Granulomatous sarcoidosis-like reactions affecting multiple organ systems at one time have infrequently been described within weeks to months after initiation of Ipilimumab. We present the first case of a 67-year-old man with isolated cutaneous granulomatous reaction involving the trunk, extremities, and face after eighteen months of treatment with ipilimumab for metastatic melanoma. This case documents the eruption of isolated cutaneous granulomatous reaction as a late treatment-related adverse effect of ipilimumab, highlighting the importance of adequate, prolonged follow-up.
Las reacciones granulomatosas «sarcoidosis-like» secundarias a ipilimumab afectarán simultáneamente a múltiples órganos y característicamente se presentarán semanas o meses después de haber iniciado el tratamiento. Paciente varón de 67 años, quien consulta por reacción cutánea granulomatosa a nivel de tronco, extremidades y rostro. Esta se presentó 18 meses después de haber iniciado tratamiento con ipilimumab, medicamento pautado por el diagnóstico de un melanoma metastásico. Reportamos reacción granulomatosa de presentación exclusivamente cutánea, como efecto adverso medicamentoso tardío secundario al tratamiento con ipilimumab. Así mismo resaltamos la necesidad de realizar en los pacientes tratados con ipilimumab un seguimiento prolongado.
A 67-year-old man attended the dermatology clinic for mild generalized pruritus with onset 6 months earlier, accompanied by multiple coalescent papular lesions that had presented initially on the back but subsequently spread rapidly to the face, arms, legs, backs of the hands