Primary cutaneous T-cell lymphomas other than mycosis fungoides, Sézary syndrome, and lymphoproliferative CD30+ disorders are few, accounting for less than 5% of all cutaneous lymphomas. A cytotoxic phenotype is characteristic of these tumors, and their clinical behavior is usually aggressive. Patients often present with extracutaneous symptoms or develop them shortly after diagnosis. Management is usually multidisciplinary, and intensive systemic therapy and bone marrow transplantation should be considered. Cutaneous B-cell lymphomas account for approximately 30% of primary cutaneous lymphomas. They make up a heterogeneous group of tumors that have different clinical and pathological features. Clinical course also varies. Presenting as papules, nodules, or tumors of variable reddish–violaceous coloring, the lesions may be solitary or multiple and occasionally form clusters. There may also be generalized lesions, present at multiple sites on the trunk, head, or extremities. Three well-defined groups of primary cutaneous lymphoma have been reported: follicle center lymphoma; marginal zone lymphoma, which follows an indolent course; and a diffuse large B-cell lymphoma, leg type, which follows an aggressive course.
Los linfomas cutáneos primarios de células T distintos de la micosis fungoide, el síndrome de Sézary y los procesos linfoproliferativos cutáneos positivos para CD30 son poco frecuentes, representan menos del 5% de todos los linfomas cutáneos; generalmente se caracterizan por un fenotipo citotóxico y habitualmente presentan un comportamiento clínico agresivo. A menudo, los pacientes presentan o desarrollan enfermedad extracutánea poco después del diagnóstico. El manejo comúnmente incluye un enfoque multidisciplinario, se debe considerar un tratamiento sistémico intensivo y un trasplante de médula ósea. Los linfomas cutáneos primarios de células B representan aproximadamente el 30% de los linfomas cutáneos primarios. Incluyen un grupo heterogéneo de entidades con diferentes características clinicopatológicas y evolutivas. Suelen presentarse como pápulas, nódulos o tumores de coloración variable (rojo-violeta), solitarios o múltiples, que aparecen ocasionalmente agrupados o como lesiones generalizadas multifocales en el tronco, la cabeza o las extremidades. Se pueden distinguir 3grupos bien definidos: el linfoma cutáneo primario de células del centro del folicular y el linfoma cutáneo primario de células de la zona marginal, que siguen un curso clínico indolente, y el linfoma difuso cutáneo primario de células B grandes del tipo de las piernas, de curso agresivo.
Primary cutaneous T-cell lymphomas, other than mycosis fungoides, Sézary syndrome, and cutaneous CD30+ lymphoproliferative disorders are uncommon, accounting for less than 5% of all cutaneous lymphomas. They are generally characterized by a cytotoxic phenotype and usually have an aggressive clinical behavior. Often, patients present with extracutaneous disease or develop it shortly after diagnosis. Management involves a multidisciplinary approach and intensive systemic treatment and even hematopoietic stem cell transplantation may be considered.
