The patient was an 87-year-old woman with multiple myeloma who was receiving treatment with bendamustine and prednisone. She was seen for a lesion on her upper lip that had first appeared 2 weeks previously, coinciding with the last cycle of chemotherapy. She had previously had painful vesicles on the hard palate and tip of the tongue.
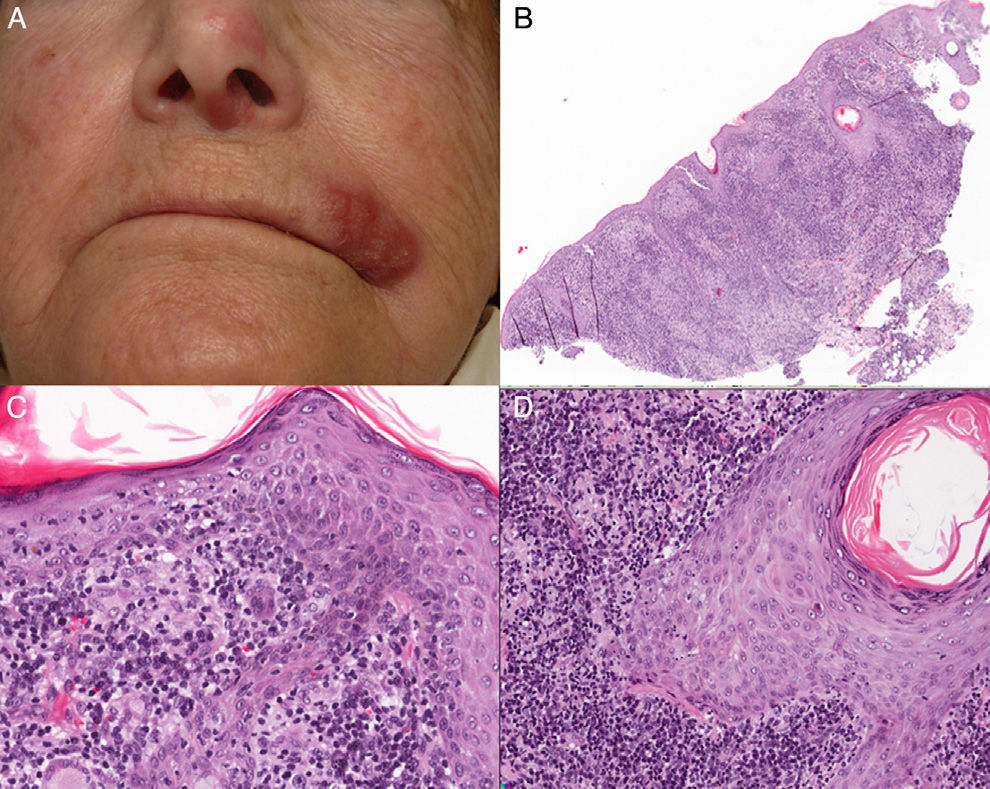
Physical ExaminationPhysical examination revealed an indurated erythematous-edematous plaque and crusting vesicles on the left side of the upper lip and an erythematous macule on the nasal septum and left nasal ala (Fig. 1A).
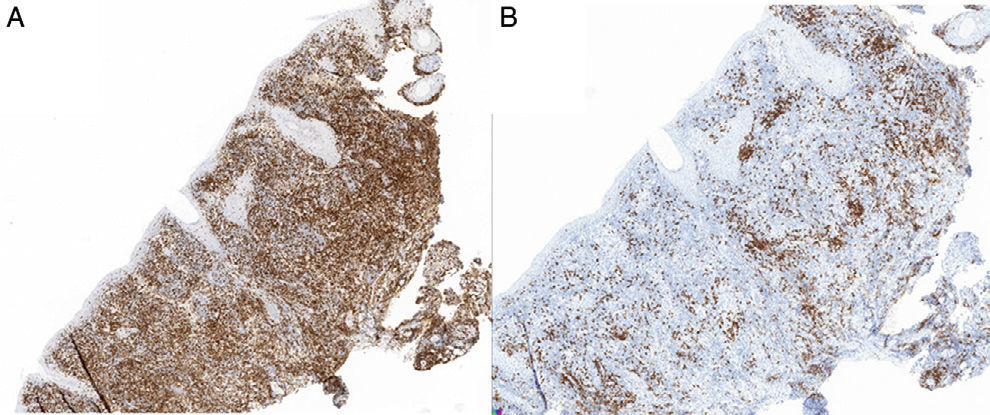
HistopathologyHistopathology revealed an epidermis with mild acanthosis, spongiosis, and exocytosis in the absence of cytopathic changes. The follicular epithelium contained foci of vacuolar degeneration and solitary apoptotic keratinocytes. A dense lymphoplasmacytic infiltrate with noncaseating granulomas was visible in the dermis (Figs. 1B-D). Histochemistry did not reveal germs. The lymphoid infiltrate comprised predominantly CD3 cells (Figs. 2A and B), with CD30+ cells and no light chain restriction. Immunohistochemistry for herpes simplex virus (HSV) types 1 and 2 was negative. Polymerase chain reaction of the exudate for determination of HSV was negative, although it proved to be positive for HSV-1 in the biopsy specimen.