and feet, groin, and hips. Of note in his medical history was diagnosis of metastatic melanoma (stage IV). The primary tumor had been diagnosed in the lumbar skin and the patient had metastases to the lung, liver, and retroperitoneal lymph nodes. Treatment with ipilimumab had induced complete remission for 2 years. Ipilimumab was administered initially at high doses (10mg/kg) every 3 weeks for 4 cycles (induction phase) and then with a regimen of infusion every 3 months (maintenance phase). Dry skin, alopecia, and vitiligo were reported as adverse drug reactions to ipilimumab. All these were grade 1 and so no dose reductions were required. In addition to ipilimumab, the patient also received treatment with venlafaxine, olmesartan, fexofenadine, and ranitidine. He had no known drug allergies.
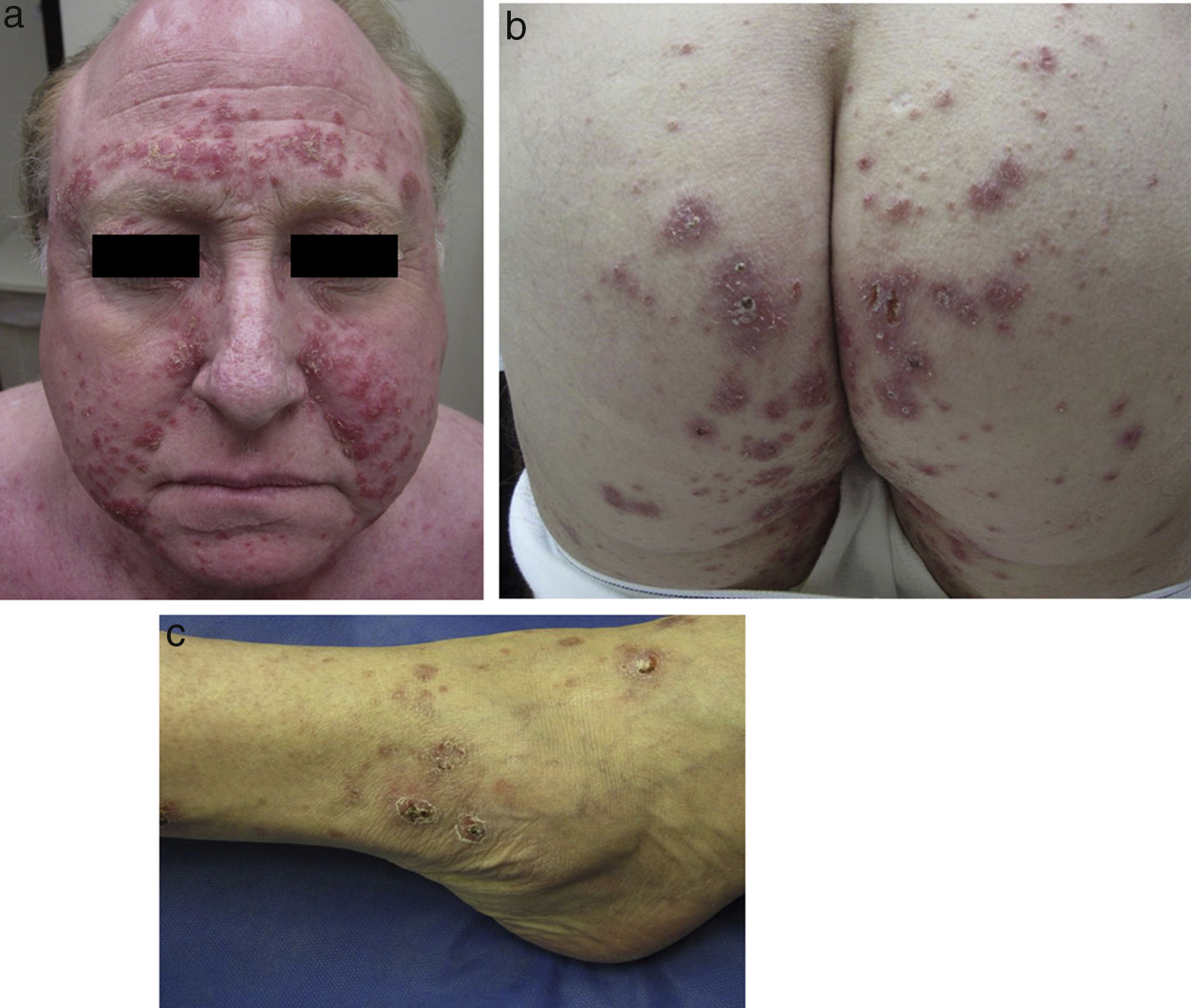
Physical examination revealed multiple inflammatory grouped papules with a crusty appearance, distributed symmetrically on the face (predominantly in the nasolabial folds, supraorbital arches, and glabella) (Fig. 1a) and on the neck, ears, scalp, groin, back, hips (Fig. 1b), elbows, wrists, and ankles (Fig. 1c). In the laboratory analysis including blood chemistry, hematology, urinary calcium, and angiotensin converting enzyme, parameters were within normal range. Cultures for mycobacteria, bacteria, and fungi were negative, as were purified protein derivative and rapid plasma reagin tests. Chest X-ray and computed tomography-positron emission tomography (CT-PET) did not show any pathological findings.
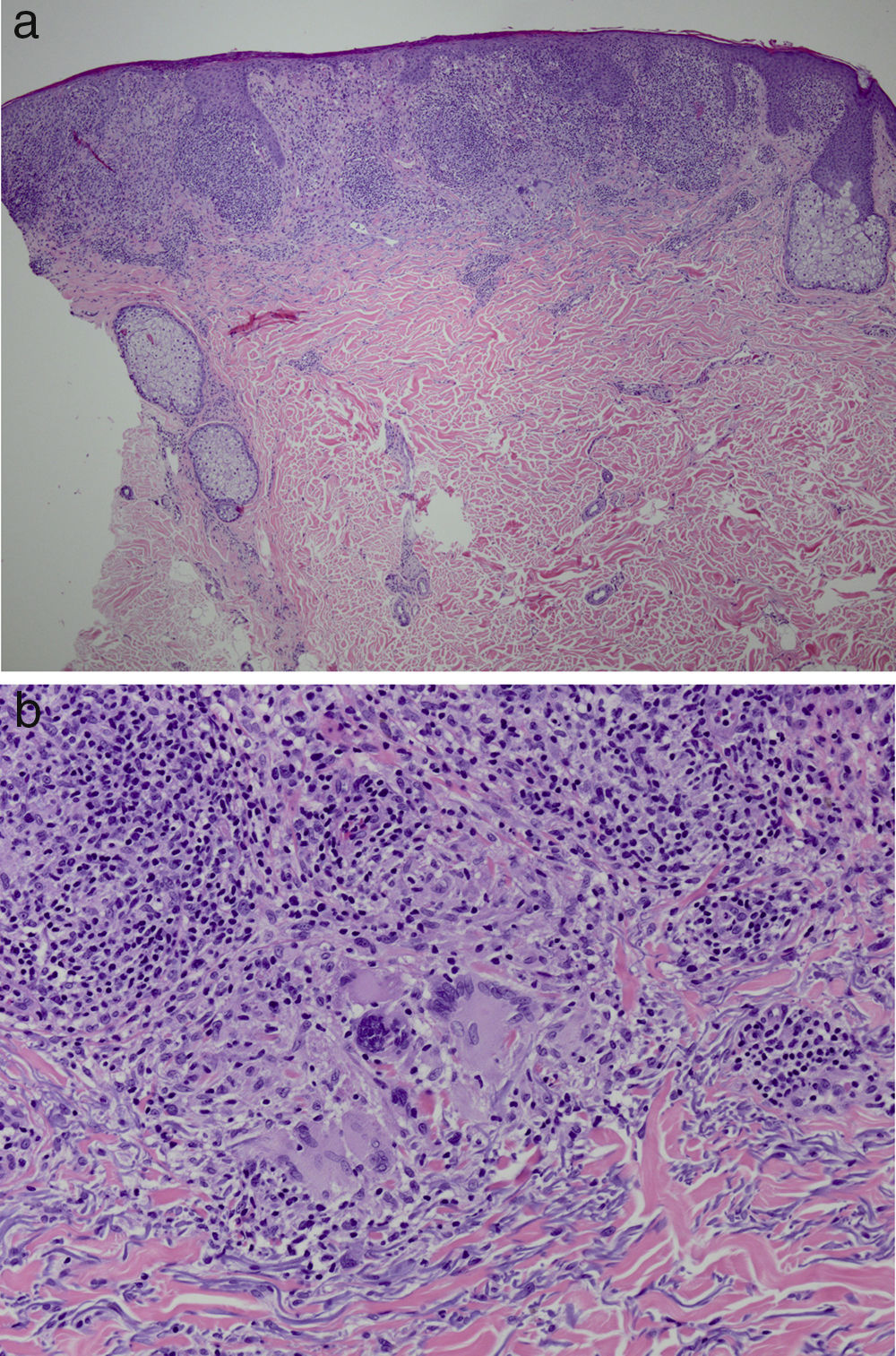
