Prurigo nodularis is a chronic inflammatory skin disease characterized by highly pruritic nodular lesions that cause constant itching and scratching and significant quality-of-life impairment. It has been described in a range of conditions, including skin diseases (mainly atopic dermatitis) and metabolic, neurological, and psychiatric disorders. The pathophysiological mechanisms are largely unknown. Various modalities of phototherapy have been described as appropriate and safe treatments for achieving clinical control and alleviating symptoms. In this article, we describe our experience with phototherapy in patients with prurigo nodularis.
Material and methodsRetrospective observational study of patients who received their first cycle of phototherapy to treat prurigo nodularis between March 2011 and October 2019. Information was collected on epidemiological and clinical characteristics, concomitant treatments, type and duration of phototherapy, maximum dose reached, and response to treatment.
ResultsWe studied 44 patients (30 women and 14 men) with a median age of 65.5 years. The most common form of phototherapy used was narrowband UV-B phototherapy (34 cycles, 77.27%) followed by a combination of UV-B and UV-A phototherapy (8 cycles). Response to treatment was considered satisfactory (clearance rate of ≥ 75%) in 24 patients (55.4%).
ConclusionsPhototherapy is a suitable treatment for prurigo nodularis in a considerable proportion of patients. It can be used as monotherapy or combined with other treatments.
El Prurigo Nodular (PN) es una dermatosis inflamatoria crónica caracterizada por la presencia de lesiones nodulares, intensamente pruriginosas, con gran impacto en la calidad de vida. El intenso prurito y el rascado son rasgos constantes en esta enfermedad, que se ha descrito en el contexto de diversos procesos dermatológicos – fundamentalmente dermatitis atópica-, metabólicos, neurológicos o psiquiátricos, aunque la fisiopatogenia es en buena parte desconocida. La fototerapia, en diversas de sus variantes, se ha propuesto como una opción terapéutica adecuada en el control clínico y sintomático, con un buen perfil de seguridad. En el presente estudio, describimos nuestra experiencia con el uso de fototerapia en el tratamiento del PN.
Material y métodoEstudio observacional retrospectivo en el que se incluyeron pacientes que habían recibido su primer ciclo de fototerapia entre marzo 2011 y octubre 2019 para el tratamiento de su PN. Se recogieron las siguientes variables: características epidemiológicas y clínicas de los pacientes, tratamientos concomitantes, tipo de fototerapia, duración y dosis alcanzadas, y la respuesta obtenida.
ResultadosIncluimos un total de 44 pacientes (30 mujeres y 14 hombres, con mediana de edad de 65.5 años). La variante de fototerapia más empleada fue UVBBE (34 ciclos, 77.27%) seguida de la combinación de UVB + UVA (8 ciclos). La respuesta se consideró satisfactoria (respuestas ≥75%) en 24 pacientes (55.4%).
ConclusionesLa fototerapia es una opción terapéutica adecuada en un porcentaje considerable de pacientes con PN, que puede emplearse en monoterapia o combinada con diversos tratamientos.
