Sonidegib is an antagonist of the transmembrane protein Smoothened in the Hedgehog signaling pathway. It is indicated for the treatment of locally advanced basal cell carcinoma (BCC) that is not amenable to curative surgery or radiotherapy. Sonidegib’s efficacy and safety were demonstrated in the phase 2 BOLT trial, where 61% (95% CI, 48%–72%) of patients with locally advanced BCC treated with sonidegib 200 mg achieved an objective response to treatment with a mean time to response of 4 months. The median duration of response was 26.1 months and the median progression-free survival was 22.1 months. The most common adverse events were muscle spasms (54.4%), hair loss (49.4%), and loss of taste (44.3%); most events were grade 1 or 2. In this review, we summarize the main findings on the efficacy, safety, and tolerability of sonidegib and discuss the management of locally advanced BCC with this drug.
Sonidegib es un inhibidor del receptor transmembrana Smoothened (SMO) de la vía de señalización de Hedgehog indicado para el tratamiento del carcinoma basocelular localmente avanzado (CBCla) no susceptible a cirugía curativa ni a radioterapia. Sonidegib ha demostrado su eficacia y seguridad en pacientes con CBCla en el ensayo de fase II (BOLT), donde el 61% (IC95%: 48; 72) de los pacientes tratados con 200 mg de sonidegib tuvo una respuesta objetiva al tratamiento, con un tiempo medio hasta la respuesta de 4 meses. La mediana de duración de respuesta fue de 26,1 meses y la mediana de supervivencia libre de progresión fue de 22,1 meses. Los eventos adversos más frecuentes fueron los espasmos musculares (54,4%), alopecia (49,4%) y disgeusia (44,3%), siendo principalmente de grado 1 y 2. Esta revisión proporciona un resumen sobre la eficacia, seguridad y tolerabilidad de sonidegib, así como consideraciones sobre su uso en el manejo de pacientes con CBCla.
Basal cell carcinoma (BCC) is the most frequent malignant neoplasm in humans and it accounts for 75% of all skin cancers. Its annual incidence is highest in Australia, followed by the United States, and Europe, and ranges from 70-150/100000 population, with increasing incidence with age.1 It is estimated that the incidence of BCC increases annually by 6.8% (95% CI, 5.3%-8.3%) in men and 7.9% (95% CI, 6.2%-9.7%) in women, due to increased exposure to ultraviolet (UV) radiation in sunlight and artificial light sources,2 increased outdoor sporting and recreational activities,3 increased life expectancy, and depletion of the ozone layer.4,5 In Spain, the estimated incidence of BCC in 2016 was 113.05/100000 population; this figure only includes histologically confirmed primary tumors in each patient.6 In addition to exposure to UV radiation and age, BCC has been associated with other risk factors, such as fair skin phototype (I and II), family history, exposure to ionizing radiation, arsenic intake, immunosuppression, and some genodermatoses.7
BCC is characterized as an extremely heterogeneous tumor with several clinical presentations and histologic subtypes. It generally does not follow an aggressive course, with slow and localized growth. It usually develops in parts of the body exposed to sun, with the head and neck being the most frequently affected (80%). The tumor is derived from undifferentiated and pluripotent stem cells in the basal cell layer of the epidermis and pilosebaceous follicles, regulated by the Hedgehog (Hh) signaling pathway. Basal epithelial tumor cells form groups surrounded by stroma with different growth patterns, giving rise either to slow-growing BCC with nodular and superficial subtypes or aggressive and invasive growth with morpheaform, invasive, micronodular, and basosquamous subtypes.7–9 The tumor can present with a single histologic pattern or a combination of patterns, in which case it is denoted as being of mixed histology.7
Prognosis for BCC is usually favorable and treatment is essentially surgical, although depending on different factors, radiotherapy, photodynamic therapy, and topical imiquimod can be used. However, BCC can at times progress locally, invading adjacent structures (locally advanced BCC [laBCC]) or metastasizing (metastatic BCC). It is estimated that up to 0.8% of all cases of BCC correspond to laBCC. In Spain, in 2017, laBCC was estimated to be present in at least 500 people.6 Patients with laBCC usually have a long history of untreated BCC or repeated treatment failures and recurrence, characterized by the difficulty or impossibility of eliminating the tumor by means of surgery or radiotherapy (Table 1).10,11 In these cases, the usual therapeutic options are ineffective, leading to major functional, emotional, and cosmetic impact, with a negative effect on the quality of life of the affected patients. Invasion of locoregional structures may lead to functional impotence, pain, and chronic insomnia that is difficult to control and a negative esthetic impact, often in the cephalic pole, causing anxiety, depression, and social exclusion.12 For these reasons, other tools are needed for control of the tumor, such as inhibitors of Hh signaling.
