Secukinumab, an immunoglobulin G1/κ monoclonal antibody that selectively targets interleukin 17a, is used to treat moderate to severe plaque psoriasis in adults who are eligible for systemic treatment. Indirect comparisons of the efficacy of secukinumab, ustekinumab, and anti-tumor necrosis factor agents have found lower drug survival rates for patients on secukinumab, in spite of that biologic’s rapid onset of action and efficacy as demonstrated by the large number of patients reaching a Psoriasis Area and Severity Index of 90 or 100. We present data from a retrospective study of 171 patients treated with doses of 300 mg or 150 mg of secukinumab every 4 weeks in 5 hospitals in the Spanish autonomous community of Andalusia. Eighty-seven percent continued on treatment at 132 weeks, contrasting with reports from previously published case series.
Secukinumab es un anticuerpo monoclonal selectivo a la interleucina 17A de tipo IgG1/κ para el tratamiento de la psoriasis en placas de moderada a grave en adultos candidatos a tratamientos sistémicos. Las comparaciones indirectas de eficacia entre fármacos anti-TNF, ustekinumab y secukinumab nos han ido revelando que este último presenta menores tasas de supervivencia libre de enfermedad a pesar de su rápido inicio de acción y eficacia, pues un elevado número de pacientes alcanzan PASI90 y PASI100. Aportamos los datos de un estudio retrospectivo de 5 hospitales de la comunidad autónoma andaluza que incluyen 171 pacientes con una supervivencia global de secukinumab del 87% incluyendo pacientes con dosis de 300 y 150 mg/4 semanas. Estos datos contrastan con las series previamente publicadas en la literatura.
Secukinumab is a recombinant fully human monoclonal anti-interleukin 17A antibody of the IgG1/κ isotype. It has been approved by the regulatory agencies since 2015 for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic treatment.1 The survival on treatment with different biologic agents is derived from randomized clinical trials and extension studies. However, the population included in those trials required a series of entry baseline characteristics that differ from those of patients who we treat in everyday clinical practice. Recent studies have compared anti-TNF agents, ustekinumab, and secukinumab, and shown that secukinumab has a lower survival on treatment despite the rapid onset of action and high percentage of patients who achieve PASI 90 and PASI 100.2
We describe the results of a retrospective, multicenter study of 5 hospitals in Andalusia, Spain, between November 2015 and November 2018. The objective of our study was to assess the estimated survival on treatment with secukinumab up to week 150 in patients with moderate to severe psoriasis. The study included 171 patients over 18 years of age who had been treated with 300 mg doses (n = 125 patients) or 150 mg doses (n = 46 patients) of secukinumab in weeks 0 to 5, and then every 4 weeks according to the judgment of the prescribing physician. The 150 mg regimen was not used in patients with exclusively psoriatic arthritis. In those patients, the dose used was that prescribed by the treating physician taking into account baseline characteristics. Demographic variables (age, sex, duration of psoriasis, and weight and height) and comorbidities (psoriatic arthritis, diabetes mellitus, hypertension, dyslipidemia, depression, fatty liver, neoplasms, and major cardiovascular events) were recorded. Survival was assessed using a Kaplan-Meier approach with the SSPS® statistical package, version 25. Patients were considered censured (active) if they continued treatment, and not censured (event) if treatment was discontinued for any reason (loss of efficacy [primary failure, if the patient had not achieved at least PASI 75 and secondary failure if the patient, despite achieving an initial response better than PASI 75, subsequently lost response], adverse events, loss to follow-up, or patient decision because of improvement in their disease).
This approach was used for both doses combined and also separately. To see if there were differences in survival time between the 2 doses, a log rank comparison test (modified Chi-squared) was performed to calculate the statistical significance of the comparison. If the P value was below .05, we can say with 95% confidence that there are statistically significant differences in survival time between the 2 doses.