Chronic prurigo nodularis (PN) is the best known subtype of chronic prurigo. The latter was recently defined by a consensus of experts as a distinct dermatosis, characterized by chronic pruritus of 6 or more weeks duration, a history of repeated scratching, and the presence of multiple pruritic lesions.1
PN lesions are excoriated, scaly, or crusted nodules or papules, with a pinkish-white center and a hyperpigmented border.1–3 These lesions are usually located in areas of skin that are accessible to patients, are symmetrically distributed, and rarely affect the palms or the face.1,2 Skin between lesions may be normal or lichenified.1,2,4 Pruritus has been described as the initial sign, followed by the development of skin lesions, and is usually accompanied by other sensations (eg burning, pain, tightness).1,4 Another characteristic of this entity is a marked impact on the quality of life of affected patients, including increased rates of anxiety, insomnia, and depression.5
PN most often affects the elderly6 and African-Americans,7,8 although cases have been described in children and adolescents.9 There is no consensus regarding the distribution between the sexes.6
Although the pathogenesis of PN is currently unknown, chronic pruritus and prolonged scratching appear to play an essential role, resulting in an itch–scratch cycle that leads to the development and perpetuation of chronic prurigo skin lesions.1 The etiology of chronic pruritus can be dermatological, systemic, neurological, psychiatric/psychosomatic, multifactorial, or idiopathic.1,6
PN is a difficult-to-manage condition: there are no specific or curative treatments currently available, and therefore the primary objective is to alleviate symptoms and interrupt the itch–scratch cycle.2,3 The first-line treatment consists of topical corticosteroids and selective topical calcineurin inhibitors, administered either alone or with antihistamines, followed by phototherapy and other systemic treatments (gabapentin, antidepressants, or immunosuppressants).3,10,11
Phototherapy has been described as an effective tool in the management of the disease, producing satisfactory results in most cases.3,12–25 As described for other dermatoses, phototherapy reduces pruritus in these patients.13,14,26 However, reports on its use to treat PN are scarce. We evaluated the use of phototherapy to treat PN and sought to identify variables predictive of a better treatment response.
Material and MethodsIn this observational retrospective study we analyzed data extracted from the phototherapy database of the Hospital Universitari Germans Trias i Pujol (HUGTIP), Barcelona, as well as data acquired through a systematic search of patients’ medical records. This database was constructed systematically and includes data on all patients who underwent phototherapy at the dermatology department of HUGTIP. We selected patients who were diagnosed with PN and received a first cycle of phototherapy between March 2011 and October 2019. Specifically, epidemiological, clinical, and phototherapy-related variables were analyzed. All patients underwent phototherapy in accordance with the consensus guidelines of the Spanish Photobiology Group (GEF) for atopic dermatitis.27 In patients who received more than one phototherapy cycle, the first cycle was analyzed.
The epidemiological and clinical variables studied were sex, age at treatment, and the presence of the following comorbidities: psychological/psychiatric disease, diabetes mellitus, chronic renal failure, anemia, thyroid diseases, hepatobiliary diseases, acquired immunodeficiency virus, neoplasms (hematological and non-hematological), and other dermatoses (concomitant or previous).
Among the patient management variables recorded were diagnostic biopsy, previous and concomitant systemic treatments, and time from diagnosis to phototherapy.
Phototherapy-related variables included the type of radiation used, duration of the cycle, number of sessions per cycle, maximum dose achieved, appearance of adverse effects, and response to treatment as evaluated by the physician in charge. The response to phototherapy classified as follows: good (≥ 75%); moderate (50–74%); or therapeutic failure (< 50%).
Using the R statistical program (version 3.6.1), contingency tables were constructed to describe the variables of interest. Analyses were performed to compare the distribution of some of these variables as a function of the response to phototherapy. The variables analyzed were age, sex, the presence of comorbidities, duration of disease prior to phototherapy, and duration of the first phototherapy cycle. Patients were divided into 2 groups, depending on whether treatment elicited a response (≥ 50%) or failed (< 50%). For this analysis, p-values < 0.05 were considered statistically significant. A Shapiro-Wilks test demonstrated that quantitative variables were not normally distributed, and therefore these variables were analyzed using the nonparametric Wilcoxon test. Qualitative variables were analyzed using Fisher’s exact test.
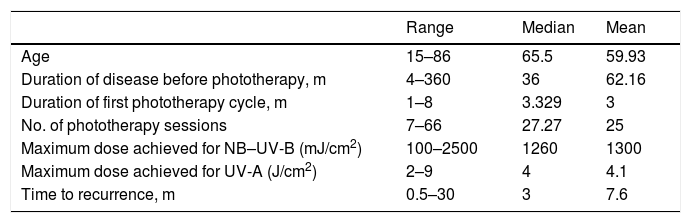
ResultsThe study population consisted of 44 patients (14 men and 30 women), aged 15 to 86 years (median age, 65.5 y) (Tables 1 and 2).
