieved, so the study was early finished.73 Data with tildrakizumab are sparse. The largest series with 9 patients showed its potential therapeutic usage in HS.74
Anti-PDE-4 agentsApremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor, demonstrated its efficacy in a 3:1 trial at a dose of 30mg/12h compared to placebo, reaching 53.3% the HiSCR objective versus 0% in the group placebo.75 These responses were confirmed at the two-year follow-up.76 These results have been supported by other small series but so far, we lack comparative prospective studies with more evidence to support its widespread use.
Complement inhibitorsThe involvement of the complement pathway in the pathogenesis of HS has been demonstrated. Vilobelimab (IFX-1), an anti-C5a monoclonal antibody, showed target HiSCR rates of 75% in an open-label trial.77 Subsequently, in a phase 2 trial (NCT03487276) it has shown a HiSCR of 51.5% at a dose of 800mg IV every 4 weeks, although the response with placebo was 47.1%.78 Avacopan, another oral C5a inhibitor already used in ANCA vasculitis and atypical hemolytic uremic syndrome, is being evaluated in a trial (NCT03852472) against placebo but the results of this study are yet to be published.79
JAK/STAT pathwayWith increasing use in dermatology, a key role of stress signaling and Janus Kinase (JAK)/Signal transducer and activator of transcription 1 (STAT1) activation in disease progression of HS has been described, turning this pathway into a possible therapeutic target. Tofacitinib has been described as a successful treatment of HS in isolated cases.80 In a recent real-life retrospective cohort study, upadacitinib demonstrated 75% (n=15/20) individuals achieved HiSCR50 at week 4, increasing to 100% at week 12 and maintained at week 24, promising results.81 There is an ongoing phase 2 clinical trial evaluating upadacitinib in HS (NCT04430855).82 Only one case of HS treatment with baricitinib has been reported in the literature.83 INCB054707 (povorcitinib) is a new JAK-1 inhibitor that is being evaluated in 3 clinical trials (NCT03569371, NCT03607487 and NCT04476043). In NCT03607487, higher response rates were demonstrated in the 90mg/day group (HiSCR 88%) versus placebo (HiSCR 57%), although the adverse effect rate was substantial, with up to one third of patients grade 3 or 4.84 Lately, its usefulness has also been postulated topically with ruxolitinib,85 as has already been postulated in other entities such as vitiligo or alopecia areata. Its efficacy is being tested in two clinical trials, one of which is in the recruitment phase and the other in progress, using 1.5% topical ruxolitinib.86
Other targetsThere are numerous new targets that are being evaluated for the treatment of HS.87,88 IRAK-4 is a multiple immune regulator, primarily of the innate immune system. KT-474, an IRAK-4 inhibitor, is being evaluated for the treatment of HS in a phase 1 trial (NCT04772885), as well as a phase 2 trial (NCT04092452), a trial with three anti-kinase drugs that also includes Ropasacitinib (PF-06826647), a tyrosine kinase 2 (TYK2) inhibitor, and Brepocitinib (PF-06700841) dual TYK2/JAK1 inhibitor.89 We have not found any studies evaluating tocilizumab or another anti-IL-6 drug in the treatment of HS. IL-36 has been implicated in the pathogenesis of autoinflammatory diseases, autoimmune diseases, and infectious diseases. Its elevation in hidradenitis suppurativa could represent a new therapeutic target for spesolimab and imsidolimab, two anti-IL-36 monoclonal antibodies employed in generalized pustular psoriasis. Both drugs are being evaluated in two phase 2 clinical trials (NCT04762277 spesolimab90 and NCT04856930 imsidolimab91). Rituximab, a monoclonal anti-CD-20 agent, is postulated as a possible therapeutic weapon, its effectiveness being postulated in 5 cases described in the literature.92 Iscalimab (CFZ533) is a monoclonal antibody that blocks the CD-40 pathway, and it is being studied in the treatment of the HS on a phase II trial (NCT03827798).93 LYS 006, an oral leukotriene A4 hydrolase (LTA4) inhibitor, is being evaluated in a phase II trial for the treatment of HS (NCT03827798).93 LY 3041658, a monoclonal antibody that blocks chemokines that bind to CXCR1 and CXCR2 receptors, is also being evaluated in a phase 2 trial (NCT04493502).94 Finally, CSL 324 is a phase 1 anti-G-CSF antibody for the treatment of HS and palmoplantar pustulosis (NCT03972280).95