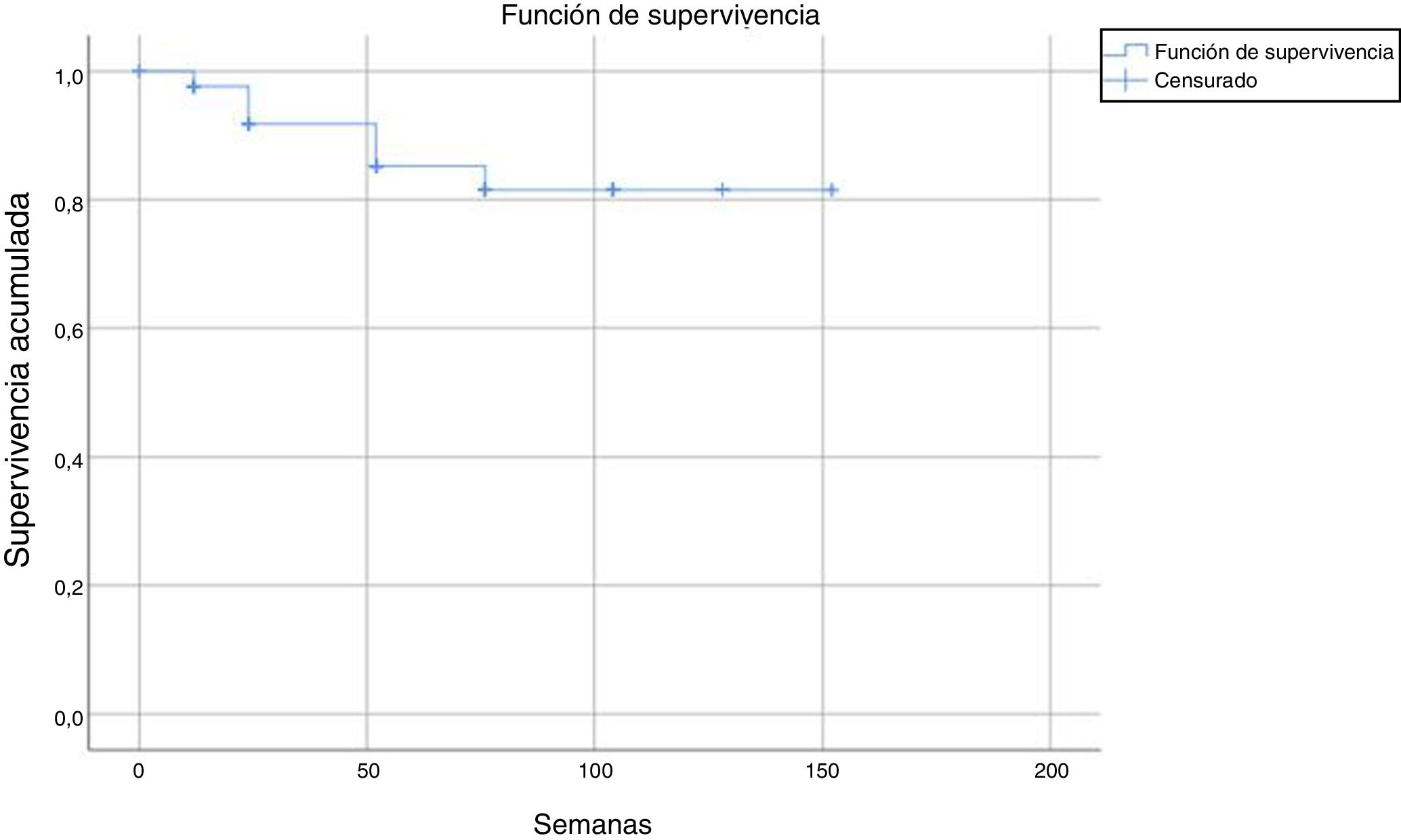
Tests for independence were applied beforehand to check for association between variables, doses, status, and number of weeks. In the Chi-squared test to check for independence of dose/status, the following result was obtained: P = .323; Z = 0.976. In the Mann-Whitney tests for independence between the number of weeks and the variable dose and status, the following result was obtained: P = 0, U = 1603.5 and P = .046, U = 1236.5, respectively. Subsequently, a binary logistic regression analysis was performed taking the status variable as the dependent variable and the number of weeks and doses and the independent one. In this regression, the overall percentage prediction was 87.1%, with this percentage being 100% in the group without discontinuation; statistical significance was also obtained in the omnibus test (P = .008) and the Wald test (P = .000) and so variables of dose and number of weeks were good predictors for the status variable. In addition, the Cox and Snell R-squared number (0.505) and Nagelkerke R-squared number (0.602) were calculated; these indicate the part of the variance of the dependent variable explained by the model and in this case it varied between 50.5% and 60.2% (Fig. 1).