Quantitative Variables
| Range | Median | Mean | |
|---|---|---|---|
| Age | 15–86 | 65.5 | 59.93 |
| Duration of disease before phototherapy, m | 4–360 | 36 | 62.16 |
| Duration of first phototherapy cycle, m | 1–8 | 3.329 | 3 |
| No. of phototherapy sessions | 7–66 | 27.27 | 25 |
| Maximum dose achieved for NB–UV-B (mJ/cm2) | 100–2500 | 1260 | 1300 |
| Maximum dose achieved for UV-A (J/cm2) | 2–9 | 4 | 4.1 |
| Time to recurrence, m | 0.5–30 | 3 | 7.6 |
Abbreviations: NB–UV-B, narrowband ultraviolet B; UV-A, ultraviolet A.
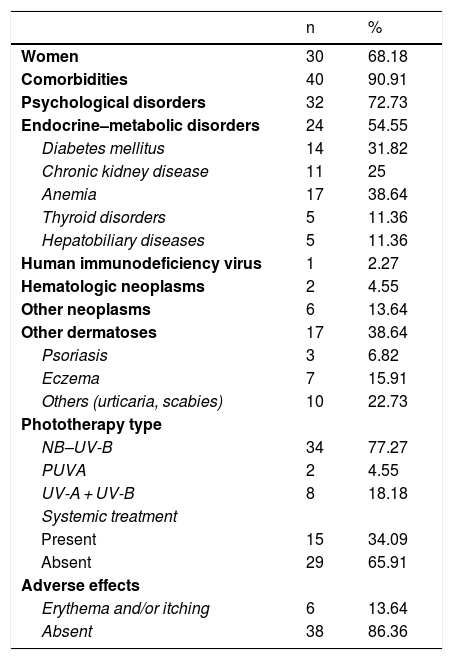
Qualitative Variables.
| n | % | |
|---|---|---|
| Women | 30 | 68.18 |
| Comorbidities | 40 | 90.91 |
| Psychological disorders | 32 | 72.73 |
| Endocrine–metabolic disorders | 24 | 54.55 |
| Diabetes mellitus | 14 | 31.82 |
| Chronic kidney disease | 11 | 25 |
| Anemia | 17 | 38.64 |
| Thyroid disorders | 5 | 11.36 |
| Hepatobiliary diseases | 5 | 11.36 |
| Human immunodeficiency virus | 1 | 2.27 |
| Hematologic neoplasms | 2 | 4.55 |
| Other neoplasms | 6 | 13.64 |
| Other dermatoses | 17 | 38.64 |
| Psoriasis | 3 | 6.82 |
| Eczema | 7 | 15.91 |
| Others (urticaria, scabies) | 10 | 22.73 |
| Phototherapy type | ||
| NB–UV-B | 34 | 77.27 |
| PUVA | 2 | 4.55 |
| UV-A + UV-B | 8 | 18.18 |
| Systemic treatment | ||
| Present | 15 | 34.09 |
| Absent | 29 | 65.91 |
| Adverse effects | ||
| Erythema and/or itching | 6 | 13.64 |
| Absent | 38 | 86.36 |
Abbreviations: NB–UV-B, narrowband ultraviolet B; PUVA, psoralen plus UV-A; UV-A, ultraviolet A; UV-B, ultraviolet B.
Comorbid conditions were present in 91% of patients (Table 2), and more half of all patients (n = 20) had more than 1 comorbidity. The most frequent comorbid conditions were psychological disorders (72% of patients) followed by endocrine–metabolic disorders (54.5%). Previous or concomitant dermatoses were recorded in 38% of patients. Atopy was the most common skin condition (16%). One patient was positive for human immunodeficiency virus, 2 had hematological malignancies, and 6 had a history of other neoplasms.