Subcutaneous Panniculitis-Like T-Cell LymphomaSubcutaneous panniculitis-like T-cell lymphoma (SPTCL) is an uncommon subtype characterized clinically by lesions resembling those in panniculitis. The neoplastic cells in this lymphoma correspond to CD8+/CD4–/CD56–/TCR-αβ T cells with a cytotoxic phenotype (TIA-1, granzyme B, perforin).1 Patients usually experience recurring inflammatory nodular cutaneous lesions over the course of months or years (with 5-year survival greater than 80%). Extracutaneous spread is uncommon, although it may exceptionally be associated with autoimmune processes (particularly lupus erythematosus).2 Recently, the presence of HAVCR2/TIM3 mutations have been reported in a group of patients with SPTCL.3 Hemophagocytic syndrome (pancytopenia, fever, and hepatosplenomegaly) may also be present. Histologically, a dense lymphoid infiltrate is observed almost exclusively in subcutaneous cell tissue. The infiltrate affects both the septa and the lobes, with a characteristic marginal distribution of atypical malignant cells around adipocytes, with processes of phagocytosis of nuclear detritus and red blood cells (hemophagocytosis). It is not associated with Epstein–Barr virus (EBV). Differential diagnosis should include other PCTCLs with predominant involvement of subcutaneous cell tissue, especially γδ-PCTCL, or secondary cutaneous forms of systemic T-cell lymphomas. Treatment is usually with low-dose oral corticosteroids, methotrexate, interferon, or even localized radiotherapy.1
Phenotype of Cutaneous γδ T-Cell Lymphomasγδ-PCTCL is a lymphoid neoplasm with a typically aggressive course. The clinical presentation is usually heterogeneous in the form of nodules or plaques with a tendency to ulcerate. These lesions may be solitary or multiple, and the site of presentation is variable. Often, extranodal extracutaneous involvement (lungs, central nervous system, intestines, etc.) or a hemophagocytic syndrome characteristically develops.4,5 Histopathologically, there is evidence of lymphoid proliferation (nodular or diffuse) formed of pleomorphic cells at times with epidermotropic predominance and at others in the form of intense infiltration of subcutaneous cell tissue, resembling an SPTCL. Histiocytes can be observed with hemophagocytic processes. Neoplastic cells do not usually express either CD4 or CD8 antigen and are γδ-TCR/CD2+/CD3+/CD56+/EBV–.6 Aggressive treatment is not usually considered for management of γδ-PCTL and cytotoxic cutaneous lymphomas in general, in elderly patients or those with associated comorbidities, whereas in other patients, the approach should consider the possibility of allogeneic stem cell transplantation. The recently described role of SETD2 mutations may open the possibility in the future to new targeted therapeutic options.7
Extranodal NK/T-Cell Lymphoma, Nasal TypeNatural killer (NK) T-cell lymphomas or extranodal NK/T lymphoma, nasal type, has an etiology clearly related to EBV infection. This group of lymphomas is more prevalent in areas of South East Asia, Central America, and South America.8 The cutaneous site is the second most frequent after the nasal cavity. The lymphoma is clinically aggressive, with rapid dissemination to extranodal organs (skin, digestive tract, etc.) and a 5-year survival of 20%.9 Clinically, the lesions are usually erythematous or multiple violaceous plaques or nodules with a tendency to ulcerate and, in cases with a nasal site, a destructive nodular lesion in the central area of the face (oral cavity and upper respiratory tract) with clinical manifestations of nasal obstruction, epistaxis, head and neck edema/inflammation (lethal midline granuloma).8 Histology shows a dense dermo-hypodermal infiltrate with frequent phenomena of angiodestruction and extensive areas of necrosis formed from cytoplasmic CD56+/CD2–/CD3ε+ cells with a cytotoxic phenotype (TIA-1+).
Hydroa Vacciniforme-Like Lymphoproliferative DisorderThe new classification of the WHO-EORTC defines the concept of chronic EBV infection that includes hydroa vacciniforme (HV)-like lesions. This is a CD8+ T-lymphoproliferative disorder often associated with a frequent history of extensive exposure to mosquito bites and expression of NK cell markers. It is observed in certain ethnic groups and specific geographic areas such as Latin America and Asia. HV-like processes are usually observed in children or young adults and are manifest in the form of infiltrated papules or plaques, scabs/ulceration, blisters, and edema, which usually resolve leaving varioliform scars in exposed areas. There is a risk of progression to lymphoma as an adult. The process can be associated with systemic symptoms such as fever, weight loss, general malaise, swollen lymph nodes, hepatosplenomegaly, and anemia.6,10