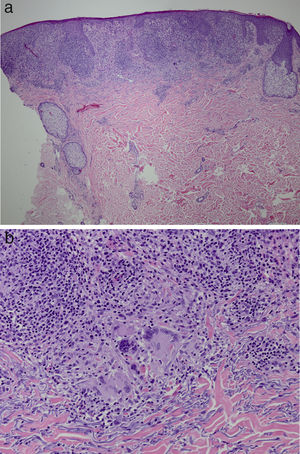
It was decided to obtain a biopsy of the lesions. Pathology study showed presence of nodular epithelioid granulomas surrounded by T lymphocytes in the dermis. In addition, areas were observed with lymphocytic epidermotropism, consistent with granulomatous dermatitis (Fig. 2a and b). Limited CD20+ B lymphocytes were detected, while CD39, CD56, CD1a, and S100 were negative. CD58 was positive for giant cells and also for some macrophages. Most of the lymphocytes of the infiltrate were marked with CD3, CD4, and CD7. The CD4:CD8 ratio was 2:1. Periodic acid-Schiff, Gomori methenamine silver, Giemsa, gram, and Fite stains were negative. Specific stains for Treponema were also negative.
In view of these clinical and histological findings, granulomatous dermatitis induced by ipilimumab was diagnosed. Treatment was initiated with oral prednisone, at a dose of 60mg/d, and the lesions showed initial improvement. However, after prednisone tapering, the lesions worsened once again. Intramuscular and topical corticosteroids were administered, and the patient showed a relative clinical improvement. In agreement with the oncologist, it was decided to suspend ipilimumab. Six months after discontinuing treatment, the skin lesions had completely resolved. Administration of additional drugs was not required. In the most recent visit, the patient was asymptomatic and in follow-up with both the oncology department and the dermatology department with the aim of early detection of any tumor relapse. This involves a CT-PET scan every 3 months as well as follow-up visits every 6 months.