In terms of the management and diagnosis, 24 of the 44 patients had biopsy findings consistent with a clinical diagnosis of PN. In most patients the disease followed an insidious course. The mean time between symptom onset and the start of phototherapy was 3 years (Table 1). Fifteen patients received systemic treatments (eg cyclosporine, corticosteroids, methotrexate) prior to phototherapy. The most frequent concomitant treatments included topical corticosteroids (31 patients) and oral antihistamines (36 patients). Among patients who received antihistamines, most (22 patients) received both first- and second-generation drugs, 10 received only second-generation compounds, and 4 received only first-generation compounds. Only 2 patients received other systemic treatments (cyclosporine and corticosteroids, respectively).
The most commonly used phototherapy modality was narrow-band ultraviolet B (NB–UV-B) therapy (77.27% of patients), followed by combination (UV-A + UV-B) phototherapy (18.18%; Table 2). The mean duration of the first phototherapy cycle was 3 months, and the mean number of sessions per cycle was 25. The mean maximum dose of NB–UV-B achieved per session was 1300 mJ/cm2 (range, 200–2500 mJ/cm2) (Table 2). Only 6 patients experienced adverse effects, which consisted of pruritus and/or erythema after the phototherapy sessions. These effects resolved spontaneously within a few hours and in no case necessitated treatment discontinuation.
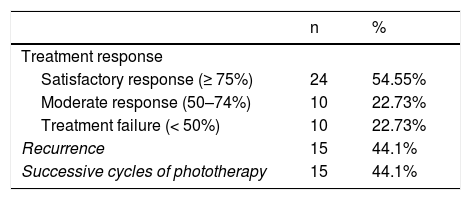
After the first cycle of phototherapy, 54.5% of patients achieved a satisfactory response, resulting in resolution of ≥ 75% of lesions. A complete response was observed in half of these patients. Treatment failure occurred in 10 patients. Of the 34 patients who responded to the first cycle of phototherapy, 15 experienced recurrence (median time to recurrence, 3 m) and required successive cycles of treatment (Table 3).
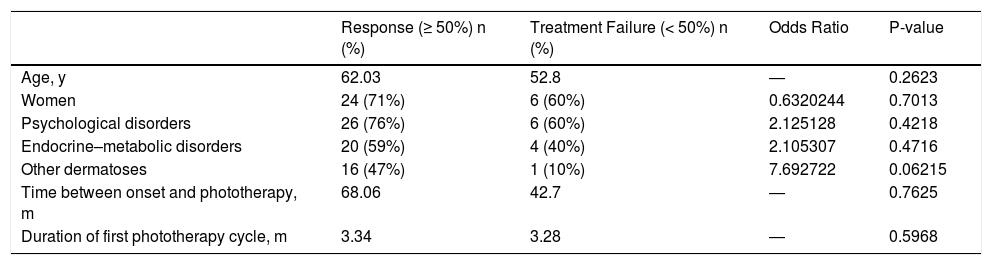
Comparison of age, sex, and the presence of comorbidities between patients who responded to phototherapy and those who did not revealed significant differences (Table 4). Although previous or concomitant dermatoses were more frequent among patients who responded to treatment, this difference was not significant. Similarly, we observed no differences in the time between symptom onset and phototherapy, nor in the duration of the phototherapy cycle (Table 4).