Criteria for Defining When Surgery and Radiation Are Considered Inappropriate in laBCC11.
| > 5 BCCs, if the patient has genetic syndromes |
| BCC > 10 mm, recurrence after 2 operations at critical sites (for example, periocular and perioral area) |
| BCC with invasion of bone/cartilage/other structures and curative resection unlikely to be successful |
| BCC recurrent after multiple operations and/or radiotherapy |
| laBCC in patients who cannot undergo general anesthetic |
Abbreviations: BBC, basal cell carcinoma; laBCC, locally advanced basal cell carcinoma.
The Hh signaling pathway was first observed in 1980 in larvae of Drosophila melanogaster, in which mutations to the Patched and Hedgehog genes, which encode this signaling pathway, led to abnormal embryonic development.13 In different species, this pathway plays a crucial role in organogenesis during embryogenesis, but in adults, it is only active in hair follicles, skin, and stem cells, where it plays a major role in maintaining tissue homeostasis and cell repair.14
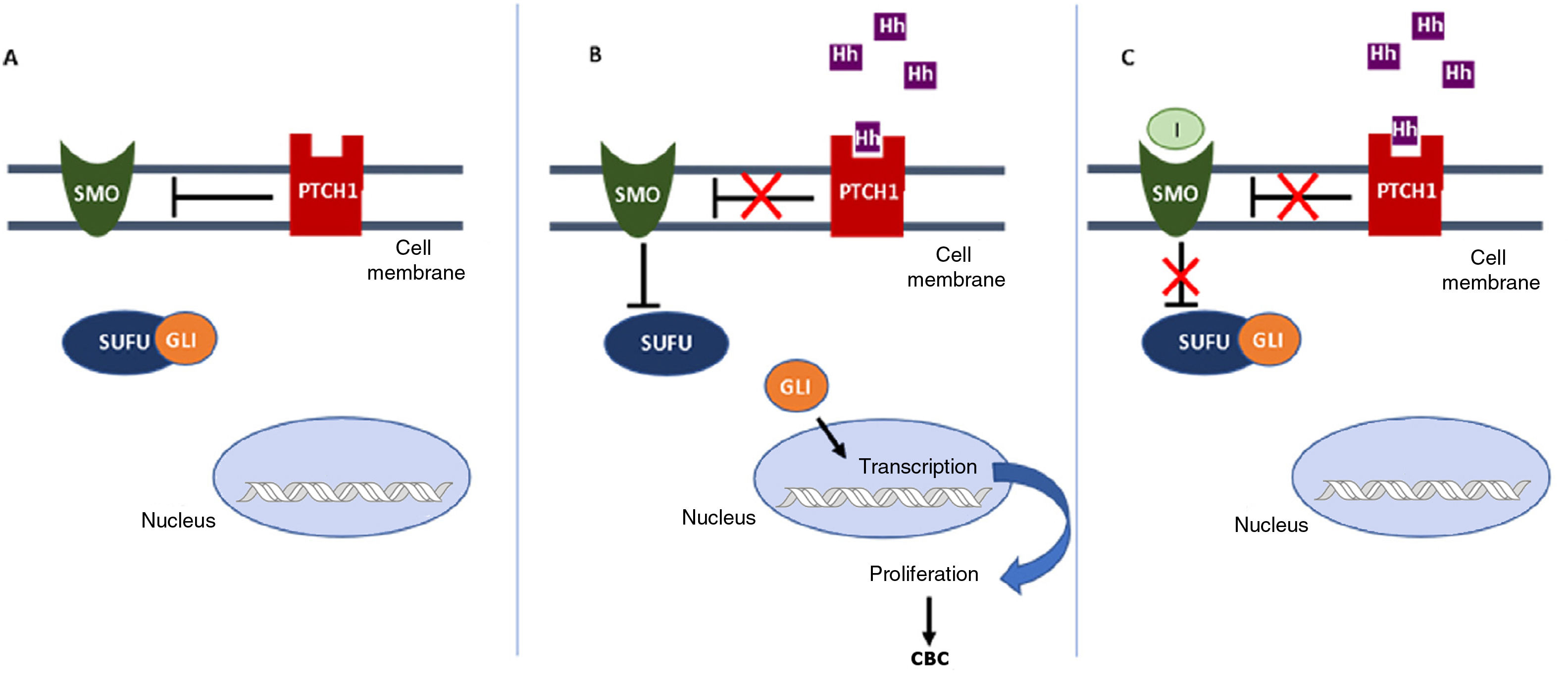
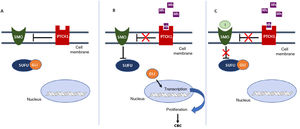
In the Hh pathway, the Patched 1 (PTCH1) transmembrane receptor, located in the primary cilia of the cells, in absence of ligand, inhibits the Smoothened (SMO) protein, a transmembrane receptor that acts as a signal transducer (Fig. 1A). When an Hh ligand binds to PTCH1, SMO inhibition disappears. SMO acts on the suppressor of fused homolog (SUFU) mediator protein, enhancing the activation of GLI transcription factors and inducing transcription of genes implicated in increased cell survival and mitosis (Fig. 1B).15
Activation and deactivation of the Hedgehog (Hh) signaling pathway. A, In absence of Hh ligands, the PTCH1 receptor inhibits SMO, allowing the SUFU protein to bind and inhibit the GLI transcription factors. B, Binding of Hh ligands to PTCH1 suppresses SMO inhibition; as a result SUFU is inhibited, dissociating the complex and activating the GLI transcription factors, which translocate to the nucleus and transcription of genes associated with Hh signaling implicated in increased cell survival and mitosis is induced. C, In presence of Hh ligands, which activate the Hh pathway, inhibitors block SMO activation, allowing suppression of the GLI transcription factors by SUFU and transcription of genes associated with the Hh pathway.