DiscussionGranulomatous dermatoses are characterized by a predominantly dermal, reactive, non-neoplastic inflammatory infiltrate, composed principally of tissue macrophages (histiocytes), epithelioid cells, and giant multinucleated cells.1 Granulomas of noninfectious etiology can be observed in granuloma annulare, annular elastolytic giant cell granuloma, necrobiosis lipoidica, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, sarcoidosis, foreign body reaction, and malignant neoplasms (for example, Sézary syndrome, Hodgkin disease, chronic B cell lymphocytic leukemia, melanoma, and other neoplasms [of lung, breast, etc]) among others.2 Due to overlap of both clinical and pathological characteristics, diagnosis of this entity is often a challenge for clinicians.
Ipilimumab is a human recombinant monoclonal antibody (IgG1) that acts by inhibiting cytotoxic T-lyphocyte associated antigen 4 (CTL-4), a negative regulator of T lymphocyte activation, thanks to blockade of binding to B7 receptors. This leads to an increase in both activation/proliferation and production of IL-2, thereby amplifying immune response and enhancing antitumor immunity.3,4 Ipilimumab was approved by the United States Food and Drug Administration (FDA) for treatment of unresectable or metastatic melanoma. The most frequent immune-related adverse drug reactions include colitis/diarrhea, dermatitis,5 hepatitis, endocrinopathies, blepharitis,5 transaminitis,5 uveitis, hypophysitis,6 nephritis,7–9 thyroiditis,5 and inflammatory myopathy.10–12 The cutaneous adverse drug reactions observed most frequently are morbilliform rash, vitiligo, and alopecia areata.13 Morbilliform rash will present mainly on the trunk and limbs. Histologically, epidermal spongiosis; edema in the papillary dermis; perivascular infiltrate composed of lymphocytes and eosinophils; and predominance of CD4 T lymphocytes in the dermis can be observed.10,11 Other cutaneous adverse drug reactions which present with a lower frequency are presence of hypopigmentation, prurigo, acneiform and lichenoid rash, pyoderma gangrenosum-like ulcerations, skin toxicity in previously irradiated areas, photosensitivity reactions, and drug rash with eosinophilia and systemic symptoms (DRESS syndrome),13 as well as Stevens-Johnson syndrome/toxic epidermal necrolysis.13