Statistical Analyses.
| Response (≥ 50%) n (%) | Treatment Failure (< 50%) n (%) | Odds Ratio | P-value | |
|---|---|---|---|---|
| Age, y | 62.03 | 52.8 | ― | 0.2623 |
| Women | 24 (71%) | 6 (60%) | 0.6320244 | 0.7013 |
| Psychological disorders | 26 (76%) | 6 (60%) | 2.125128 | 0.4218 |
| Endocrine–metabolic disorders | 20 (59%) | 4 (40%) | 2.105307 | 0.4716 |
| Other dermatoses | 16 (47%) | 1 (10%) | 7.692722 | 0.06215 |
| Time between onset and phototherapy, m | 68.06 | 42.7 | ― | 0.7625 |
| Duration of first phototherapy cycle, m | 3.34 | 3.28 | ― | 0.5968 |
The epidemiological and clinical characteristics of our study population were similar to those previously described in PN patients. The mean age was in the sixth decade of life, and most patients had underlying comorbidities.6 The predominance of female patients in our series is not a consistent finding in the literature.8 Psychological and psychiatric disorders, which have been previously associated with PN,2,5,6,10 were the most frequent comorbidities in our patients.
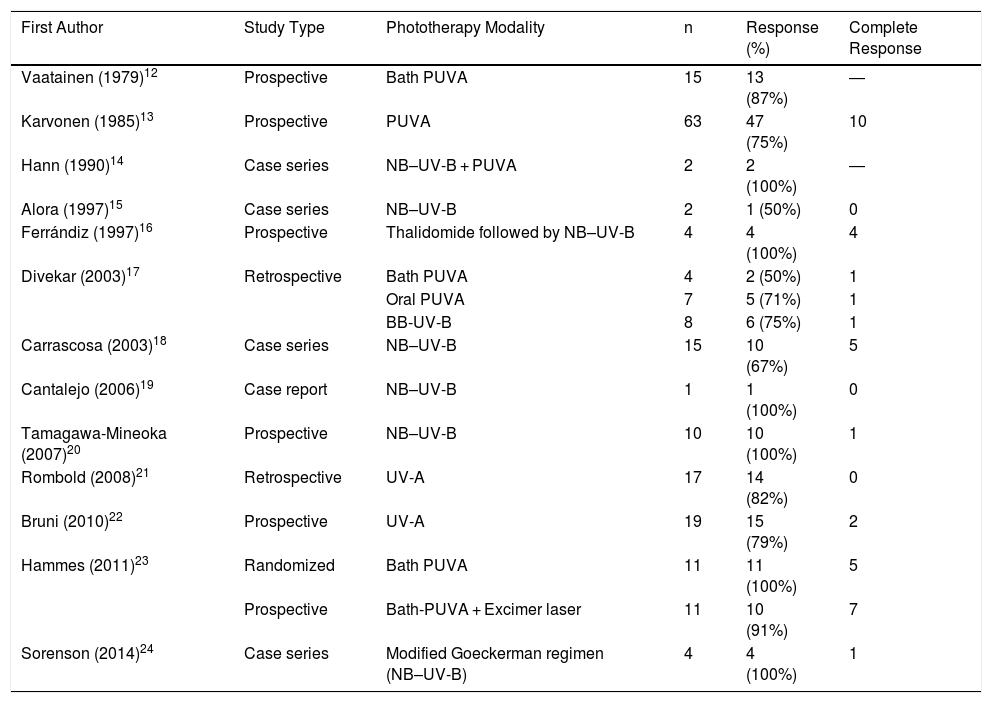
Phototherapy resulted in a satisfactory response, with resolution of at least 75% of lesions and symptom improvement in about half of all patients, an appreciable proportion given the therapeutic difficulties posed by this disease. A search of the published literature (Table 5) revealed a mean rate of treatment response, including both partial and complete responses, of 83%, with a complete response obtained in approximately 20% of patients.12–24 Despite these published results, it should be noted that corresponding studies are scarce, and consist mainly of case series or nonrandomized trials with small sample sizes. Furthermore, there is considerable variation in the literature as regards the phototherapy protocol applied and the manner in which it is evaluated. Concomitant treatment of patients with other systemic treatments appeared not to influence our findings: the 2 patients who received such treatments showed phototherapy responses of less than 50%, perhaps because they presented more recalcitrant lesions, which in turn justified systemic treatment. In most studies, adverse effects were either absent or few, and were limited to mild erythema or pruritus.12–24
Previous Studies of Phototherapy in Prurigo Nodularis
| First Author | Study Type | Phototherapy Modality | n | Response (%) | Complete Response |
|---|---|---|---|---|---|
| Vaatainen (1979)12 | Prospective | Bath PUVA | 15 | 13 (87%) | ― |
| Karvonen (1985)13 | Prospective | PUVA | 63 | 47 (75%) | 10 |
| Hann (1990)14 | Case series | NB–UV-B + PUVA | 2 | 2 (100%) | ― |
| Alora (1997)15 | Case series | NB–UV-B | 2 | 1 (50%) | 0 |
| Ferrándiz (1997)16 | Prospective | Thalidomide followed by NB–UV-B | 4 | 4 (100%) | 4 |
| Divekar (2003)17 | Retrospective | Bath PUVA | 4 | 2 (50%) | 1 |
| Oral PUVA | 7 | 5 (71%) | 1 | ||