Nonspecific Cutaneous Peripheral T-Cell LymphomasNonspecific cutaneous T-cell lymphomas include those cutaneous lymphoproliferative disorders that cannot be readily included in the previous categories given their clinical-pathological characteristics and poorly defined immunophenotypes. In general, they correspond to aggressive lymphomas with morphology or expression of cytotoxic markers and with variable expression of T-cell markers.11
Provisional EntitiesPrimary Cutaneous CD8+ Aggressive Epidermotropic Cytotoxic T-Cell LymphomaPrimary cutaneous CD8+ aggressive cytotoxic epidermotropic T-cell lymphoma (CD8+ PCACETL) is manifest as generalized macules, plaques, and tumors, often ulcerated, and hemorrhagic lesions often with mucosal involvement (oral, genital), although indolent psoriasiform lesions or even those resembling mycosis fungoides (MF) have been described. CD8+ PCACETL follows an aggressive course with rapid extracutaneous extranodal spread (lungs, central nervous system, etc.) and an extremely poor prognosis. Histologically, nodular or diffuse proliferation of atypical lymphocytes is observed with marked epidermotropism, necrosis, and epidermal ulceration with invasion and occasional destruction of skin appendages and vascular structures. The neoplastic cells correspond to lymphocytes with a cytotoxic CD3+/CD7–/CD8+/CD45RA+ (CD2–/CD4–/CD5–/CD30–)/TIA-1+ phenotype. Differential diagnosis should essentially be considered with other cytotoxic lymphomas and different indolent processes of CD8+ phenotype, such as pagetoid reticulosis, some cases of CD8+ MF, lymphomatoid papulosis type D, or primary cutaneous CD30+ anaplastic large-cell lymphoma.12 Early intensive systemic treatment is required, and the option of hematopoietic stem cell transplantation can be considered.13
Primary Cutaneous Acral CD8+ T-Cell LymphomaAcral CD8+ PCTL presents with solitary nodular lesions or lesions in a bilateral and symmetric distribution on the outer ears and other acral areas (nose, fingers). Growth is slow and the course is not aggressive. The site and characteristics of the lesions have led to suggestions of the possible reactive nature of this entity. The histopathological findings correspond to a dense, diffuse monomorphic, nonepidermotropic infiltrate consisting of atypical small to medium sized T cells diffusely infiltrating the dermis and subcutaneous cell tissue. Tumor cells express a cytotoxic CD8+/TIA1+/granzyme B – immunophenotype.14 The dotted perinuclear expression of the CD68 antigen is a possible differential characteristic not present in other aggressive cytotoxic T-cell lymphomas.
Small/Medium Sized Pleomorphic CD4+ T-Cell Lymphoproliferative DisorderThe concept of small/medium sized pleomorphic CD4+ T-cell lymphoproliferative disorder covers cases of cutaneous T-cell proliferations with an indolent course that present as solitary nodules or a limited number of nodules, grouped on the cephalic pole or upper trunk and whose histology shows a dense dermal lymphoid infiltrate of CD3+/CD4+/CD5+/CD7–/CD30– T cells that also express follicle center cell markers such as BCL6+/CD10+/CXCL13/ICOS. The PD1 antigen, although nonspecific, is the main marker necessary for diagnosis of this entity.5,15,16 In the current WHO classification of hematologic cancers, it is not considered a true lymphoma but rather a T lymphoproliferative disorder very likely of reactive nature.
Primary Cutaneous B-Cell LymphomasPrimary cutaneous B-cell lymphomas (PCBCLs) account for approximately 30% of primary cutaneous lymphomas. They include a very heterogeneous group of entities with differentiated clinicopathologic features and varying clinical course. PCBCLs include follicle center lymphomas (PCFCL), marginal zone lymphomas (PCMZLs), and diffuse large B-cell lymphoma, leg type (DLBCLLT).5,17
PCMZL and PCFCL are lymphoproliferative disorders with a clinically indolent course, whereas DLBCLLT follows an aggressive course and the clinical approach is similar to that of systemic B-cell lymphomas.18,19 The study of initial extent of PCBCL should rule out skin involvement of a systemic lymphoma (secondary cutaneous lymphoma) (Table 1).17,19 Differential diagnosis between PCMZL, PCFCL, reactive lymphoid hyperplasia (RLH), or a CD4+ small/medium pleomorphic T-cell lymphoproliferative disorder may occasionally present difficulties because clinical and histopathological aspects may overlap (mixed population of T and B cells, variable cytomorphology with small cell predominance).18
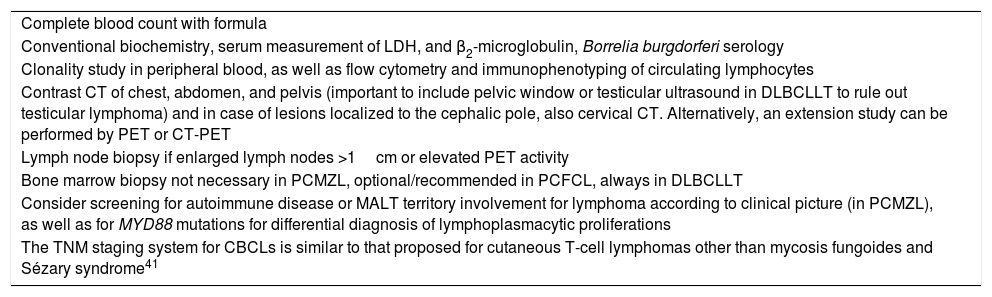
Additional Tests for Diagnosis and Staging of Cutaneous B-Cell Lymphomas.