Sarcoidosis-like granulomatous reactions caused by ipilimumab are increasingly frequent.12 Sarcoidosis is a multisystemic granulomatous entity of unknown etiology. It is usually associated with hematological neoplasms, solid tumors, and also some cancer treatments. Granuloma formation is thought to be due to an uncontrolled response of T helper 1 cells to unknown antigens. Overproduction of IL-2 and IFN-γ may also contribute to triggering this reaction.14 The absence of blockade of T cell proliferation, for example because of the effects of an anti-CTLA-4 agent, might explain the development of these granulomatous lesions, as is the case with self-reactive T cells in sarcoidosis.15 The exact pathogenic mechanism by which ipilimumab induces a sarcoidosis-like granulomatous reaction is still unknown.16
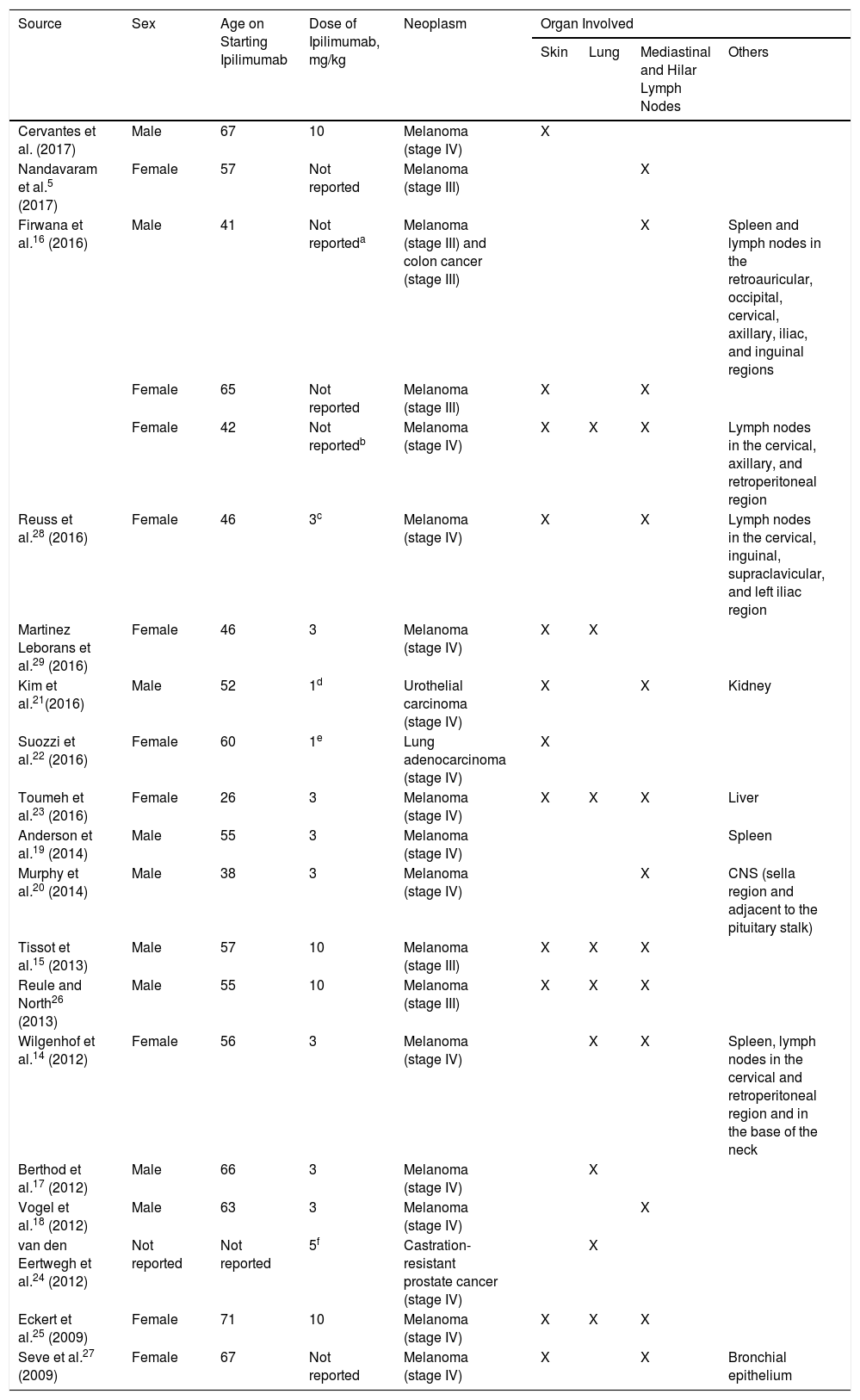
The development of sarcoidosis-like granulomatous lesions, localized both in the mediastinum and lung as well as in other organs, had already been described as an adverse drug reaction5,14,16–24 (Table 1). Andersen et al.19 recently reported a case of a male patient who, 20 months after starting treatment with ipilimumab, presented granulomatous lesions localized only in the spleen. However, although there are several publications of sarcoidosis-like lesions induced by ipilimumab,15,16,21–23,25,–29 (Table 1), exclusive involvement of the skin is considered exceptional. In addition, unlike other cases, in which the granulomatous reaction presents in the first weeks or months, in our patient, presentation occurred 18 months after initiation of ipilimumab, in the form of a diffuse cutaneous granulomatous reaction. Clinical improvement was only achieved after suspension of ipilimumab.