| BB-UV-B | 8 | 6 (75%) | 1 | ||
| Carrascosa (2003)18 | Case series | NB–UV-B | 15 | 10 (67%) | 5 |
| Cantalejo (2006)19 | Case report | NB–UV-B | 1 | 1 (100%) | 0 |
| Tamagawa-Mineoka (2007)20 | Prospective | NB–UV-B | 10 | 10 (100%) | 1 |
| Rombold (2008)21 | Retrospective | UV-A | 17 | 14 (82%) | 0 |
| Bruni (2010)22 | Prospective | UV-A | 19 | 15 (79%) | 2 |
| Hammes (2011)23 | Randomized | Bath PUVA | 11 | 11 (100%) | 5 |
| Prospective | Bath-PUVA + Excimer laser | 11 | 10 (91%) | 7 | |
| Sorenson (2014)24 | Case series | Modified Goeckerman regimen (NB–UV-B) | 4 | 4 (100%) | 1 |
Abbreviations: BB-UV-B, broad band ultraviolet B; NB–UV-B, narrowband ultraviolet B; PUVA, psoralen plus UV-A; UV-A, ultraviolet A; UV-B, ultraviolet B.
PN therapy should include management of the many comorbid processes that can influence the development or worsening of PN; such comorbidities were present in 55% of patients in published series and in 91% of our patient population.6 It should be noted that in our series patients with previous or concomitant dermatoses tended to show a better therapeutic response, although this effect was not significant. By contrast, in patients with PN of psychogenic origin the disease frequently followed a much more chronic course, with multiple recurrences and poorer responses to treatment. Nonetheless, we should not underestimate the positive effect of phototherapy in this group of patients: the partial reduction in pruritus, together with close follow-up enabled by regular visits, allowed patients to express their emotions about the disease and to better accept it.
We may be at the cusp of a change in the prognosis of PN, conditioned by better knowledge of the pathogenies of pruritus and the development of drugs directed against specific therapeutic targets. Indeed, several recently developed drugs, including a neurokinin-1 receptor inhibitor (serlopitant), an interleukin (IL) -31 inhibitor (nemolizumab), and a µ-opioid receptor inhibitor have shown encouraging results in the treatment of severe chronic pruritus.28–30 Calugareanu et al31 reported very good responses to dupilumab treatment, which in another study resulted complete remission in 50% of cases.32 The appearance and eventual approval of these drugs could provide treatment options that are more efficacious and convenient than phototherapy.33 However, phototherapy not only promises an acceptable treatment response rate, but also has an excellent safety profile, especially in patients who have high comorbidities, are polymedicated, or are pregnant, and can be administered as monotherapy or in combination with other treatment options.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Arrieta A, Jaka A, del Alcázar E, Blanco M, Carrascosa JM. Fototerapia en el prurigo nodular. Experiencia propia y revisión de la literatura. Actas Dermosifiliogr. 2021;112:339–344.