Abbreviations: GLI, GLI transcription factor; Hh, Hedgehog ligand; I, SMO inhibitors such as sonidegib and vismodegib; PTCH1, Patched 1 transmembrane receptor; SMO, Smoothened protein; SUFU, suppressor of fused homolog protein.
Aberrant activation of the Hh signaling pathway is the trigger for the pathogenic process in more than 90% of BCCs and is also responsible for triggering other types of tumor such as neuroblastomas, gliomas, and rhabdomyosarcomas.16 Abnormalities that can arise within the pathway include increased expression of the Hh ligand, which directly increases signaling, or genetic alteration of the regulatory proteins PTCH1 and SMO, giving rise to activated receptors, also leading to increased signal transduction. Most BCCs arise due to sporadic mutations in one of the implicated genes, with the most frequent being those in the PTCH1 (80%) and SMO (10%-20%) genes.16 There are also hereditary cases, such as Gorlin syndrome (GS) or basal cell nevus syndrome.17 GS is a hereditary disease of autosomal dominant transmission characterized by development of multiple BCCs at young ages, due mainly to mutations in the PTCH1 or SUFU gene.18 Most patients with GS are born with a mutation inherited from one of the alleles of PTCH1 and, subsequently, a second acquired mutation in a healthy allele because of external factors such as UV light, which is responsible for development of tumors.19
Inhibition of the Hh signaling pathway is a key therapeutic target in the treatment of laBCC, where surgery and radiotherapy cannot be used. Vismodegib and sonidegib inhibit the Hh pathway by binding to and deactivating the SMO protein, thus suppressing tumor growth (Fig. 1C). This review will summarize the efficacy, safety, and tolerability of sonidegib, as well as consider some points of importance in its use to treat patients with laBCC.
Sonidegib: Hh Pathway InhibitorSonidegib, marketed as Odomzo®, was approved by the United States Food and Drug Administration (FDA) and the European Commission after assessment by the European Medicines Agency (EMA) in July and August, respectively, of 2015. It is indicated for treatment of adult patients with laBCC who are not candidates for curative surgery or radiotherapy. The recommended dose is 200 mg of sonidegib, once a day, administered orally, separated from meals.