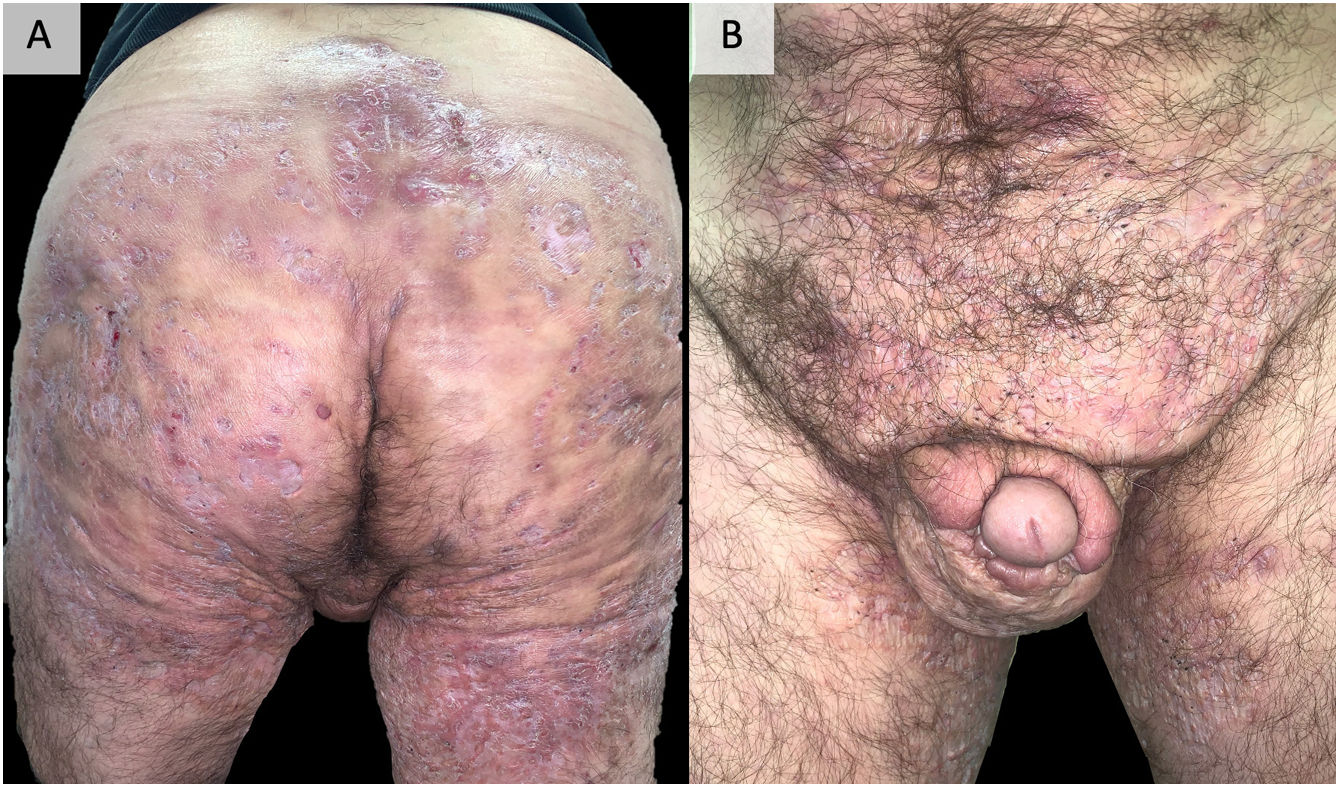
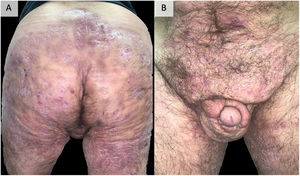
DiscussionEarly recognition and treatment of HS is essential to prevent its progression to a chronic phase, with the presence of organized fistulous tracts that are sometimes refractory to all types of medical treatment and require surgery that is sometimes mutilating96 (Fig. 2). Added to the disease and its severity, its multiple comorbidities, and the highest unemployment rate among those who suffer from it make this impact even more severe, affecting all aspects of quality of life.97 The accurate management of this disease requires a multifactorial approach, starting from patient education, insisting on the abolition of triggering and precipitating factors, especially tobacco and obesity. In most cases, topical management or small courses of oral antibiotics satisfactorily control the disease. In addition, in our experience, performing surgeries in the form of drainage or deroofing helps to clinically resolve the lesions. However, in those patients who do not respond to these measures, the use of biological or small molecule drugs is required, highlighting adalimumab as the only drug currently approved by the main drug regulatory agencies. When adalimumab fails, or is contraindicated, we find a gap in the literature, being complex patients and treated with great variability between centers, using different targets, including anti-IL-17, IL-23, IL-1 drugs, inhibitors of the JAK or PDE-4 pathway or numerous other targets under study.86,87 The literature provides imprecise results for the different targets. The recent favorable results obtained for secukinumab46,47 and bimekizumab55,56 in phase 3 clinical trials suggest that they may be the next drugs to be approved by the FDA and the EMA for the treatment of severe hidradenitis suppurativa. In this way, IL-17 would be placed as the next target after anti-TNF failure. Comparative studies between both alternatives would be necessary. Other options with outstanding results, although in lower-level studies and therefore far from being approved, are infliximab34–37 (especially at high doses of 10mg/kg/4 weeks37) and ustekinumab59 (particularly with the first intravenous administration and with doses of illness inflammatory bowel60–62). The results obtained by apremilast75,76 are also promising, even though its trial has a small number of patients or, in relation to newer targets, JAK kinase inhibitors, particularly upadacitinib81,82 and povorcitinib.84 In this last sense, it is also worth noting the possibility of using small molecule topical treatment with ruxolitinib,85 a trial that is still ongoing.86 This range of therapies is a faithful reflection of the multifactoriality of the disease and highlights the need for new clinical trials and prospective studies that allow clarifying the therapeutic horizon of severe hidradenitis suppurativa with failure or contraindication to adalimumab. The therapeutic future of HS is promising.
Example of extremely serious hidradenitis suppurativa. A 48-year-old man with HS of >20 years’ duration, refractory to multiple treatments, including high-dose adalimumab, infliximab, and ustekinumab. Currently, in treatment with bimekizumab. Photographs taken and published with the oral and written consent of the patient.
The present review is limited by being narrative and not a systematic review of the literature or a meta-analysis of the main studies. In addition, except for the most widely used drugs, the rest of the therapeutic arsenal presented here is often based on small case series or clinical trials in very preliminary phases.
ConclusionsHidradenitis suppurativa is a very complex multifactorial disease, and its approach requires a combination of topical, systemic, and surgical medical treatment. Currently, the only biologic or small molecule drug approved for its treatment is adalimumab. In patients with failure or contraindication for adalimumab, there are numerous therapeutic options that cover various targets, most of them pro-inflammatory cytokines. New prospective studies and comparative trials are needed to elucidate a standardized therapeutic algorithm for patients with severe hidradenitis suppurativa.
Conflicts of interestThe authors declare that they have no conflicts of interest.
None.