Sjögren–Larsson syndrome (SLS) is a rare genetic disorder with autosomal recessive inheritance,1,2 characterized by clinical triad of congenital ichthyosis, spastic diplegia or tetraplegia and mental retardation.1,3,4 We report a new patient affected by SLS due to two unreported mutations.
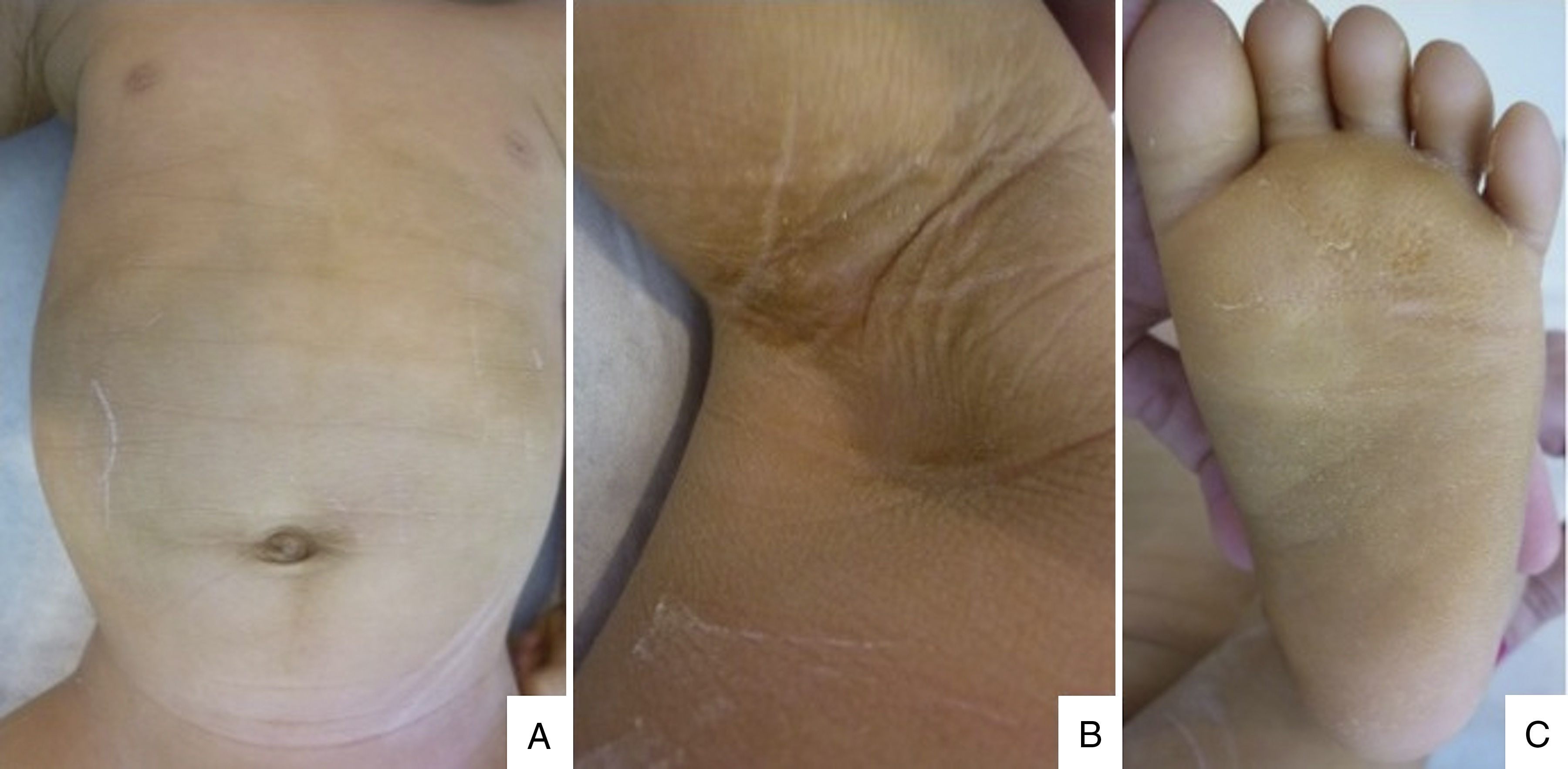
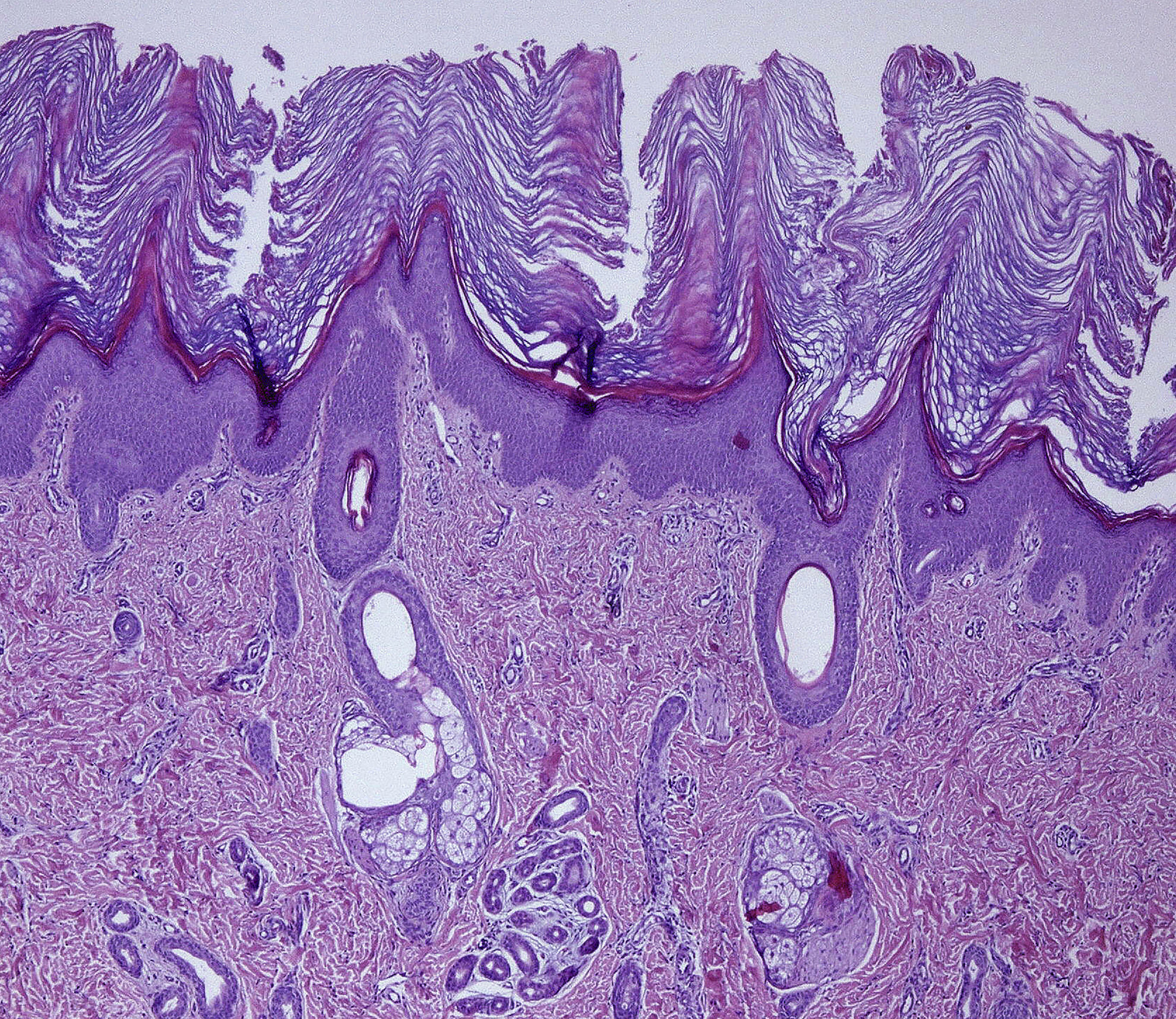
A 6 months male child was referred to our hospital because of congenital erythroderma and subsequent development of generalized fine scaling and persistent pruritus. He was the first child of non-consanguineous parents, born at 33 weeks of gestation. There was no history suggestive of a collodion membrane at birth, and no family history of ichthyosis. On physical examination we observed generalized fine desquamation on the limbs and trunk without underlying erythema (Fig. 1A) and hyperkeratotic skin on the armpits (Fig. 1B), palms and soles (Fig. 1C). Hair and nails appeared normal. Given that an ichthyosis was the diagnosis suspected, a skin biopsy from an armpit was made. Histological study showed hyperkeratosis, psoriasiform epidermal hyperplasia, a prominent granulous layer in some areas of the epidermis and mild perivascular inflammatory infiltrate of mononuclear cells in papillary dermis (Fig. 2), resulting compatible with a congenital ichthyosis. The rest of the physical exam showed the patient had macrocephaly, mild psychomotor retardation, mild axial hypotonia, incipient signs of spasticity in the lower limbs, and occasional spasms. A transfontanelar ultrasound showed benign external hydrocephaly, which was confirmed in a brain magnetic resonance imaging where others anomalies were not observed. Given the coexistence of congenital ichthyosis and neurological signs, our clinical suspicion was a neurocutaneous disorder. Ophthalmologic examination, peripheral