| Complete blood count with formula |
| Conventional biochemistry, serum measurement of LDH, and β2-microglobulin, Borrelia burgdorferi serology |
| Clonality study in peripheral blood, as well as flow cytometry and immunophenotyping of circulating lymphocytes |
| Contrast CT of chest, abdomen, and pelvis (important to include pelvic window or testicular ultrasound in DLBCLLT to rule out testicular lymphoma) and in case of lesions localized to the cephalic pole, also cervical CT. Alternatively, an extension study can be performed by PET or CT-PET |
| Lymph node biopsy if enlarged lymph nodes >1cm or elevated PET activity |
| Bone marrow biopsy not necessary in PCMZL, optional/recommended in PCFCL, always in DLBCLLT |
| Consider screening for autoimmune disease or MALT territory involvement for lymphoma according to clinical picture (in PCMZL), as well as for MYD88 mutations for differential diagnosis of lymphoplasmacytic proliferations |
| The TNM staging system for CBCLs is similar to that proposed for cutaneous T-cell lymphomas other than mycosis fungoides and Sézary syndrome41 |
Source: Senff et al.17
PCFCL is the most frequent type of PCBCL accounting for 50%-60% of such these lymphomas. It is manifest clinically in adults in the form of erythematous papules, plaques, or nodules with no tendency to ulcerate, as solitary or multiple lesions, occasionally grouped, often on the cephalic pole or trunk. Extracutaneous involvement may be observed in up to 10% of cases and it is usually a lymphoma with a favorable prognosis, with 5-year survival greater than 90%.17,20 Recurrences limited to the skin are not uncommon.
Histologically, PCFCLs are characterized by a dermal lymphoid infiltrate with a diffuse or mixed nodular follicular pattern (formation of expanded and confluent lymphoid follicles), sparing the papillary dermis and occasionally spreading to the subcutaneous cell tissue. Irregular lymphoid follicles, superimposed, with recognizable germinal centers consisting of centrocyte and centroblast aggregates on a network of well-structured dendritic cells are observed. They do not show polarity (clear and dark zones) and have well-formed mantles. In the variants with a diffuse pattern, the formation of lymphoid follicles is not observed.20 Neoplastic cells correspond to B cells (CD19+, CD20+, CD79a+, PAX-5+), which express germinal center cell markers (BCL-6, CD10) and, unlike nodal follicle center lymphomas, they are usually BCL2–. They do not express activated B-cell markers (MUM1–/FOXP1–), and