Clinical Efficacy of Sonidegib in Locally Advanced BCCThe efficacy and safety of sonidegib were evaluated in a phase I trial and a pivotal phase II trial, BOLT. This review will focus on the results of sonidegib at a dose of 200 mg in patients with laBCC, as this is the approved dose and patient population.
The phase I trial was a multicenter dose-escalation study to assess the efficacy and safety of sonidegib in a total of 103 patients with advanced solid tumors, including 16 patients with BCC. The maximum tolerated dose (MTD) was 800 mg per day, as a single dose, or 250 mg twice a day. Moreover, sonidegib caused a dose-dependent reduction in expression of GLI1 messenger RNA, explaining its antitumor activity. Objective tumor response was reported in 6 patients (37.5%), and this response was associated with evidence of Hh pathway acitvation.20
The BOLT trial was designed to evaluate the efficacy and safety of sonidegib in patients with laBCC or metastatic BCC who were not candidates for curative surgery or radiotherapy.21–24 Patients were randomized (1:2) to receive a daily dose of sonidegib 200 mg or 800 mg until disease progression or unacceptable toxicity. Overall, 230 patients with advanced BCC were included in the trial. Of these, 194 (84.3%) had laBCC. The primary efficacy outcome measure was objective response rate (ORR), assessed by central review, according to the modified response evaluation criteria in solid tumors (mRECIST), which are stricter than the RECIST criteria, for detection of evidence of disease.11 However, the response was also assessed in a predefined analysis with criteria similar to RECIST, used in the pivotal trial of vismodegib (ERIVANCE).
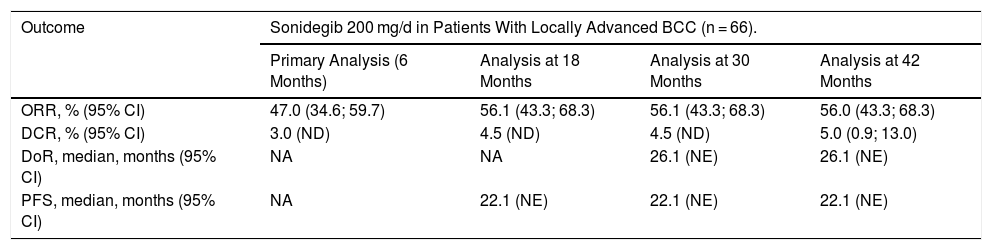
According to the final analysis at 42 months in the sonidegib 200 mg arm, by central review, 56% (95% CI, 43%-68%) and 61% (95%, 48%-72%) of patients with laBCC had objective response (ORR), assessed by the mRECIST and RECIST criteria, respectively (Table 2). The investigator-assessed ORR was higher: 60% (95% CI, 47%-72%) and 74.2% (95% CI, 62%-84%), according to the mRECIST and RECIST criteria, respectively.23,25 Efficacy was similar among patients with laBCC with aggressive and nonaggresive histologic subtypes.21 The median duration of response (DoR) was 26.1 months and the median progression-free survival (PFS) was 22.1 months. The mean time until tumor response was 4 months and the mean follow-up duration was 50 months, the longest follow-up published to date for an Hh inhibitor. The efficacy results were consistent during the course of the trial (Table 3).23 Additionally, patients treated with sonidegib maintained or improved their quality of life, an aspect of great importance in a tumor with a high morbidity but low mortality.26
Comparison of Tumor Response to Sonidegib 200 mg Assessed by Central Review According to mRECIST and RECIST Criteria in Patients with Locally Advanced BCC (Analysis at 42 Months)25,35.
| Efficacy Outcome | Sonidegib 200 mg/d in Patients With Locally Advanced BCC (n = 66). Central Review at 42 Months Follow-up | |
|---|---|---|
| mRECIST Criteria | RECIST Criteria | |
| ORR, % (95% CI) | 56.1 (43.3; 68.3) | 61 (48; 72) |
| CR, % (95% CI) | 4.5 (0.9; 12.7) | 21 (12; 33) |
| PR, % | 51.5 | 39 |
| SD, % | 34.8 | 30 |
| DCR, % | 90.9 | 91 |
Abbreviations: BCC, basal cell carcinoma; CI, confidence interval; CR, complete response; DCR, disease control rate; mRECIST, modified response evaluation criteria in solid tumors; ORR, objective response rate; PR, partial response; RECIST, response evaluation criteria in solid tumors; SD: stable disease.