blood smear, peripheral blood analysis and abdominal ultrasound were normal. Genetic analysis of the patient confirmed the diagnosis of Sjögren–Larsson syndrome (SLS) by identifying two unreported heterozygous ALDH3A2 mutations, a deletion mutation c.154_155delAG (p.Ser52Stop) in exon 2 and a missense mutation c.536A>T (p.Asp179Val) in exon 4. The c.154_155delAG mutation is considered of pathogenic nature, and the variant c.536A>T is likely to cause disease by several in silico analysis. Despite the genetic counseling, the patient's parents did not want to perform the genetic study in that moment. Progressively, the neurological symptoms were worsening by detecting hyperreflexia, spasticity and delay speech in our patient.
In 1957, Karl Gustaf Torsten Sjögren, on collaboration with Tage K. Larsson, established the clinical and genetic profile of the Sjögren–Larsson syndrome (SLS).5 SLS is a recessively inherited neurocutaneous disorder characterized by a triad of congenital ichthyosis, mild to moderate mental retardation and spastic diplegia or tetraplegia, caused by a fatty aldehyde dehydrogenase (FALDH) deficiency.4,6,7 It occurs in all races and its prevalence worldwide has been estimated as 0.4:100,000 live births.1,4
SLS is caused by mutation in the ALDH3A2 gene on the short arm of chromosome 17 (17p11.2), that is the gene for FALDH which catalyzes oxidation of long chain aliphatic alcohols to corresponding fatty acids.3,8 The consequent accumulation of fatty aldehyde precursors, including fatty alcohols, caused by the FALDH deficiency, is postulated to affect the normal formation of multilamellar membranes in the stratum corneum and myelin, and to result in the symptoms.7