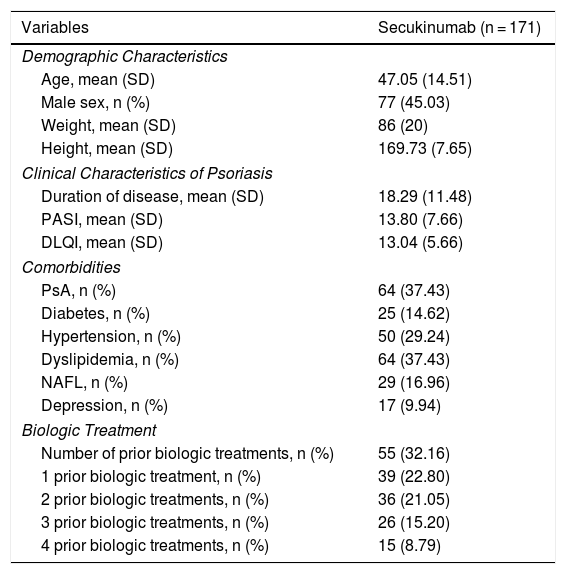
The clinical and demographic characteristics of the patients are shown in Table 1.
Clinical and Demographic Characteristics of Patients in Our Series.
| Variables | Secukinumab (n = 171) |
|---|---|
| Demographic Characteristics | |
| Age, mean (SD) | 47.05 (14.51) |
| Male sex, n (%) | 77 (45.03) |
| Weight, mean (SD) | 86 (20) |
| Height, mean (SD) | 169.73 (7.65) |
| Clinical Characteristics of Psoriasis | |
| Duration of disease, mean (SD) | 18.29 (11.48) |
| PASI, mean (SD) | 13.80 (7.66) |
| DLQI, mean (SD) | 13.04 (5.66) |
| Comorbidities | |
| PsA, n (%) | 64 (37.43) |
| Diabetes, n (%) | 25 (14.62) |
| Hypertension, n (%) | 50 (29.24) |
| Dyslipidemia, n (%) | 64 (37.43) |
| NAFL, n (%) | 29 (16.96) |
| Depression, n (%) | 17 (9.94) |
| Biologic Treatment | |
| Number of prior biologic treatments, n (%) | 55 (32.16) |
| 1 prior biologic treatment, n (%) | 39 (22.80) |
| 2 prior biologic treatments, n (%) | 36 (21.05) |
| 3 prior biologic treatments, n (%) | 26 (15.20) |
| 4 prior biologic treatments, n (%) | 15 (8.79) |
Abbreviations; DLQI, dermatology life quality index; NAFL, nonalcoholic fatty liver; PASI, psoriasis activity skin index; PsA, psoriatic arthritis.
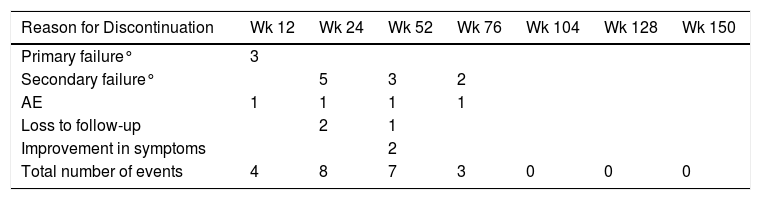
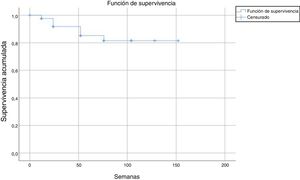
After a median follow-up of 123 weeks, 22 patients (13%) had to discontinue treatment due to loss of efficacy (primary or secondary failure, adverse events, or loss to follow-up). Most of the discontinuations occurred between weeks 24 and 52, with no discontinuations after weeks 104, 128, and 150 (Table 2 and Fig. 2).