so can be differentiated from DLBCLLT. The presence of not very intense (<50%) proliferation marker in germinal cells supports diagnosis. With expression of BCL2, systemic follicular lymphoma, PCMZL, or DLBCLLT can be ruled out.21,22 Specific genetic mutations have not been identified. The (14;18) (q32;q21)-IgH/BCL2 translocation is not usually detected, although in some series it has been detected in 10% of cases. Occasionally, translocations between the IgH and BCL6 genes, amplifications at 2p16.31-REL, or deletions at 14q32.32 have been described.23,24 Differential diagnosis of some PCFCLs should be established with B-cell RHL, especially with a follicular pattern. However, the lymphoid follicles observed in RHL are usually more monomorphic and better formed than those of PCFCLs with polarity and they do not show follicular center cell expansion toward interfollicular zones. Clonality studies can help determine the monoclonal or reactive origin of the process.21
Primary Cutaneous Marginal Zone B-Cell LymphomaPCMZL is not recognized as a separate entity in the current WHO classification of hematological neoplasms, but is rather included within the mucosa-associated lymphoid tissue (MALT) type lymphomas, which develop in extranodal or mucosal sites such as the stomach, saliva glands, orbit, thyroids, breasts, or lungs.5,18,19,25 MALT type lymphomas appear to develop in tissues in which there is persistent lymphoid activation as a result of chronic antigen stimulation, such as with Helicobacter pylori in gastric MALT lymphomas, Campylobacer jejuni in intestinal lymphomas, or Borrelia burgdorferi infection, vaccines, or tattoos in cutaneous processes. PCMZLs are characterized clinically by the presence of solitary or multiple papules, plaques, or nodules on the trunk or limbs of adults. Exclusively cutaneous recurrences are observed in 50% of these patients, although extracutaneous involvement is uncommon. Mortality is low (100% survival at 5 years).17,25–27
Histologically, the process is characterized by nodular, diffuse infiltrate, often periadnexal, in the reticular dermis and subcutaneous cell tissue, sparing the papillary dermis and epidermis, formed of small or medium size lymphocytes (monocytes), with indented nuclei and clear cytoplasm, similar to marginal zone cells. Often, large cells (similar to centroblasts) and a variable number of lymphocytes with plasmacytoid morphology and plasma cells are observed, thus facilitating diagnosis. Reactive germ cells and expansion of