Efficacy of Sonidegib 200 mg in Patients With Locally Advanced BCC Assessed by Central Review21,23.
| Outcome | Sonidegib 200 mg/d in Patients With Locally Advanced BCC (n = 66). | |||
|---|---|---|---|---|
| Primary Analysis (6 Months) | Analysis at 18 Months | Analysis at 30 Months | Analysis at 42 Months | |
| ORR, % (95% CI) | 47.0 (34.6; 59.7) | 56.1 (43.3; 68.3) | 56.1 (43.3; 68.3) | 56.0 (43.3; 68.3) |
| DCR, % (95% CI) | 3.0 (ND) | 4.5 (ND) | 4.5 (ND) | 5.0 (0.9; 13.0) |
| DoR, median, months (95% CI) | NA | NA | 26.1 (NE) | 26.1 (NE) |
| PFS, median, months (95% CI) | NA | 22.1 (NE) | 22.1 (NE) | 22.1 (NE) |
Abbreviations: BCC, basal cell carcinoma; CRR, complete response rate; DoR, duration of response; NA, not available; NE, not evaluable; NR, not reached; ORR, objective response rate; PFS, progression-free survival.
Moreover, the BOLT trial initially showed that sonidegib was effective in GS; 8 of 13 patients with this tumor in the 800 mg group had complete clinical remission of BCC.21 Efficacy was confirmed recently in a randomized exploratory trial that evaluated the efficacy and safety of sonidegib in 10 patients with GS over 12 weeks. All patients (n = 7) treated with 400 mg sonidegib per day achieved complete clinical remission (n = 3, 43%) or partial clinical remission (n = 4, 57%) of BCC assessed at 16 weeks.27
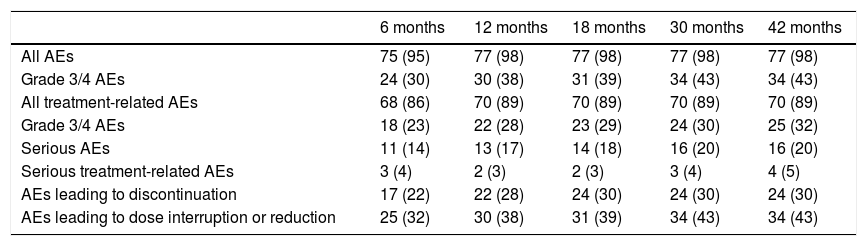
Safety and Tolerability Profile of SonidegibThe safety and tolerability of sonidegib were assessed in the BOLT trial, with the 200 mg dose of sonidegib better tolerated than the 800 mg dose. In the 200 mg arm, the median duration of exposure to the drug was 11 months. Overall, 98% of patients experienced at least one adverse event (AE), although these were mainly grade 1 or 2, manageable, and reversible on temporarily interrupting treatment or reducing the dose. The most frequently reported AEs were muscle spasms (54.4%), alopecia (49.4%), and dysgeusia (44.3%).23 The incidence of AEs was similar to that observed in analyses prior to the trial (Table 4).
Adverse Events Reported With 200 mg Sonidegib During the BOLT Trial23.
| 6 months | 12 months | 18 months | 30 months | 42 months | |
|---|---|---|---|---|---|
| All AEs | 75 (95) | 77 (98) | 77 (98) | 77 (98) | 77 (98) |
| Grade 3/4 AEs | 24 (30) | 30 (38) | 31 (39) | 34 (43) | 34 (43) |
| All treatment-related AEs | 68 (86) | 70 (89) | 70 (89) | 70 (89) | 70 (89) |
| Grade 3/4 AEs | 18 (23) | 22 (28) | 23 (29) | 24 (30) | 25 (32) |
| Serious AEs | 11 (14) | 13 (17) | 14 (18) | 16 (20) | 16 (20) |
| Serious treatment-related AEs | 3 (4) | 2 (3) | 2 (3) | 3 (4) | 4 (5) |
| AEs leading to discontinuation | 17 (22) | 22 (28) | 24 (30) | 24 (30) | 24 (30) |
| AEs leading to dose interruption or reduction | 25 (32) | 30 (38) | 31 (39) | 34 (43) | 34 (43) |
Data expressed as n (%). The results are derived from the safety population.