What is your diagnosis?
DiagnosisAtypical granulomatous herpes simplex infection.
Clinical Course and TreatmentGiven the clinical suspicion of HSV infection in an immunosuppressed patient, treatment was started with famciclovir 750mg/d for 14 days. The patient's clinical condition improved, with mild residual erythema remaining.
CommentCutaneous eruptions caused by HSV-1, HSV-2, and varicella-zoster virus are common. Diagnosis is generally based on the presence of characteristic vesicles. All 3 viruses are histopathologically indistinguishable. The most commonly observed entities are balloon-like basal keratinocytes with multinuclear nuclei, ground glass appearance, and a vacuolated eosinophilic cytoplasm. Secondary acantholysis, epidermal necrosis, ulceration, and leukocytoclastic vasculitis are also observed.1 However, in immunosuppressed patients and patients with cancer and blood diseases in particular, lesions can also present as plaques, papules, nodules, tumors, and ulcers that tend to be chronic. In such cases, therefore, it is common practice to take biopsy specimens,2 and histology findings may be similar to those of benign and malignant lesions, with little or no epidermal involvement. Various findings have been proposed as diagnostic criteria in these patients, including involvement of adnexal structures with a vacuolar interface pattern and periadnexal infiltrate, as well as keratinocytic necrosis, multinucleation, syringotropism, and perineural symptoms.1,3,4
The lesion rarely presents as granulomatous dermatitis. In fact, in a recent review that analyzed the histopathologic characteristics of HSV, this presentation was found in only 1 out of 65 cases studied.3
Furthermore, in some series, the presence of atypical lymphocytes has been reported in more than 65% of cases. These cells can express CD30, thus mimicking CD30+ lymphoproliferative disorders.3 In rare cases, HSV infection leads to a CD56+ lymphocyte–rich infiltrate, thus mimicking NK/T lymphoma; in other cases it mimics B-cell lymphoma and plasmacytoma. Herpes infection can also mimic inflammatory conditions such as lupus, insect bites and stings, rosacea, erythema multiforme, and linear IgA bullous dermatosis. In these conditions, confirming a diagnosis requires a high degree of suspicion, and performance of immunohistochemistry testing for herpes can help, although its sensitivity is lower in the absence of vesicles, as is the case for polymerase chain reaction.4
Furthermore, there have been reports of inflammatory oncotaxis in areas affected by herpesvirus infection in patients with leukemia (especially B-cell chronic lymphocytic leukemia) and neoplastic plasma cell infiltrate in affected areas of patients with myeloma (ruled out in the present case). It was also necessary to differentiate our findings from the Wolf isotopic response, where several types of dermatosis, mainly granulomatous dermatitis, affect an area that had previously been infected by herpesvirus, mainly herpes zoster infection, but that was now healed.5 The latency period of both conditions ranges from a few weeks to years.6 The underlying mechanism of this entity could be a delayed hypersensitivity reaction, since viral DNA is not usually identified and outcome is not affected by antiviral treatment.6
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We are grateful to the staff of the Histopathology Service of Hospital General Universitario de Ciudad Real for their help.
Please cite this article as: Ramos-Rodríguez C, González-López L, García-Arpa M. Placa infiltrada en labio superior de paciente con mieloma múltiple. Actas Dermosifiliogr. 2017;108:61–62.