neoplastic marginal zone cells toward the interfollicular areas are observed.17,31 These are mature B cells (CD20+, CD22+, CD79a), with aberrant CD43+ expression. They express BCL2 and are CD10–/BCL6–. Often, there is a prominent or predominant accompanying T lymphoid population, which can cause diagnostic difficulties when it comes to differentiating from RHL lesions or from a small/medium sized pleomorphic CD4+ T-cell lymphoproliferative disorder. Two subgroups of PCMZL have been described: an uncommon group with IgM/CXCR3 expression, similar to other MALT type lymphomas, and a group with IgG+/IgG4/CXCR3– expression, also known as class-switched. A monotypic expression of short chains of the immunoglobulins can be demonstrated in most cases.28
Clonality study by PCR detects the presence of a dominant clone in most cases. It is generally not be necessary to perform bone marrow biopsy; underlying infection or a situation of chronic antigen stimulation, including autoimmune diseases, should be ruled out. Likewise, the presence of lymphoid proliferation in other MALT territories or MYD88 mutations should also be ruled out for differential diagnosis with other lymphoplasmacytic disorders with secondary cutaneous presentation. Chronic stimulation of this lymphoid tissue makes it genetically unstable, facilitating a build-up of genetic abnormalities such as trisomy 3, trisomy 18, t(1;14) (p22;q32), t(11;18) (q21;q21), t(14;18) (q32;q21)-IgH/MALT, t(3;14) (q27;q32), or t(3;14) (p14.1;q32), thereby giving rise to lymphomatous transformation. These genetic abnormalities are detected in a limited number of PCMZLs (10–15%).29
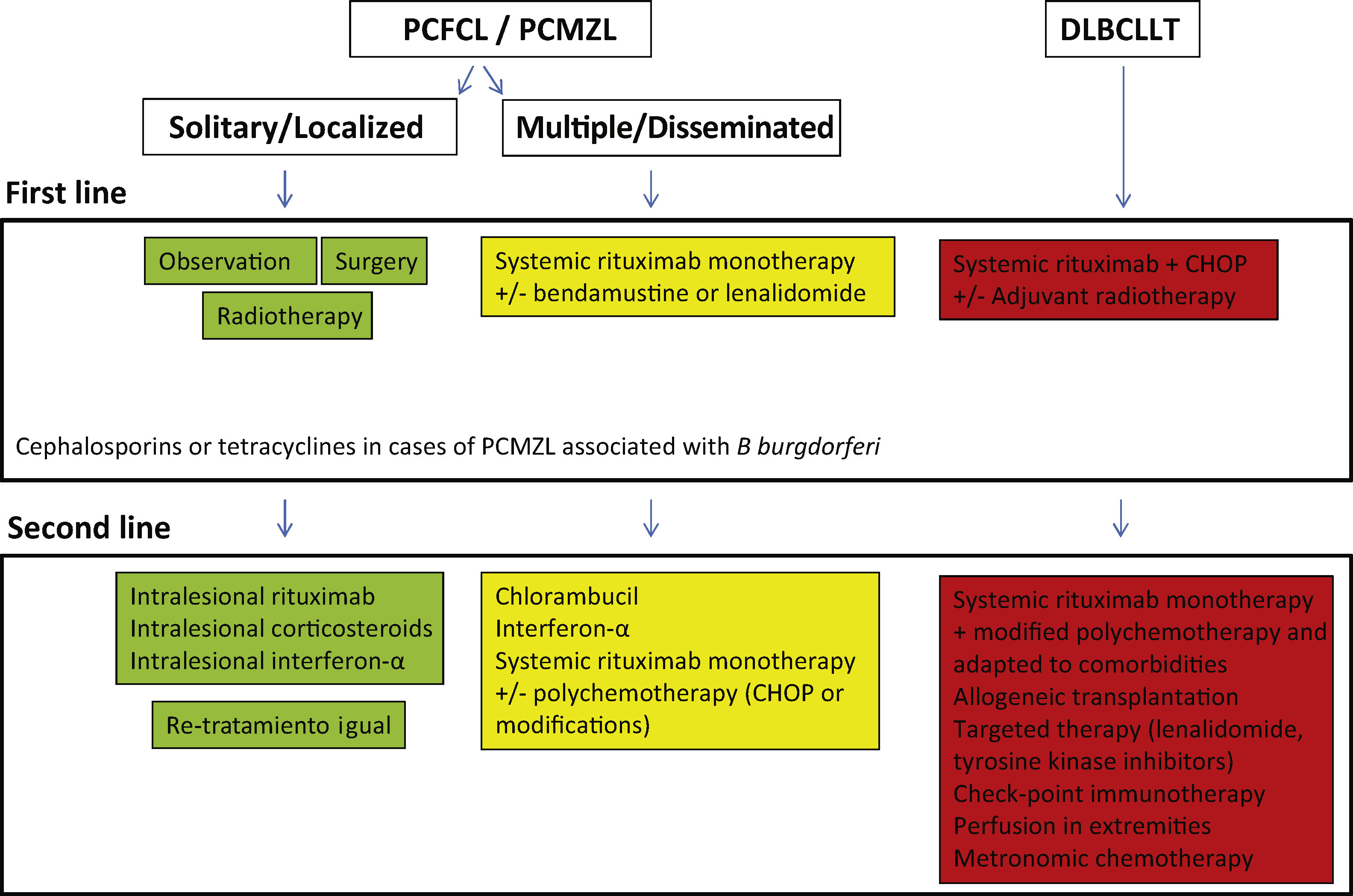
Treatment of Indolent Primary Cutaneous B-Cell LymphomasTreatment of indolent PCBCLs (PCFCL and PCMZL) is very similar. These are highly radiosensitive lymphomas, and so local electrons or photon radiotherapy is a therapeutic option for solitary tumor lesions or grouped lesions that would require complex surgery (Fig. 1). Doses of 20 to 36Gy are recommended for PCMZL and 30Gy for PCFCL, although, recently, treatment regimens with low radiation doses have been proposed to reduce toxicity.30,31 The option of intralesional rituximab has been shown to be safe and effective for those cases in which other options are not possible, thus avoiding surgical scarring or postradiotherapy alopecia.32 In patients with multiple lesions, systemic treatments are usually recommended (intravenous rituximab in monotherapy or associated with chemotherapy).