Abbreviation: AE, adverse events.
Overall, AEs were responsible for 30% of treatment discontinuations, with more than half being grade 1 or 2.21 The AEs that most frequently led to treatment discontinuation were muscle spasms (5%), asthenia (4%), dysgeusia (4%), and nausea (4%). Early-onset AEs were fatigue and muscle spasms (time to onset, 1.08 and 2.07 months, respectively) while late-onset AEs were weight loss and diarrhea (time to onset, 6.47 months in both cases).11
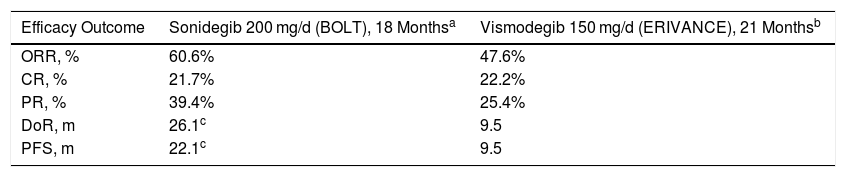
Efficacy and Safety of Sonidegib, in PerspectiveThe availability of Hh pathway inhibitors, vismodegib and sonidegib, has transformed the management of patients with laBCC, as reflected by the reference clinical guidelines.10 However, given the lack of randomized, controlled trials with standard treatment or head-to-head trials, clinical differences between the 2 have not been established. Therefore, a committee of internationally renowned experts in the management of BCC undertook a comparative assessment based on the evidence available for the 2 inhibitors.11 In the comparison of efficacy data, according to the centrally-assessed RECIST criteria from the pivotal BOLT trial (sonidegib) and ERIVANCE trial (vismodegib),28 sonidegib and vismodegib showed an ORR of 60.6% and 47.6%, respectively. Despite the methodological difficulties in making comparisons, notable differences between sonidegib and vismodegib were also observed for DoR (26.1 and 9.5 months, respectively) and PFS (22.1 and 9.5 months, respectively) (Table 5).
Comparison of the Centrally Assessed Main Efficacy Outcome Measures Between Sonidegib (BOLT) and Vismodegib (ERIVANCE) Trials According to the RECIST Criteria11.
| Efficacy Outcome | Sonidegib 200 mg/d (BOLT), 18 Monthsa | Vismodegib 150 mg/d (ERIVANCE), 21 Monthsb |
|---|---|---|
| ORR, % | 60.6% | 47.6% |
| CR, % | 21.7% | 22.2% |
| PR, % | 39.4% | 25.4% |
| DoR, m | 26.1c | 9.5 |
| PFS, m | 22.1c | 9.5 |
Abbreviations: CR, complete response; DoR, duration of response; ORR, objective response rate; PFS, progression-free survival; PR, partial response.
In the case of tolerability, both the profile of AEs and percentage of treatment withdrawals due to AEs were similar for both agents, with a slightly lower incidence and severity and slightly higher time to onset in most categories for sonidegib (Table 6). Even so, the biggest difference between sonidegib and vismodegib lies in their pharmacokinetic profile, where the evidence suggests that sonidegib is more widely distributed in the skin than vismodegib, and this could explain the observed differences in efficacy and safety in favor of sonidegib.11 With the available evidence, the authors recognized the difficulty in drawing conclusions and suggest that this analysis is to provide context for the results. Nevertheless, there have been reports of complex cases treated with sonidegib,29,30 notably including patients treated with vismodegib as first-line therapy, in whom replacement by sonidegib enabled AEs to be controlled and treatment continued.31,32
Frequency and Time to Onset of the Most Common AEs with Sonidegib (BOLT, 30 Months) and Vismodegib (ERIVANCE, 21 Months)11.