The disorder presents at birth or in the neonatal period with varying degrees of erythema and ichthyosis, but a collodion membrane is rarely seen. Ichthyosis has a generalized distribution across the trunk, flexures and nape of neck, although the central face is spared in most cases.1 Palmoplantar keratoderma is seen in 50% of cases.3 The nails and hair are interestingly normal.6 Persistent pruritus is common, which is mostly absent in other forms of ichthyosis.1,4,8 The histological findings of hyperkeratosis, papillomatosis, acanthosis, and a mildly thickened granular layer are nonspecific.5
The diagnosis of SLS is delayed until the onset of neurological symptoms, because only cutaneous manifestations are present at birth.3 The neurological symptoms appear in the first or second year of life,3,4 and include cognitive impairment, brain magnetic resonance imaging (MRI) findings, speech–language development and spasticity.1 One-third of patients present with perifoveal glistening white dots in the ocular fundus which appear after several years of age,3 and their occurrence strongly suggests SLS.4 Macrocephaly is not a characteristic or common finding in SLS.
Mutation analysis of the ALDH3A2 gene is a highly sensitive method of confirming the diagnosis of SLS.4 More than 90 pathogenic variants of ALDH3A2 have been identified to date.1 The diagnosis of SLS can be confirmed by measurement of enzyme activity in cultured skin fibroblasts or leukocytes.
There is no permanent cure for SLS and no specific therapy, so that a multidisciplinary approach is necessary.3
In conclusion, we report a new case of SLS caused by two novel mutations, supporting the rich mutational heterogeneity associated with this syndrome. High index of suspicion is necessary for the diagnosis of SLS, so that in a neonate or infant with congenital ichthyosis and neurological symptoms we must rule out this neurocutaneous disorder.
Conflict of interestsThe authors declare no conflict of interest.