Reasons for Discontinuation at Different Time Points.
| Reason for Discontinuation | Wk 12 | Wk 24 | Wk 52 | Wk 76 | Wk 104 | Wk 128 | Wk 150 |
|---|---|---|---|---|---|---|---|
| Primary failure° | 3 | ||||||
| Secondary failure° | 5 | 3 | 2 | ||||
| AE | 1 | 1 | 1 | 1 | |||
| Loss to follow-up | 2 | 1 | |||||
| Improvement in symptoms | 2 | ||||||
| Total number of events | 4 | 8 | 7 | 3 | 0 | 0 | 0 |
Abbreviation: AE, adverse event.
The estimated survival until drug discontinuation was 87% at 132 weeks.
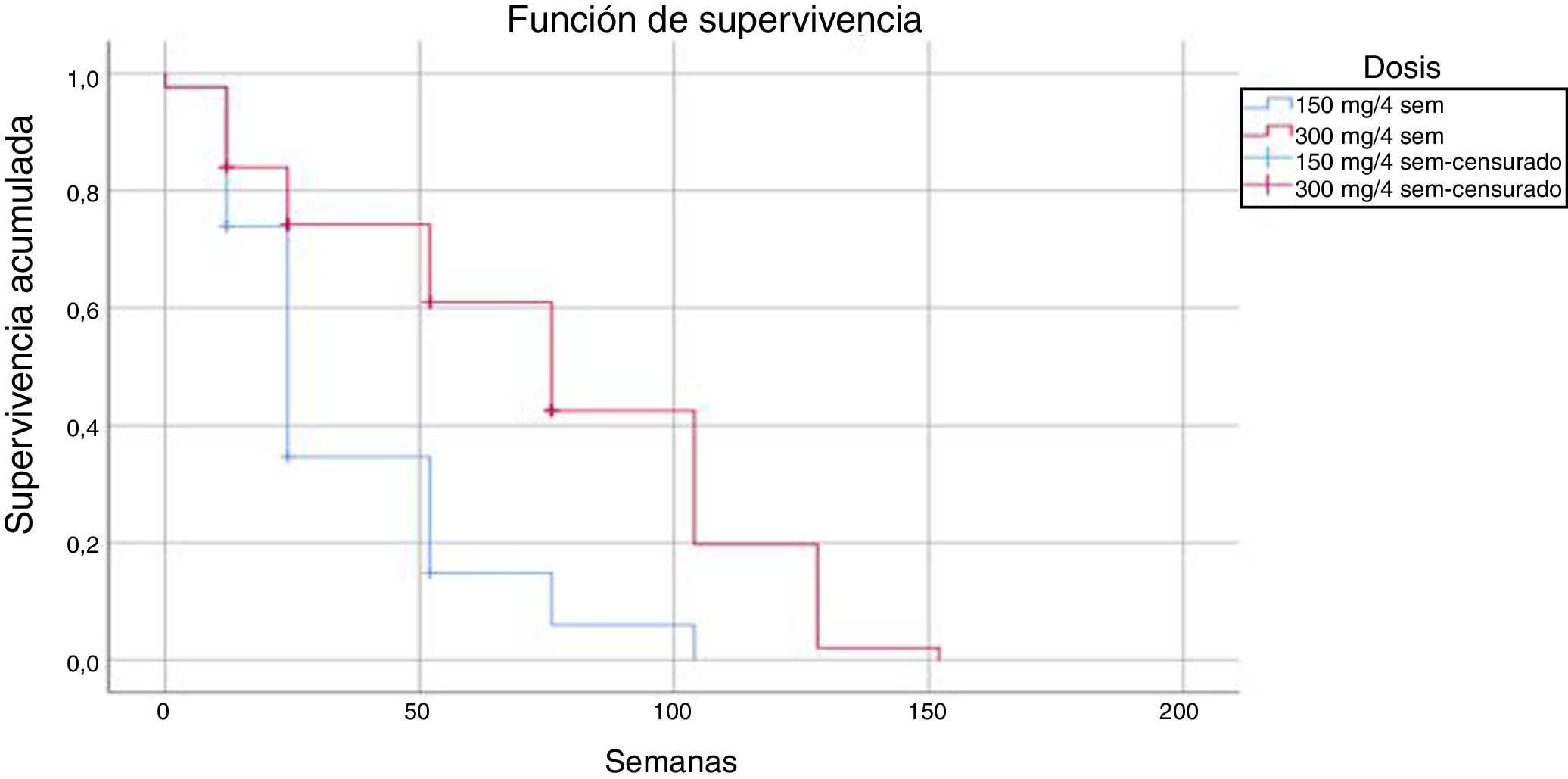
Comparison of the 2 regimens showed that the regimen of 300 mg every 4 weeks had 18 events (14.4%) compared with 4 (9%) in the regimen of 150 mg every 4 weeks; these differences were statistically significant (log-rank P < .05).
The mean time until drug discontinuation due to adverse events was 118 weeks for patients treated with the regimen of 300 mg every 4 weeks and 86 weeks for the regimen of 150 mg every 4 weeks.
In the randomized clinical trials, survival on treatment at 52 weeks varied according to the type of study (SCULPTURE, ERASURE, and FIXTURE) with values of 78.2%, 80.5%, and 80.5%, respectively.1,3 Different series from clinical practice point to a clear decrease in efficacy of secukinumab compared with the pivotal trials, although the results are broadly inconsistent. The figures closest to the values in the clinical trials are from the study by Sotiriou et al.,4 who in a series of 42 patients with one year of follow-up reported a survival expressed as maintenance of PASI 75 of 82.4% and survival on treatment of 78.6%, with no significant differences between patients who had and had not received prior treatment with a biologic agent. The mean survival time was 110 weeks regardless of cause for discontinuation.