| Adverse Event | Sonidegib 200 mg/d (BOLT), 30 Months | Vismodegib 150 mg/d (ERIVANCE), 21 Months | ||
|---|---|---|---|---|
| Frequency (All Grades), % | Mean Time to Onset of AE, Months (95% CI) | Frequency (All Grades), % | Mean Time to Onset of AE, Months (95% CI) | |
| Muscle spasms | 54.4 | 2.07 (1.87; 3.19) | 71.2 | 1.89 (1.35; 2.73) |
| Alopecia | 49.4 | 5.55 (4.70; 6.41) | 65.4 | 3.38 (2.83; 4.11) |
| Dysgeusia | 44.3 | 3.71 (2.76; 4.90) | 53.8 | 1.48 (0.99; 2.07) |
| Nausea | 39.2 | 3.22 (1.51; 4.63) | 32.7 | 2.14 (0.59; 6.67) |
| Weight loss | 30.4 | 6.47 (4.70; 8.31) | 50.0 | 6.13 (4.50; 7.36) |
| Elevated CPK | 15.2 | 2.58 (0.95; 5.59) | Not measured | Not measured |
| Fatigue | 30.4 | 1.08 (0.53; 3.68) | 40.4 | 2.79 (1.35; 3.75) |
| Diarrhea | 31.6 | 6.47 (1.35; 10.32) | 26.0 | 4.47 (2.27; 6.51) |
| Loss of appetite | 22.8 | 3.60 (1.02; 6.51) | 22.8 | 2.87 (1.38; 4.50) |
Abbreviations: AE, adverse event; CI, confidence interval; CPK, creatinine phosphokinase.
The frequency and importance of AEs associated with inhibition of the Hh pathway could be a limiting factor in treatment continuity. Dose adjustment or temporary treatment interruption are ready ways to improve patient compliance with treatment. During the BOLT trial, temporary dose interruptions or reductions did not have a negative impact on the efficacy of sonidegib.33 Specifically, similar ORRs assessed by central review were observed in subgroups with (50%) or without (57%) interruptions or dose reductions. Sonidegib is the only Hh inhibitor whose Summary of Product Characteristics includes a treatment regimen with dosing on alternate days to manage AEs.34
Use in Special PopulationsThe available data on efficacy and safety for the use of sonidegib suggest that dose adjustment is not necessary in special populations. Nevertheless, given that no data are available in patients with severe liver or kidney failure, it is recommended to individualize treatment in these patients.34 There are no data on the safety and efficacy of sonidegib in patients under 18 years of age.34
ConclusionsSonidegib is an inhibitor of Hh signaling pathway that has been shown to be effective in the treatment of laBCC, with benefit observed in a significant proportion of patients, and short time to response to enable early detection of patients who respond and with favorable DoR and PFS. Its safety profile has been sustained in the long term, in the trial with the longest follow-up to date of an Hh inhibitor, while its tolerability is improved with a regimen of administration on alternative days, as stated in the Summary of Product Characteristics, thereby helping to reduce the number of treatment discontinuations.
Without doubt, sonidegib opens up a range of therapeutic possibilities for treatment of laBCC, although in absence of a head-to-head trial with the other molecule in its class, we need to wait for results of the 2 drugs in clinical practice to determine true differences and define the optimal profile of patients who respond to sonidegib, adjust dosing to minimizes adverse effects, and delay the onset of resistance to Hh inhibitors.
FundingThis article was funded by Sunpharma.
Conflicts of interestRafael Botella-Estrada: Advisory board, and/or speaker, and/or participated in clinical trials for Pfizer, Abbvie, Almirall, Novartis, Janssen, Leo Pharma, Lilly, Celgene, Roche, SunPharma. Susana Puig: Advisory board or speaker or research grants or non-financial support or clinical trials for Abbvie, Almirall, Amgen, Avene, BMS, Canfield, Cantabria, ISDIN, La Roche Posay, Leo Pharma, MSD, Novartis, Pellepharma, Pfizer, Polychem, Regeneron, Roche, Sanofi. The remaining authors declare that they have no conflicts of interest.
The authors would like to thank Content Ed Net (Madrid) for collaborating in the drafting and editorial management of the article.
Please cite this article as: San Martín O, Llombart B, Carretero Hernandez G, Flórez Menéndez A, Botella-Estrada R, Herrera Ceballos E, et al. Sonidegib en el tratamiento del carcinoma basocelular localmente avanzado. Actas Dermosifiliogr. 2021;112:295–301.