Diffuse Large B-Cell Lymphoma, Leg TypeDLBCLLT is a malignant lymphoid lymphoproliferative disorder involving large cells (centroblasts, immunoblasts) with an activated B-cell phenotype. It is usually observed in elderly individuals and 5-year survival is 50%.5,18–20 DLBCLLT is usually manifest as solitary or grouped nodular lesions or tumors, with a tendency to form ulcers. Although it was initially described on one or both legs, it can develop at other sites.5,18–20,33,34 Histopathological study shows a diffuse monomorphic infiltrate that usually occupies the entire dermis, occasionally with subcutaneous cell tissue involvement. The infiltrate is formed of large rounded BCL-2+/MUM-1+/FOXP1+/BCL6+ cells. The cells usually express MYC, IgM, and P63.5,18–20,22,33The genetic profile of DLBCLLT is similar to that observed in systemic large diffuse B-cell lymphomas, with mutations in genes implicated in the NF-κB signaling pathway (CD79B, PM1CARD11, and MYD88 in 60%-75% of cases), translocations or amplifications of IgH, BCL6, MYC, or FOXP1 genes, as well as deletions or hypermethylation of the p16 promotor.30,33,35,36
DLBCLLT is treated in similar fashion to systemic diffuse lymphomas with rituximab in combination with polychemotherapy (CHOP) or regimens adapted to the patients’ comorbidities. Local radiotherapy is used as adjuvant or palliative treatment. Extensive knowledge of specific molecular pathways in these aggressive lymphomas opens up the possibility of targeted therapies. In addition, immunotherapy with check-point inhibitors has been developed and hematopoietic stem cell transplantation is an option in some patients.5,17,19,20
Primary Cutaneous Intravascular Large B-Cell LymphomaPrimary cutaneous intravascular large B-cell lymphoma (PCIVLBCL) is an uncommon process with an aggressive course whose particular feature is a malignant lymphoid infiltration of activated MUM-1+/BCL2+ B cells inside vascular lumens.37 PCIVLBCL tends to affect different organs and specifically the skin (macules, telangiectases) and central nervous system. In a significant number of cases, skin lesions are the first manifestation of this process, although most patients have systemic disease at the time of diagnosis.37
Provisional EntitiesMucocutaneous Ulcer Associated with Epstein–Barr VirusMucocutaneous ulcer caused by EBV has been included as a provisional entity within the classification of lymphoid neoplasms. It is observed in elderly individuals or those who have been treated with immunosuppressants. Clinically, it is usually manifest as one or several ulcers with well-defined borders in the oropharyngeal mucosa or gastrointestinal tract. Occasionally, these lesions may present with other skin lesions. It is usually a self-limiting process, which improves after resolution of immunosuppression.5,23,38 Histologically, it is characterized by epithelial ulceration, with pseudoepitheliomatous hyperplasia and a polymorphous dermal/submucosal infiltrate with lymphocytes, immunoblasts, Reed-Sternberg-like cells, small lymphoid cells, plasma cells, and eosinophils, along with extensive areas of necrosis. Atypical cells with a blast appearance correspond to B lymphocytes that express PAX-5+/OCT-2+/EBER+/CD30+/CD15+.38
Other Nonlymphoproliferative Hematological Processes With Predominant or Characteristic Skin InvolvementDendritic Cell Leukemia (CD4+/CD56+ Hematodermic Neoplasm)This is an aggressive malignant hematological neoplasm of precursor plasmacytoid dendritic or myeloid cells in which skin lesions are often the first manifestation of disease, although multisystemic involvement (peripheral blood, bone marrow) is usually present at the time of diagnosis or becomes evident soon afterwards.23,39 Skin lesions usually correspond to solitary or multiple nodules, characteristically purpuric and of rapid growth, located on the trunk and cephalic pole, with no tendency to ulcerate. Histopathologically, diffuse dermal infiltration of a monomorphic nonepidermotropic cell population is observed with a cytomorphology similar to lymphoblasts or myeloblasts with CD123/TCL1 expression and a CD4+/CD56+/CD8–/CD7±/CD2±/CD45RA+/CD3– phenotype.23,39 Aggressive chemotherapy can achieve complete remission, although recurrences happen early and overall survival is not usually greater than 1 year, and so hematopoietic stem cell transplantation can be considered.23 Recently, the US Food and Drug Administration has approved tagraxofusp, a fusion protein of the diphtheria toxin with interleukin 3, as a possible treatment for this disease.40
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pujol RM, Gallardo F. Linfomas cutáneos. Parte II: otros linfomas cutáneos. Actas Dermosifiliogr. 2021;112:24–31.