Lee et al.,5 in contrast, reported that only 41.7% (n = 48) of patients who received secukinumab in their series did not require combination treatment or switching to alternative therapy; this represents the lowest on-treatment survival published to date. They attributed the greater rate of discontinuations to the longer duration of follow-up in their series.
Georgakopoulos et al.6 reported survival of 68.3% at 52 weeks in their series of 41 patients who were treated according to the Summary of Product Characteristics and maintained PASI 75 and PGA 0-1, with loss of efficacy being the main reason for treatment discontinuation. Finally, van den Reek et al.7 reported similar results with a larger number of patients (n = 196) with 76% survival at 52 weeks.
The results obtained in our series in everyday clinical practice show high survival in patients treated with secukinumab with both doses, and a lower number of discontinuations with the dose of 150 mg every 4 weeks. This observation may be the result of the lower number of patients and shorter follow-up at this dose, as well as the use of the dose indicated in the Summary of Product Characteristics in more complex patients, with greater risk of therapeutic failure. We should remember that patients who did not continue and who were being treated with the 150 mg dose every 4 weeks had a shorter survival on treatment than those treated with the 300 mg dose every 4 weeks (35 vs. 74 weeks, respectively).
The main limitation in all the published series, and ours is no exception, is the lack of a control group to contextualize the findings. These preliminary data suggest we should perform subanalyses of survival according to the presence or absence of psoriatic arthritis or whether the patient has or has not been previously treated with other biologics.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ruiz-Villaverde R, Rodriguez Fernández-Freire L, Galán-Gutiérrez M, Armario-Hita JC, Martinez-Pilar L. Secukinumab: supervivencia en práctica clínica real. Actas Dermosifiliogr. 2021;112:361–364.