Clinical Cases of Sarcoidosis-Like Granulomatous Reaction in Patients Diagnosed with Melanoma or Other Neoplastic Processes Treated With Ipilimumab.
| Source | Sex | Age on Starting Ipilimumab | Dose of Ipilimumab, mg/kg | Neoplasm | Organ Involved | |||
|---|---|---|---|---|---|---|---|---|
| Skin | Lung | Mediastinal and Hilar Lymph Nodes | Others | |||||
| Cervantes et al. (2017) | Male | 67 | 10 | Melanoma (stage IV) | X | |||
| Nandavaram et al.5 (2017) | Female | 57 | Not reported | Melanoma (stage III) | X | |||
| Firwana et al.16 (2016) | Male | 41 | Not reporteda | Melanoma (stage III) and colon cancer (stage III) | X | Spleen and lymph nodes in the retroauricular, occipital, cervical, axillary, iliac, and inguinal regions | ||
| Female | 65 | Not reported | Melanoma (stage III) | X | X | |||
| Female | 42 | Not reportedb | Melanoma (stage IV) | X | X | X | Lymph nodes in the cervical, axillary, and retroperitoneal region | |
| Reuss et al.28 (2016) | Female | 46 | 3c | Melanoma (stage IV) | X | X | Lymph nodes in the cervical, inguinal, supraclavicular, and left iliac region | |
| Martinez Leborans et al.29 (2016) | Female | 46 | 3 | Melanoma (stage IV) | X | X | ||
| Kim et al.21(2016) | Male | 52 | 1d | Urothelial carcinoma (stage IV) | X | X | Kidney | |
| Suozzi et al.22 (2016) | Female | 60 | 1e | Lung adenocarcinoma (stage IV) | X | |||
| Toumeh et al.23 (2016) | Female | 26 | 3 | Melanoma (stage IV) | X | X | X | Liver |
| Anderson et al.19 (2014) | Male | 55 | 3 | Melanoma (stage IV) | Spleen | |||
| Murphy et al.20 (2014) | Male | 38 | 3 | Melanoma (stage IV) | X | CNS (sella region and adjacent to the pituitary stalk) | ||
| Tissot et al.15 (2013) | Male | 57 | 10 | Melanoma (stage III) | X | X | X | |
| Reule and North26 (2013) | Male | 55 | 10 | Melanoma (stage III) | X | X | X | |
| Wilgenhof et al.14 (2012) | Female | 56 | 3 | Melanoma (stage IV) | X | X | Spleen, lymph nodes in the cervical and retroperitoneal region and in the base of the neck | |
| Berthod et al.17 (2012) | Male | 66 | 3 | Melanoma (stage IV) | X | |||
| Vogel et al.18 (2012) | Male | 63 | 3 | Melanoma (stage IV) | X | |||
| van den Eertwegh et al.24 (2012) | Not reported | Not reported | 5f | Castration-resistant prostate cancer (stage IV) | X | |||
| Eckert et al.25 (2009) | Female | 71 | 10 | Melanoma (stage IV) | X | X | X | |
| Seve et al.27 (2009) | Female | 67 | Not reported | Melanoma (stage IV) | X | X | Bronchial epithelium | |
In summary, this case can be considered both a diagnostic and therapeutic challenge, as clinically, the patient was refractory to high doses of corticosteroids and his condition only improved after suspending ipilimumab. We also highlight the importance of close follow-up of patients treated with ipilimumab as there has been shown to be a risk of developing sarcoidosis-like granulomatous lesions as an adverse drug reaction. To the best of our knowledge, this is the first case described in a patient diagnosed with melanoma who presented a granulomatous reaction confined to the skin as a side effect of ipilimumab.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank the patient for authorizing the publication of this case. Likewise, we thank Dr. George W. Elgart, of the Dermatology and Cutaneous Surgery Department, Faculty of Medicine of the Miller University in Miami, United States, who supported us with pathology images and Dr. Sadegh Amini of the Dermatology and Cutaneous Surgery Department, Faculty of Medicine of the Miller University in Miami, United States, who helped us with this case. Those acknowledged did not receive any recompense.
Please cite this article as: Cervantes J, Rosen A, Dehesa L, Dickinson G, Alonso-Llamazares J. Granulomatous Reaction in a Patient With Metastatic Melanoma Treated With Ipilimumab: First Case Reported With Isolated Cutaneous Findings. Actas Dermosifiliogr. 2019;110:43–49.